Deck 18: Electrochemistry: The Quest for Clean Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/167
Play
Full screen (f)
Deck 18: Electrochemistry: The Quest for Clean Energy
1
In the smelting of iron from iron oxide according to the equation  what is the change in oxidation number for iron?
what is the change in oxidation number for iron?
A)(3)
B)(3)
C)(2)
D)(2)
E)0
 what is the change in oxidation number for iron?
what is the change in oxidation number for iron?A)(3)
B)(3)
C)(2)
D)(2)
E)0
(3)
2
Sodium carbonate is produced using the Solvay process, which involves a final step where sodium bicarbonate is heated to produce sodium carbonate, water, and carbon dioxide. What are the oxidation numbers of the sodium, carbon, and oxygen, respectively, in sodium carbonate?
A)(1, 4, and 2)
B)(1, 2, and 2)
C)0, 2, and 2)
D)(1, 2, and 2)
E)(1, 2, and 0)
A)(1, 4, and 2)
B)(1, 2, and 2)
C)0, 2, and 2)
D)(1, 2, and 2)
E)(1, 2, and 0)
(1, 4, and 2)
3
In the reaction below, ________ is the oxidizing agent. 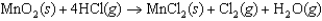
A)MnO2(s)
B)HCl(g)
C)MnCl2(s)
D)Cl2(g)
E)H2O(g)
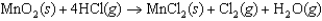
A)MnO2(s)
B)HCl(g)
C)MnCl2(s)
D)Cl2(g)
E)H2O(g)
MnO2(s)
4
Oxidation refers to ________
A)an increase in oxidation number.
B)a decrease in oxidation number.
C)a gain in the number of protons.
D)an increase in the atomic number.
E)an increase in mass.
A)an increase in oxidation number.
B)a decrease in oxidation number.
C)a gain in the number of protons.
D)an increase in the atomic number.
E)an increase in mass.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
5
The thermite reaction, shown below, is very exothermic and is often used as an initiator in fireworks. In this reaction, ________ is the reducing agent. 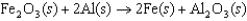
A)Fe2O3(s)
B)Al(s)
C)Fe(s)
D)Al2O3(s)
E)H2(g)
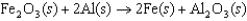
A)Fe2O3(s)
B)Al(s)
C)Fe(s)
D)Al2O3(s)
E)H2(g)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
6
Oxidation is the ________
A)gain of electrons.
B)loss of electrons.
C)gain of protons.
D)loss of protons.
E)loss of mass.
A)gain of electrons.
B)loss of electrons.
C)gain of protons.
D)loss of protons.
E)loss of mass.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
7
Where in the periodic table do you find the elements that typically do not participate in redox processes?
A)in group 1
B)in group 2
C)in group 16
D)in group 17
E)in group 18
A)in group 1
B)in group 2
C)in group 16
D)in group 17
E)in group 18

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
8
When aluminum metal is obtained from aluminum oxide (Al2O3), ________ moles of electrons must be transferred for each mole of aluminum oxide processed.
A)2
B)3
C)4
D)6
E)9
A)2
B)3
C)4
D)6
E)9

