Deck 4: Solution Chemistry: The Hydrosphere
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/148
Play
Full screen (f)
Deck 4: Solution Chemistry: The Hydrosphere
1
The amount of blood in the human body is around 5.0 L, and this amount of blood contains about 0.88 g of potassium ions. What is the concentration of potassium ions in blood?
A)0.88 mM
B)0.18 mM
C)23 mM
D)4.5 mM
E)2.3 mM
A)0.88 mM
B)0.18 mM
C)23 mM
D)4.5 mM
E)2.3 mM
4.5 mM
2
If there are 0.505 g of NaCl left in a beaker that originally contained 75.0 mL of saltwater, what must have been the concentration of the original solution?
A)0.00647 M
B)0.0115 M
C)0.0673 M
D)0.115 M
E)0.673 M
A)0.00647 M
B)0.0115 M
C)0.0673 M
D)0.115 M
E)0.673 M
0.115 M
3
Concentrated sulfuric acid contains 4 g of water for every 100 g of solution. The solvent is ________
A)water.
B)sulfuric acid.
C)concentrated.
D)the same as the solution.
E)the same as the solute in this case.
A)water.
B)sulfuric acid.
C)concentrated.
D)the same as the solution.
E)the same as the solute in this case.
sulfuric acid.
4
Human blood contains about 0.18 g potassium ions per liter and has a density of 1.06 g/mL. What is the concentration of potassium ions in blood expressed in parts per million?
A)18 ppm
B)180 ppm
C)170 ppm
D)88 ppm
E)880 ppm
A)18 ppm
B)180 ppm
C)170 ppm
D)88 ppm
E)880 ppm

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
5
Lead is the least toxic metal ion to fish compared to zinc, copper, and mercury ions. Fish can tolerate a lead concentration slightly above 0.850 mM. What is this concentration in ppm? Assume the density of the water is 1.00 g/mL.
A)176 ppm
B)83 ppm
C)352 ppm
D)0.176 ppm
E)0.352 ppm
A)176 ppm
B)83 ppm
C)352 ppm
D)0.176 ppm
E)0.352 ppm

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
6
The National Primary Drinking Water Regulations limit the concentration of lead in drinking water to 0.015 mg/L, which is ________
A)0.015 ppb.
B)15 ppm.
C)1.5 ppb.
D)15 ppb.
E)150 ppb.
A)0.015 ppb.
B)15 ppm.
C)1.5 ppb.
D)15 ppb.
E)150 ppb.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
7
Assuming that the density of water is 1.00 g/mL, how many moles of water are there in a liter of water?
A)1.00 moles
B)0.0180 moles
C)55.5 moles
D)18.0 moles
E)100 moles
A)1.00 moles
B)0.0180 moles
C)55.5 moles
D)18.0 moles
E)100 moles

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
8
What is the concentration, in g/L, of a 0.100 M sodium chloride solution?
A)1.71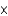
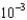 g/L
g/L
B)5.84 g/L
C)584 g/L
D)11.7 g/L
E)3.42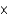
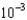 g/L
g/L
A)1.71
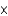
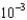 g/L
g/LB)5.84 g/L
C)584 g/L
D)11.7 g/L
E)3.42
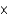
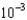 g/L
g/L
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
9
What are the units of molar concentration, M ?
A)mol/L
B)g/L
C)mol/kg
D)ppm
E)mol/mL
A)mol/L
B)g/L
C)mol/kg
D)ppm
E)mol/mL

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
10
Determine the molar concentration of an aqueous solution of lead(II) nitrate solution that is 26 ppb (parts per billion) Pb(NO3)2. Since the solution is very dilute, assume the density is that of water, 1.00 g/mL.
A)7.85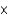
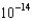 M
M
B)2.60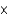
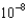 M
M
C)4.26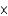
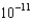 M
M
D)7.85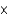
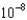 M
M
E)7.85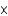
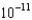 M
M
A)7.85
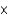
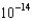 M
MB)2.60
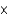
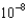 M
MC)4.26
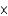
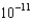 M
MD)7.85
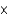
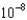 M
ME)7.85
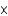
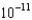 M
M
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
11
A chemistry student attempted to make a 0.2000 M solution of NaCl using a 100.0 mL volumetric flask. She added exactly 100.0 mL of water to the flask, then added 0.02000 mol of NaCl, and found that the total volume in the flask was above the 100.0 mL mark. What was the concentration of the solution?
A)exactly 0.2000 M
B)a bit less than 0.2000 M
C)a bit more than 0.2000 M
D)exactly 0.2002 M
E)There is insufficient information to select one of the above responses.
A)exactly 0.2000 M
B)a bit less than 0.2000 M
C)a bit more than 0.2000 M
D)exactly 0.2002 M
E)There is insufficient information to select one of the above responses.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
12
Molarity, M, is defined as ________
A)moles of solute dissolved in 1 mol of solvent.
B)moles of solute dissolved in 1 kg of solvent.
C)moles of solute dissolved in 1 L of solvent.
D)moles of solute dissolved in 1 L of solution.
E)moles of solute dissolved in the solution.
A)moles of solute dissolved in 1 mol of solvent.
B)moles of solute dissolved in 1 kg of solvent.
C)moles of solute dissolved in 1 L of solvent.
D)moles of solute dissolved in 1 L of solution.
E)moles of solute dissolved in the solution.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
13
A concentrated aqueous ammonia solution has a density of 0.90 g/mL and is 28.0% ammonia by mass. Determine the molar concentration of this solution.
A)15 M
B)1.5 M
C)0.032 M
D)31 M
E)3.0 M
A)15 M
B)1.5 M
C)0.032 M
D)31 M
E)3.0 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
14
Sodium fluoride (NaF) is added to drinking water in some municipalities to protect teeth against cavities. The idea is to convert hydroxyapatite, Ca10(PO4)6(OH)2, into more stable fluorapatite, Ca10(PO4)6F2. The molar mass of hydroxyapatite is 502 g/mol. What is the molarity of a 10.0 mg/L sodium fluoride solution?
A)2.38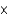
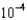 M
M
B)1.99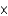
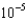 M
M
C)2.63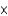
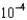 M
M
D)1.19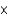
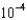 M
M
E)0.263 M
A)2.38
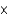
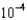 M
MB)1.99
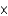
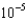 M
MC)2.63
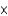
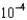 M
MD)1.19
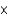
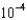 M
ME)0.263 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
15
A homogeneous mixture of two or more substances is called ________
A)a compound.
B)an electrolyte.
C)a solution.
D)a solvent.
E)a mess.
A)a compound.
B)an electrolyte.
C)a solution.
D)a solvent.
E)a mess.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following mixtures is not a solution?
A)white vinegar
B)bottled spring water
C)filtered dry air
D)clear tea
E)pulpy orange juice
A)white vinegar
B)bottled spring water
C)filtered dry air
D)clear tea
E)pulpy orange juice

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
17
Mercury is one of the most toxic metal ions to fish, as they can only tolerate a concentration slightly above 1.5 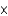
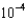 mM. What is the mercury concentration in g/L?
mM. What is the mercury concentration in g/L?
A)6.0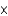
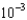 g/L
g/L
B)12 g/L
C)30 g/L
D)75 g/L
E)1.5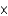
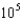 g/L
g/L
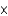
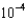 mM. What is the mercury concentration in g/L?
mM. What is the mercury concentration in g/L?A)6.0
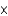
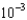 g/L
g/LB)12 g/L
C)30 g/L
D)75 g/L
E)1.5
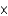
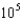 g/L
g/L
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
18
If 120 g of NaOH were used to prepare 500 mL of solution, what would the concentration be?
A)1 M
B)2 M
C)3 M
D)4 M
E)6 M
A)1 M
B)2 M
C)3 M
D)4 M
E)6 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following cartoons depicts a solution?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
20
A medical saline solution is prepared by mixing 55 mg of morphine (C17H19NO3) with water to make 75.0 mL of solution. What is the millimolar concentration of this solution?
A)1.45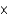
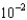 mM
mM
B)2.57 mM
C)3.69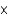
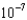 mM
mM
D)14.5 mM
E)2.09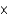
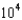 mM
mM
A)1.45
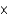
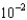 mM
mMB)2.57 mM
C)3.69
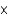
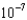 mM
mMD)14.5 mM
E)2.09
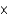
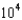 mM
mM
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
21
If 100 mL of 3.0 M solution were diluted to 250 mL, what would the concentration be?
A)0.012 M
B)0.12 M
C)1.2 M
D)12 M
E)120 M
A)0.012 M
B)0.12 M
C)1.2 M
D)12 M
E)120 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
22
What volume of 12 M HCl solution needs to be diluted to produce 275 mL of 2.1 M HCl solution?
A)1.8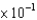 mL
mL
B)9.2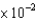 mL
mL
C)5.7 mL
D)23 mL
E)48 mL
A)1.8
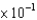 mL
mLB)9.2
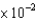 mL
mLC)5.7 mL
D)23 mL
E)48 mL