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
9
Reduction refers to ________
A)a decrease in oxidation number.
B)an increase in oxidation number.
C)a gain in the number of protons.
D)a decrease in the atomic number.
E)loss of mass.
A)a decrease in oxidation number.
B)an increase in oxidation number.
C)a gain in the number of protons.
D)a decrease in the atomic number.
E)loss of mass.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
10
What is the oxidation number of chromium in the ionic compound ammonium dichromate, (NH4)2Cr2O7?
A)(3)
B)(4)
C)(5)
D)(6)
E)(7)
A)(3)
B)(4)
C)(5)
D)(6)
E)(7)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
11
The following reaction occurs in basic solution. Identify the reducing agent. Note that the reaction equation is not balanced. 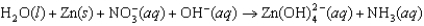
A)Zn(s)
B)NO3(aq)
C)OH(aq)
D)H2O(l )
E)Zn(OH)42(aq)
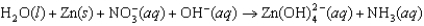
A)Zn(s)
B)NO3(aq)
C)OH(aq)
D)H2O(l )
E)Zn(OH)42(aq)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
12
The following reaction occurs in basic solution. Identify the oxidizing agent. Note that the reaction equation is not balanced. 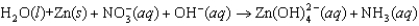
A)Zn(s)
B)NO3(aq)
C)OH(aq)
D)H2O(l)
E)NH3(aq)
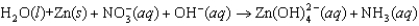
A)Zn(s)
B)NO3(aq)
C)OH(aq)
D)H2O(l)
E)NH3(aq)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
13
The following reaction occurs in a new battery called the super iron battery. In this reaction, ________ is oxidized. 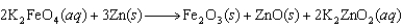
A)Fe in K2FeO4
B)Zn metal
C)Fe in Fe2O3
D)Zn in ZnO
E)Zn in K2ZnO2
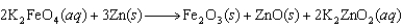
A)Fe in K2FeO4
B)Zn metal
C)Fe in Fe2O3
D)Zn in ZnO
E)Zn in K2ZnO2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following items does not characterize a reducing agent?
A)A reducing agent loses electrons.
B)A reducing agent causes another species to be reduced.
C)The oxidation number of a reducing agent increases.
D)A good reducing agent is a metal in a high oxidation state, such as Mn7.
E)An example of a good reducing agent is an alkali metal, such as Na.
A)A reducing agent loses electrons.
B)A reducing agent causes another species to be reduced.
C)The oxidation number of a reducing agent increases.
D)A good reducing agent is a metal in a high oxidation state, such as Mn7.
E)An example of a good reducing agent is an alkali metal, such as Na.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
15
Glancing at a periodic table, where do you expect to find elements that are good oxidizing agents?
A)on the right (except for the last group)
B)in the middle left
C)in the top left
D)at the bottom
E)in the transition metals
A)on the right (except for the last group)
B)in the middle left
C)in the top left
D)at the bottom
E)in the transition metals

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following items does not characterize an oxidizing agent?
A)An oxidizing agent gains electrons.
B)An oxidizing agent causes another species to be oxidized.
C)The oxidation number of an oxidizing agent decreases.
D)A good oxidizing agent is a metal in a high oxidation state, such as Mn7.
E)An example of a good oxidizing agent is an alkali metal, such as Na.
A)An oxidizing agent gains electrons.
B)An oxidizing agent causes another species to be oxidized.
C)The oxidation number of an oxidizing agent decreases.
D)A good oxidizing agent is a metal in a high oxidation state, such as Mn7.
E)An example of a good oxidizing agent is an alkali metal, such as Na.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
17
Where in the periodic table do you find the elements that are the best reducing agents with the most negative standard reduction potentials?
A)in group 16
B)on the left
C)in the middle
D)at the bottom
E)in group 17
A)in group 16
B)on the left
C)in the middle
D)at the bottom
E)in group 17

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
18
In the following reaction 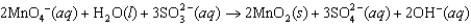 what is the change in oxidation number for S?
what is the change in oxidation number for S?
A)(3)
B)(3)
C)(2)
D)(2)
E)0
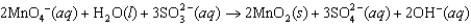 what is the change in oxidation number for S?
what is the change in oxidation number for S?A)(3)
B)(3)
C)(2)
D)(2)
E)0

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
19
The following reaction occurs in a new battery called the super iron battery. In this reaction, ________ is reduced. 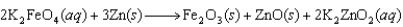
A)Fe in K2FeO4
B)Zn metal
C)Fe in Fe2O3
D)Zn in ZnO
E)Zn in K2ZnO2
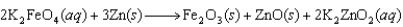
A)Fe in K2FeO4
B)Zn metal
C)Fe in Fe2O3
D)Zn in ZnO
E)Zn in K2ZnO2

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
20
Reduction is the ________
A)gain of electrons.
B)loss of electrons.
C)gain of protons.
D)loss of protons.
E)loss of mass.
A)gain of electrons.
B)loss of electrons.
C)gain of protons.
D)loss of protons.
E)loss of mass.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
21
A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the zinc nitrate on the diagram. 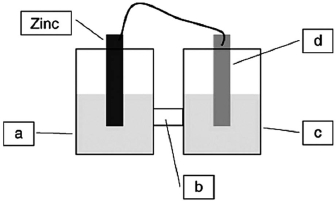
A)a
B)b
C)c
D)d