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
23
The World Health Organization recommends that the maximum allowable concentration of chromium(VI) in drinking water be limited to 0.05 mg/L. If the molar absorptivity of chromium(VI) at 345 nm is 1.5 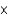 103/(M cm), what is the absorbance of a solution that contains 0.050 mg/L in a 10-cm-long cell?
103/(M cm), what is the absorbance of a solution that contains 0.050 mg/L in a 10-cm-long cell?
A)0.0014
B)0.75
C)0.014
D)0.075
E)14
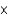 103/(M cm), what is the absorbance of a solution that contains 0.050 mg/L in a 10-cm-long cell?
103/(M cm), what is the absorbance of a solution that contains 0.050 mg/L in a 10-cm-long cell?A)0.0014
B)0.75
C)0.014
D)0.075
E)14

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
24
Commercial hydrochloric acid is 12.1 M. What volume of commercial HCl solution should be used to prepare 250.0 mL of 3.00 M HCl?
A)139 mL
B)126 mL
C)252 mL
D)62 mL
E)83 mL
A)139 mL
B)126 mL
C)252 mL
D)62 mL
E)83 mL

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds is a strong electrolyte?
A)methane, CH4
B)methanol, CH3OH
C)ammonia, NH3
D)hydrofluoric acid, HF
E)potassium chloride, KCl
A)methane, CH4
B)methanol, CH3OH
C)ammonia, NH3
D)hydrofluoric acid, HF
E)potassium chloride, KCl

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
26
Which picture best represents an atomic-level view of a weak electrolyte solution (water molecules not shown)?
A)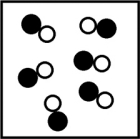
B)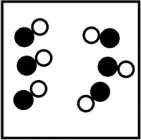
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
27
What volume of 3.0 M NaOH contains 0.15 mole of NaOH?
A)500 mL
B)50 mL
C)5.0 mL
D)0.50 mL
E)0.050 mL
A)500 mL
B)50 mL
C)5.0 mL
D)0.50 mL
E)0.050 mL

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
28
What mass of potassium iodide (molar mass = 166.0 g/mol) is needed to produce 325.0 mL of a solution that has a concentration of 0.0150 M ?
A)2.94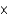
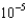 g
g
B)0.809 g
C)809 g
D)7.66 g
E)1.31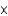
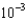 g
g
A)2.94
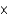
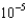 g
gB)0.809 g
C)809 g
D)7.66 g
E)1.31
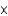
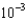 g
g
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
29
How many moles of ammonium hydroxide are present in 100 mL of a 0.17 M solution?
A)1.7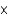
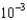 moles
moles
B)5.8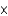
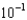 moles
moles
C)1.7 moles
D)1.7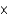
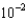 moles
moles
E)17 moles
A)1.7
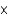
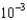 moles
molesB)5.8
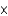
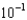 moles
molesC)1.7 moles
D)1.7
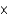
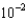 moles
molesE)17 moles

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
30
Which contains more solute particles: a 0.10 M aqueous solution of methanol (CH3OH) or a 0.10 M aqueous solution of salt (NaCl)?
A)They contain the same number of solute particles.
B)The salt solution contains twice as many particles as the methanol solution.
C)The methanol solution contains three times as many particles as the salt solution.
D)Neither solution contains solute particles.
E)The methanol solution contains twice as many particles as the salt solution.
A)They contain the same number of solute particles.
B)The salt solution contains twice as many particles as the methanol solution.
C)The methanol solution contains three times as many particles as the salt solution.
D)Neither solution contains solute particles.
E)The methanol solution contains twice as many particles as the salt solution.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
31
How many grams of solid magnesium chloride, MgCl2, are needed to make 250 mL of 0.50 M solution?
A)9.5 g
B)48 g
C)12 g
D)125 g
E)4.8 g
A)9.5 g
B)48 g
C)12 g
D)125 g
E)4.8 g