A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement about a cathode in a voltaic cell is not correct?
A)Oxidation occurs at the cathode.
B)Reduction occurs at the cathode.
C)Usually the cathode is a metal strip.
D)In the external circuit, electrons flow toward the cathode.
E)Chemical species can have their oxidation numbers decreased at the cathode.
A)Oxidation occurs at the cathode.
B)Reduction occurs at the cathode.
C)Usually the cathode is a metal strip.
D)In the external circuit, electrons flow toward the cathode.
E)Chemical species can have their oxidation numbers decreased at the cathode.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
23
Proteins containing a certain functional group (identified as RSH) can be titrated with triiodide ion to produce another functional group (identified as RSSR). The reaction equation is given below. What is oxidized and what is reduced in this reaction? 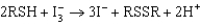
A)RSH is oxidized; I
Is reduced.
B)RSH is reduced; I is oxidized.
C)Both RSH and I are oxidized.
D)Both RSH and I are reduced.
E)This reaction is not oxidationreduction.
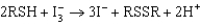
A)RSH is oxidized; I

Is reduced.
B)RSH is reduced; I is oxidized.
C)Both RSH and I are oxidized.
D)Both RSH and I are reduced.
E)This reaction is not oxidationreduction.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
24
A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the salt bridge in the diagram. 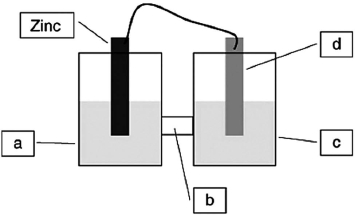
A)a
B)b
C)c
D)d

A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
25
The diagram below represents a voltaic cell. Which one of the statements about this cell is not correct? Zn(s)|Zn2(1.0 M)||Cu2(1.0 M)|Cu(s)
A)The mass of the zinc electrode decreases during discharge.
B)The copper electrode is the anode.
C)Electrons flow through the external circuit from the zinc electrode to the copper electrode.
D)Reduction occurs at the copper electrode during discharge.
E)The concentration of copper ions decreases during discharge.
A)The mass of the zinc electrode decreases during discharge.
B)The copper electrode is the anode.
C)Electrons flow through the external circuit from the zinc electrode to the copper electrode.
D)Reduction occurs at the copper electrode during discharge.
E)The concentration of copper ions decreases during discharge.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following chemical reaction used to construct a voltaic cell 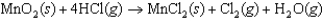 Which one of the following statements is correct?
Which one of the following statements is correct?
A)MnCl2 is produced at the anode.
B)Cl ions are reduced at the cathode.
C)MnO2 is the reducing agent.
D)Three moles of electrons are transferred in this reaction.
E)Electrons travel from Cl to Mn4.
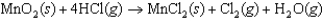 Which one of the following statements is correct?
Which one of the following statements is correct?A)MnCl2 is produced at the anode.
B)Cl ions are reduced at the cathode.
C)MnO2 is the reducing agent.
D)Three moles of electrons are transferred in this reaction.
E)Electrons travel from Cl to Mn4.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
27
Which statement about a voltaic cell is not correct?
A)Electrons are produced as a product at the cathode.
B)Reduction occurs at the cathode.
C)Usually the cathode is a metal strip.
D)In the external circuit, electrons flow toward the cathode.
E)Chemical species can have their oxidation numbers decreased at the cathode.
A)Electrons are produced as a product at the cathode.
B)Reduction occurs at the cathode.
C)Usually the cathode is a metal strip.
D)In the external circuit, electrons flow toward the cathode.
E)Chemical species can have their oxidation numbers decreased at the cathode.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
28
The electrodes on batteries are labeled and . The ________ is labeled ________, and ________ occurs there.
A)anode; positive; oxidation
B)anode; negative; reduction
C)cathode; positive; reduction
D)cathode; negative; reduction
E)cathode; positive; oxidation
A)anode; positive; oxidation
B)anode; negative; reduction
C)cathode; positive; reduction
D)cathode; negative; reduction
E)cathode; positive; oxidation