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
32
How many moles of magnesium nitrate are present in 75.0 mL of a 0.33 M solution?
A)2.5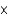
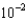 moles
moles
B)25 moles
C)4.4 moles
D)2.3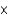
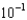 moles
moles
E)4.4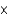
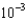 moles
moles
A)2.5
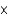
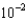 moles
molesB)25 moles
C)4.4 moles
D)2.3
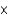
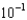 moles
molesE)4.4
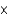
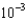 moles
moles
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
33
Which picture best represents an atomic-level view of a strong electrolyte solution (water molecules not shown)?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
34
How many grams of magnesium chloride, MgCl2, are needed to make up 500.0 mL of a solution that is 0.300 M ?
A)14.3 g
B)57.1 g
C)158 g
D)1.58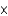
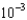 g
g
E)1.75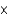
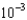 g
g
A)14.3 g
B)57.1 g
C)158 g
D)1.58
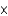
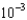 g
gE)1.75
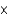
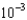 g
g
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
35
The World Health Organization recommends that the maximum allowable concentration of lead(II) in drinking water be limited to 0.015 mg/L. If the absorbance of lead(II) at 417 nm is 0.289 at this concentration, what is the molar absorptivity, assuming a 10-cm-long cell was used?'
A)4.0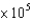 M-1
M-1
B)1.3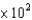 M-1cm-1
M-1cm-1
C)430 M-1cm-1
D)2.8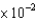 M-1cm-1
M-1cm-1
E)6.1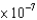 M-1cm-1
M-1cm-1
A)4.0
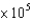 M-1
M-1B)1.3
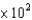 M-1cm-1
M-1cm-1C)430 M-1cm-1
D)2.8
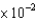 M-1cm-1
M-1cm-1E)6.1
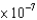 M-1cm-1
M-1cm-1
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
36
What volume of 0.25 M hydrochloric acid (HCl) solution contains 0.15 mol HCl?
A)6.0 mL
B)60 mL
C)600 mL
D)1.7 L
E)170 mL
A)6.0 mL
B)60 mL
C)600 mL
D)1.7 L
E)170 mL

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
37
Hartmann's solution is used in intravenous therapy to replace lost body fluid and mineral salts. The total ion concentration in Hartmann's solution is 276 mM. It often is prepared from a more concentrated stock solution. How much stock solution is needed to prepare 500 mL of Hartmann's solution? The total ion concentration in the stock solution is 1.38 M.
A)50 mL
B)10 mL
C)100 mL
D)500 mL
E)200 mL
A)50 mL
B)10 mL
C)100 mL
D)500 mL
E)200 mL

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
38
Which picture best represents an atomic-level view of a nonelectrolyte solution (water molecules not shown)?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
39
Determine the molar concentration of ethanol (C2H6O) in a wine that is 14% ethanol by mass. The density of this wine is 0.93 g/cm3.
A)0.063 M
B)13.0 M
C)0.14 M
D)2.8 M
E)3.0 M
A)0.063 M
B)13.0 M
C)0.14 M
D)2.8 M
E)3.0 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
40
In the dilution of 10.0 mL of a 0.10 M solution of HCl to a volume of 20.0 mL, what remains unchanged?
A)the moles of HCl in the solution
B)the concentration of the HCl solution
C)the volume of the HCl solution
D)the mass of the HCl solution
E)All of the above change.
A)the moles of HCl in the solution
B)the concentration of the HCl solution
C)the volume of the HCl solution
D)the mass of the HCl solution
E)All of the above change.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
41
What are the spectator ions in the reaction of barium hydroxide and nitric acid?
A)H3O- and NO3-
B)Ba2+ and OH-
C)H3O+ and OH-
D)Ba2+ and NO3-
E)Ba2+, NO3-, H3O+, and OH-
A)H3O- and NO3-
B)Ba2+ and OH-
C)H3O+ and OH-
D)Ba2+ and NO3-
E)Ba2+, NO3-, H3O+, and OH-

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
42
Identify a spectator ion in the following acid-base reaction. Ca(CO3)(s) 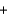 2HCl(aq)
2HCl(aq)  CaCl2(aq)
CaCl2(aq) 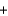 2H2O(l )
2H2O(l ) 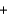 CO2(g)
CO2(g)
A)H+
B)Ca2+
C)Cl-
D)CO32-
E)H3O+
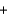 2HCl(aq)
2HCl(aq)  CaCl2(aq)
CaCl2(aq) 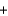 2H2O(l )
2H2O(l ) 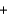 CO2(g)
CO2(g)A)H+
B)Ca2+
C)Cl-
D)CO32-
E)H3O+

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
43
If the molar concentration of sodium sulfate (Na2SO4) is 0.10 M, what is the concentration of sodium ion?
A)0.10 M
B)0.050 M
C)0.20 M
D)0.30 M
E)0.40 M
A)0.10 M
B)0.050 M
C)0.20 M
D)0.30 M
E)0.40 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
44
Which one of the following statements regarding a weak base is not correct?
A)A weak base ionizes only to a small extent in water.
B)A weak base ionizes in water to produce hydroxide ions.
C)A weak base neutralizes acids.
D)Sodium hydroxide is an example of a weak base.
E)A weak base can also be a weak electrolyte.
A)A weak base ionizes only to a small extent in water.
B)A weak base ionizes in water to produce hydroxide ions.
C)A weak base neutralizes acids.
D)Sodium hydroxide is an example of a weak base.
E)A weak base can also be a weak electrolyte.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
45
Which picture best represents an atomic-level view of hydrochloric acid, which is a strong acid, in aqueous solution (water molecules not shown)?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
46
What is the molar concentration of sodium ions in a 0.3 M sodium phosphate (Na3PO4, 164 g/mol) solution? Sodium phosphate is used as a cleaning agent, food additive, and stain remover.
A)0.1 M
B)0.3 M
C)0.6 M
D)0.9 M
E)1.0 M
A)0.1 M
B)0.3 M
C)0.6 M
D)0.9 M
E)1.0 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
47
Hartmann's solution is used in intravenous therapy to replace lost body fluid and mineral salts. Hartmann's solution contains 5.00 mM potassium chloride, 2.00 mM calcium chloride, and 102 mM sodium chloride. What is the total chloride ion concentration in Hartmann's solution?
A)109 mM
B)107 mM
C)111 mM
D)104 mM
E)218 mM
A)109 mM
B)107 mM
C)111 mM
D)104 mM
E)218 mM

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
48
In a demonstration of strong electrolytes, weak electrolytes, and nonelectrolytes, Professor Popsnorkle used a lightbulb apparatus that showed how much a solution conducted electricity by the brightness of the lightbulb. When pure water was tested, the bulb did not light. When some acetic acid was added to the water, the bulb burned dimly. When more acetic acid was added to the solution, the bulb burned a little more brightly. In his frustration to make the bulb shine brightly with acetic acid, Professor Popsnorkle started over by testing the beaker of the pure acetic acid. What was the result?
A)The bulb did not light.
B)The bulb burned dimly.
C)The bulb burned more than any of the others but still not brightly.
D)The bulb burned brightly.
E)Professor Popsnorkle was electrocuted.
A)The bulb did not light.
B)The bulb burned dimly.
C)The bulb burned more than any of the others but still not brightly.
D)The bulb burned brightly.
E)Professor Popsnorkle was electrocuted.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
49
What is the molar concentration of sodium in a 200.0 mL solution prepared from 1.223 g of sodium phosphate (Na3PO4, 163.9 g/mol), which is a cleaning agent, food additive, and stain remover?
A)0.03731 M
B)0.2486 M
C)0.7338 M
D)0.1119 M
E)0.1243 M
A)0.03731 M
B)0.2486 M
C)0.7338 M
D)0.1119 M
E)0.1243 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
50
If 50.0 mL of a 0.10 M solution of sodium chloride is mixed with 50.0 mL of 0.10 M magnesium chloride, what is the molar concentration of chloride in the resulting solution?
A)0.10 M
B)0.20 M
C)0.05 M
D)0.15 M
E)0.25 M
A)0.10 M
B)0.20 M
C)0.05 M
D)0.15 M
E)0.25 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
51
In its reaction with water, ammonia (NH3) ________
A)acts as an acid.
B)acts as a base.
C)acts neither as an acid nor as a base.
D)serves as both an acid and as a base.
E)causes a precipitate to form.
A)acts as an acid.
B)acts as a base.
C)acts neither as an acid nor as a base.
D)serves as both an acid and as a base.
E)causes a precipitate to form.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the base in the following acid-base reaction. NaNO2(aq) 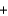 HI(aq)
HI(aq)  NaI(aq)
NaI(aq) 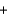 HNO2(aq)
HNO2(aq)
A)Na+
B)NO2-
C)I-
D)HI
E)HNO2
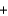 HI(aq)
HI(aq)  NaI(aq)
NaI(aq) 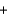 HNO2(aq)
HNO2(aq)A)Na+
B)NO2-
C)I-
D)HI
E)HNO2

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following compounds is a nonelectrolyte?
A)sulfuric acid, H2SO4
B)acetic acid, CH3COOH
C)hexane, C6H14
D)nitrous acid, HNO2
E)potassium hydroxide, KOH
A)sulfuric acid, H2SO4
B)acetic acid, CH3COOH
C)hexane, C6H14
D)nitrous acid, HNO2
E)potassium hydroxide, KOH