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
29
The diagram below represents a voltaic cell. In this cell, which species is oxidized? Cd(s)|Cd2(aq)||Fe2(aq),Fe3(aq)|Pt(s)
A)Cd(s)
B)Cd2(aq)
C)Fe2(aq)
D)Fe3(aq)
E)Pt(s)
A)Cd(s)
B)Cd2(aq)
C)Fe2(aq)
D)Fe3(aq)
E)Pt(s)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement regarding voltaic cells is not correct?
A)Reduction occurs at the cathode.
B)Anions move through the barrier or bridge toward the electrode where oxidation is occurring.
C)The electrode where reduction is occurring is represented by a positive sign.
D)Electrons flow in the external circuit from the cathode to the anode.
E)Electrons flow in the external circuit toward the electrode represented by a positive sign.
A)Reduction occurs at the cathode.
B)Anions move through the barrier or bridge toward the electrode where oxidation is occurring.
C)The electrode where reduction is occurring is represented by a positive sign.
D)Electrons flow in the external circuit from the cathode to the anode.
E)Electrons flow in the external circuit toward the electrode represented by a positive sign.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
31
The diagram below represents a voltaic cell. In this cell, the electron flow is from ________ Cd(s)|Cd2(aq)||Fe2(aq),Fe3(aq)|Pt(s)
A)Pt to Cd2.
B)Pt to Cd.
C)Fe2 to Cd2.
D)Cd2 to Fe3.
E)Cd to Fe3.
A)Pt to Cd2.
B)Pt to Cd.
C)Fe2 to Cd2.
D)Cd2 to Fe3.
E)Cd to Fe3.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
32
Which cell diagram is correct for the following electrochemical cell? 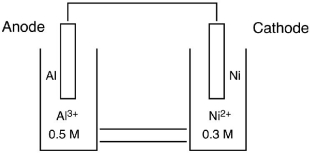
A)Al(s)|Al3(aq) (0.50 M )||Ni(s)| Ni2(aq) (0.30 M )
B)Ni(s)|Al3(aq) (0.50 M )|Ni2(aq) (0.30 M )|Al(s)
C)Al(s)|Al3(aq) (0.50 M )||Ni2(aq) (0.30 M )|Ni(s)
D)Ni2(aq) (0.30 M )|Ni(s)||Al3(aq) (0.50 M )|Al(s)
E)Ni(s)|Ni2(aq) (0.30 M )||Al(s)|Al3(aq) (0.50 M )

A)Al(s)|Al3(aq) (0.50 M )||Ni(s)| Ni2(aq) (0.30 M )
B)Ni(s)|Al3(aq) (0.50 M )|Ni2(aq) (0.30 M )|Al(s)
C)Al(s)|Al3(aq) (0.50 M )||Ni2(aq) (0.30 M )|Ni(s)
D)Ni2(aq) (0.30 M )|Ni(s)||Al3(aq) (0.50 M )|Al(s)
E)Ni(s)|Ni2(aq) (0.30 M )||Al(s)|Al3(aq) (0.50 M )

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
33
You have a job as a summer intern in an orange juice processing plant. Part of your job is to determine the amount of vitamin C in a given quantity of orange juice. To make this determination, you titrate the orange juice with I3, the triiodide ion. To make sure you know what you are doing, your supervisor asks you how many electrons are transferred in the reaction. The reaction describing the titration is given below. What is your response? ascorbate 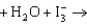 dehydroascorbate 2H 3I
dehydroascorbate 2H 3I
A)1
B)2
C)4
D)0
E)It is impossible to tell.
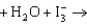 dehydroascorbate 2H 3I
dehydroascorbate 2H 3IA)1
B)2
C)4
D)0
E)It is impossible to tell.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
34
A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the silver nitrate on the diagram. 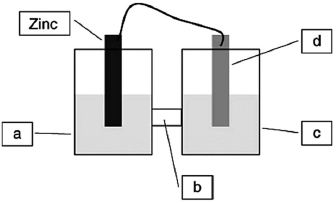
A)a
B)b
C)c
D)d