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
54
In the following reaction, H2O ________ CH3COOH(aq) 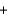 H2O(l)
H2O(l)  CH3COO-(aq)
CH3COO-(aq) 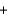 H3O+(aq)
H3O+(aq)
A)acts as an acid.
B)acts as a base.
C)acts neither as an acid nor as a base.
D)serves as both an acid and as a base.
E)causes a precipitate to form.
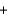 H2O(l)
H2O(l)  CH3COO-(aq)
CH3COO-(aq) 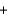 H3O+(aq)
H3O+(aq)A)acts as an acid.
B)acts as a base.
C)acts neither as an acid nor as a base.
D)serves as both an acid and as a base.
E)causes a precipitate to form.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
55
Which one of the following is the molecular equation for the reaction of hydrobromic acid with potassium hydroxide? All species are in aqueous solution.
A)H+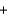 Br -
Br -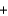 K+
K+ OH-
OH- KBr
KBr  H2O
H2O
B)H+ Br -
Br - K+
K+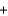 OH-
OH-  K+
K+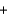 Br -
Br -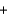 H2O
H2O
C)HBr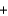 KOH
KOH  KBr
KBr 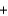 H2O
H2O
D)K+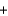 Br-
Br- KBr
KBr
E)H+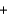 OH-
OH- H2O
H2O
A)H+
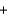 Br -
Br - K+
K+ OH-
OH- KBr
KBr  H2O
H2OB)H+
 Br -
Br - K+
K+ OH-
OH-  K+
K+ Br -
Br - H2O
H2OC)HBr
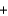 KOH
KOH  KBr
KBr 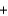 H2O
H2OD)K+
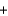 Br-
Br- KBr
KBrE)H+
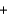 OH-
OH- H2O
H2O
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
56
In a demonstration of strong electrolytes, weak electrolytes, and nonelectrolytes, Professor Popsnorkle used a lightbulb apparatus that showed how much a solution conducted electricity by the brightness of the lightbulb. When pure water was tested, the bulb did not light. Then Professor Popsnorkle tested the following aqueous solutions. Which one caused the bulb to burn the brightest?
A)table salt, NaCl
B)ethanol, CH3CH2OH
C)table sugar, C12H22O11
D)acetic acid, CH3COOH
E)methanol, CH3OH
A)table salt, NaCl
B)ethanol, CH3CH2OH
C)table sugar, C12H22O11
D)acetic acid, CH3COOH
E)methanol, CH3OH

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
57
Which one of the following reaction equations is the net ionic equation for the reaction of hydrochloric acid with lithium hydroxide? All species are in aqueous solution.
A)HCl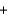 LiOH
LiOH  LiCl
LiCl 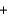 H2O
H2O
B)H+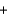 Cl-
Cl-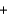 Li+
Li+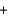 OH-
OH- Li+
Li+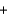 Cl-
Cl-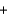 H2O
H2O
C)H+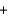 Cl-
Cl-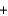 Li+ OH-
Li+ OH- LiCl
LiCl 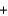 H2O
H2O
D)Li+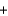 Cl-
Cl- LiCl
LiCl
E)H+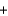 OH-
OH- H2O
H2O
A)HCl
 LiOH
LiOH  LiCl
LiCl  H2O
H2OB)H+
 Cl-
Cl- Li+
Li+ OH-
OH- Li+
Li+ Cl-
Cl- H2O
H2OC)H+
 Cl-
Cl- Li+ OH-
Li+ OH- LiCl
LiCl  H2O
H2OD)Li+
 Cl-
Cl- LiCl
LiClE)H+
 OH-
OH- H2O
H2O
Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the acid in the following acid-base reaction. PbCO3(s) 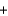 H2SO4(aq)
H2SO4(aq)  PbSO4(s)
PbSO4(s) 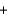 CO2(g)
CO2(g) 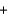 H2O(l)
H2O(l)
A)PbCO3(s)
B)CO2(g)
C)PbSO4(s)
D)H2O(l)
E)H2SO4(aq)
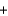 H2SO4(aq)
H2SO4(aq)  PbSO4(s)
PbSO4(s) 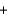 CO2(g)
CO2(g) 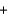 H2O(l)
H2O(l)A)PbCO3(s)
B)CO2(g)
C)PbSO4(s)
D)H2O(l)
E)H2SO4(aq)

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following statements regarding a strong acid is not correct?
A)A strong acid ionizes completely in water.
B)A strong acid ionizes in water to produce hydronium ions.
C)A strong acid neutralizes bases.
D)HCl is an example of a strong acid.
E)Acids are only strong at a high concentration.
A)A strong acid ionizes completely in water.
B)A strong acid ionizes in water to produce hydronium ions.
C)A strong acid neutralizes bases.
D)HCl is an example of a strong acid.
E)Acids are only strong at a high concentration.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
60
Chalk contains calcium carbonate. What would be the best solution for cleaning a sidewalk that a preschool class covered with smiling suns, flowers, birds, rainbows, and houses using sidewalk chalk?
A)ammonia
B)plain water
C)vinegar
D)paint thinner
E)olive oil
A)ammonia
B)plain water
C)vinegar
D)paint thinner
E)olive oil

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following phosphate compounds is soluble in water?
A)Ag3PO4
B)Ca3(PO4)2
C)(NH4)3PO4
D)AlPO4
E)Mg3(PO4)2
A)Ag3PO4
B)Ca3(PO4)2
C)(NH4)3PO4
D)AlPO4
E)Mg3(PO4)2

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
62
If 1.0 L of 1.0 M HCl spilled and needed to be neutralized, how many grams of the solid sodium carbonate (Na2CO3, 106 g/mol) would be required?
A)53 g
B)106 g
C)1,060 g
D)530 g
E)212 g
A)53 g
B)106 g
C)1,060 g
D)530 g
E)212 g

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
63
A 500 mg dietary supplement of L-lysine (an amino acid, 146.2 g/mol) required 68.4 mL of 0.100 M NaOH to reach the end point. How many protons were removed for each L-lysine molecule in this titration?
A)1
B)2
C)3
D)0.500
E)2.5
A)1
B)2
C)3
D)0.500
E)2.5

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
64
If one regular antacid tablet contains 500 mg of solid CaCO3 (100 g/mol), how many mL of 1.0 M stomach acid (HCl) could it neutralize?
A)5 mL
B)10 mL
C)50 mL
D)100 mL
E)15 mL
A)5 mL
B)10 mL
C)50 mL
D)100 mL
E)15 mL

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
65
A 125 mL sample of orange juice was titrated using a redox reaction to the equivalence point with the addition of 7.6 mL of a 0.0025M iodine (I2) solution. What is the concentration of vitamin C (C6H8O6) in this sample? C6H8O6(aq) 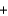 I2(aq)
I2(aq)  C6H6O6(aq)
C6H6O6(aq) 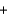 2I-(aq)
2I-(aq) 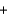 2H+(aq)
2H+(aq)
A)30 mM
B)15 mM
C)19 mM
D)0.30 mM
E)0.15 mM
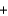 I2(aq)
I2(aq)  C6H6O6(aq)
C6H6O6(aq) 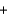 2I-(aq)
2I-(aq) 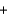 2H+(aq)
2H+(aq)A)30 mM
B)15 mM
C)19 mM
D)0.30 mM
E)0.15 mM

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
66
Most chloride salts are soluble. Identify an exception to this generalization.
A)AgCl
B)CaCl2
C)MgCl2
D)BaCl2
E)NaCl
A)AgCl
B)CaCl2
C)MgCl2
D)BaCl2
E)NaCl

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
67
In a titration, the solution of known concentration delivered by the burette is called the titrant, and the solution being titrated is called the analyte. To carry out a calculation to determine an unknown concentration of a sample from titration data, one would need all of the following data except ________
A)the volume of the titrant delivered.
B)the volume of the analyte.
C)the stoichiometry of the reaction between the titrant and the analyte.
D)the concentration of the analyte.
E)the concentration of the titrant.
A)the volume of the titrant delivered.
B)the volume of the analyte.
C)the stoichiometry of the reaction between the titrant and the analyte.
D)the concentration of the analyte.
E)the concentration of the titrant.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following compounds are soluble in water? PbCO3 , Fe2S3 , AgNO3, NH4OH
I II III IV
A)I and II only
B)II and IV only
C)III and IV only
D)I, II, and IV only
E)All compounds are soluble.
I II III IV
A)I and II only
B)II and IV only
C)III and IV only
D)I, II, and IV only
E)All compounds are soluble.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
69
Which one of the following is not a polyprotic acid?
A)sulfuric acid, H2SO4
B)phosphoric acid, H3PO4
C)carbonic acid, H2CO3
D)hydrogen sulfide, H2S
E)acetic acid, CH3COOH
A)sulfuric acid, H2SO4
B)phosphoric acid, H3PO4
C)carbonic acid, H2CO3
D)hydrogen sulfide, H2S
E)acetic acid, CH3COOH

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
70
Hard water contains Mg2+ and Ca2+ ions and tends to form a ring in a bathtub due to its reaction with the soluble anions in soap. The formation of this insoluble material is an example of ________
A)an acid-base reaction.
B)a precipitation reaction.
C)a redox reaction.
D)a bathochromic shift.
E)bad housekeeping.
A)an acid-base reaction.
B)a precipitation reaction.
C)a redox reaction.
D)a bathochromic shift.
E)bad housekeeping.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following ionic compounds is insoluble in water?
A)cesium fluoride
B)cesium chloride
C)cesium bromide
D)cesium iodide
E)All the compounds are soluble in water.
A)cesium fluoride
B)cesium chloride
C)cesium bromide
D)cesium iodide
E)All the compounds are soluble in water.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
72
How many liters of 0.200 M NaOH solution are required to completely react with 1.00 L of 0.100 M HCN solution to produce sodium cyanide and water?
A)0.25 L
B)0.50 L
C)1.00 L
D)1.50 L
E)2.00 L
A)0.25 L
B)0.50 L
C)1.00 L
D)1.50 L
E)2.00 L

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
73
Sodium thiosulfate (Na2S2O3, molar mass = 158.2 g/mol) is used in the development of photographic film. Your summer job is at a photography lab and you need to check the purity of an outdated supply. You react 40.21 mL of 0.246 M iodine solution with a 3.232 g sample. What is the percent purity of the sodium thiosulfate that you report to your boss? I2(aq) 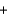 +2S2O32-(aq)
+2S2O32-(aq)  2I-(aq)
2I-(aq) 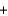 S4O62-(aq)
S4O62-(aq)
A)100%
B)48.4%
C)96.8%
D)98.6%
E)84.4%
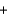 +2S2O32-(aq)
+2S2O32-(aq)  2I-(aq)
2I-(aq) 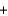 S4O62-(aq)
S4O62-(aq)A)100%
B)48.4%
C)96.8%
D)98.6%
E)84.4%

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following ionic compounds is insoluble in water?
A)BaCl2
B)BaSO4
C)NaOH
D)Ba(NO3)2
E)MgCl2
A)BaCl2
B)BaSO4
C)NaOH
D)Ba(NO3)2
E)MgCl2

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
75
Most alkaline earth salts are soluble. Identify an exception to this generalization.
A)MgSO4
B)CaS
C)Sr(OH)2
D)BaSO4
E)Ca(NO3)2
A)MgSO4
B)CaS
C)Sr(OH)2
D)BaSO4
E)Ca(NO3)2

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
76
Which one of the following water solubility guidelines A-D is not valid?
A)Salts with an alkali metal cation are soluble.
B)Salts with a nitrate anion are soluble.
C)Most ionic compounds with chloride and bromide anions are soluble.
D)Salts with the ammonium ion are soluble.
E)The guidelines A-D all are valid.
A)Salts with an alkali metal cation are soluble.
B)Salts with a nitrate anion are soluble.
C)Most ionic compounds with chloride and bromide anions are soluble.
D)Salts with the ammonium ion are soluble.
E)The guidelines A-D all are valid.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
77
Water-soluble toxic chromium compounds are waste products of electroplating operations, but the chromium can be precipitated as Cr(OH)3 to remediate the water. How much 1.0 M NaOH solution is needed to remove the chromium from 100 L of a solution that is 0.001 M in Cr3+?
A)100 mL
B)300 mL
C)10 L
D)30 L
E)33 L
A)100 mL
B)300 mL
C)10 L
D)30 L
E)33 L

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
78
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4+. If 5.00 mL of a solution of an ammonia cleaner is titrated directly with 42.6 mL of 0.5000 M HCl, what is the concentration of the NH3 in solution? (Assume that the ammonia is the only solute that reacts with the acid.)
A)0.0587 M
B)0.107 M
C)4.26 M
D)1.07 M
E)5.87 M
A)0.0587 M
B)0.107 M
C)4.26 M
D)1.07 M
E)5.87 M

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
79
In carrying out a titration of a hydrochloric acid solution with a standard sodium hydroxide solution, a student went beyond the end point before reading the volume on the burette. That is, the volume used was larger than the volume required to reach the end point. How will this error affect the calculated concentration of the hydrochloric acid?
A)The calculated concentration will be larger than the actual concentration.
B)The calculated concentration will be smaller than the actual concentration.
C)The calculated concentration will be the correct concentration.
D)There is no way to tell how this error will affect the calculation.
E)The calculated concentration will be the actual concentration.
A)The calculated concentration will be larger than the actual concentration.
B)The calculated concentration will be smaller than the actual concentration.
C)The calculated concentration will be the correct concentration.
D)There is no way to tell how this error will affect the calculation.
E)The calculated concentration will be the actual concentration.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck
80
In carrying out a titration of a potassium hydroxide solution with a standard hydrochloric acid solution, a student misread the volume on the burette. That is, the actual volume recorded was smaller than the volume required to reach the end point. How will this error affect the calculated concentration of the potassium hydroxide solution?
A)The calculated concentration will be larger than the actual concentration.
B)The calculated concentration will be smaller than the actual concentration.
C)The calculated concentration will be the correct concentration.
D)There is no way to determine how this error will affect the calculation.
E)The calculated concentration will be the actual concentration.
A)The calculated concentration will be larger than the actual concentration.
B)The calculated concentration will be smaller than the actual concentration.
C)The calculated concentration will be the correct concentration.
D)There is no way to determine how this error will affect the calculation.
E)The calculated concentration will be the actual concentration.

Unlock Deck
Unlock for access to all 148 flashcards in this deck.
Unlock Deck
k this deck