A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
35
Consider an electrochemical cell with a Zn electrode in ZnSO4(aq) and a Cu electrode in CuSO4(aq). The overall chemical reaction is 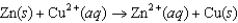 Which one of the following statements is correct?
Which one of the following statements is correct?
A)Copper is oxidized at the anode.
B)One mole of electrons is transferred in this reaction.
C)Zinc is reduced at the cathode.
D)Copper ions are reduced at the cathode.
E)Electrons travel from the Cu electrode to the Zn electrode.
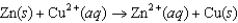 Which one of the following statements is correct?
Which one of the following statements is correct?A)Copper is oxidized at the anode.
B)One mole of electrons is transferred in this reaction.
C)Zinc is reduced at the cathode.
D)Copper ions are reduced at the cathode.
E)Electrons travel from the Cu electrode to the Zn electrode.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
36
Which statement is not correct for a voltaic cell?
A)The electron flow in the external circuit is from the negative electrode to the positive electrode.
B)The electron flow in the external circuit is from the anode to the cathode.
C)Electrons are transferred from the oxidizing agent to the reducing agent.
D)Chemical energy is transformed into electrical energy by a spontaneous redox reaction.
E)Positive ions diffuse through a porous bridge from the anode compartment to the cathode compartment.
A)The electron flow in the external circuit is from the negative electrode to the positive electrode.
B)The electron flow in the external circuit is from the anode to the cathode.
C)Electrons are transferred from the oxidizing agent to the reducing agent.
D)Chemical energy is transformed into electrical energy by a spontaneous redox reaction.
E)Positive ions diffuse through a porous bridge from the anode compartment to the cathode compartment.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
37
For the following reaction, which statement AD is not correct? 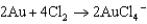
A)Au is the reducing agent.
B)Cl2 is the oxidizing agent.
C)Au is oxidized.
D)The equation is balanced.
E)More than one statement is not correct.
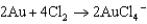
A)Au is the reducing agent.
B)Cl2 is the oxidizing agent.
C)Au is oxidized.
D)The equation is balanced.
E)More than one statement is not correct.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
38
The electrodes on batteries are labeled and . The ________ is labeled ________, and ________ occurs there.
A)anode; positive; oxidation
B)anode; negative; oxidation
C)cathode; positive; oxidation
D)cathode; negative; reduction
E)anode; positive; reduction
A)anode; positive; oxidation
B)anode; negative; oxidation
C)cathode; positive; oxidation
D)cathode; negative; reduction
E)anode; positive; reduction

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
39
Which cell diagram is correct for this electrochemical cell? 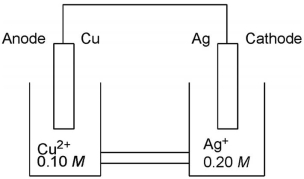
A)Ag(s)|Ag(aq) (0.20 M )||Cu(s)|Cu2(aq) (0.10 M )
B)Ag(s)|Cu2(aq) (0.10 M )||Ag(aq) (0.20 M )|Cu(s)
C)Ag(aq) (0.20 M )|Ag(s)||Cu2(aq) (0.10 M )|Cu(s)
D)Cu(s)|Cu2(aq) (0.10 M )||Ag(aq) (0.20 M )|Ag(s)
E)Cu(s)|Cu2(aq) (0.10 M )||Ag(s)| Ag(aq) (0.20 M )

A)Ag(s)|Ag(aq) (0.20 M )||Cu(s)|Cu2(aq) (0.10 M )
B)Ag(s)|Cu2(aq) (0.10 M )||Ag(aq) (0.20 M )|Cu(s)
C)Ag(aq) (0.20 M )|Ag(s)||Cu2(aq) (0.10 M )|Cu(s)
D)Cu(s)|Cu2(aq) (0.10 M )||Ag(aq) (0.20 M )|Ag(s)
E)Cu(s)|Cu2(aq) (0.10 M )||Ag(s)| Ag(aq) (0.20 M )

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
40
A voltaic cell is constructed based on the oxidation of zinc metal and the reduction of silver cations. Solutions of silver nitrate and zinc nitrate also were used. Locate the silver metal on the diagram. 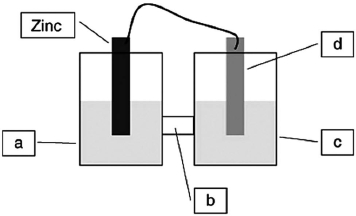
A)a
B)b
C)c
D)d

A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
41
Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest oxidizing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 1.80
1.80  1.50
1.50 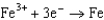 0.771
0.771 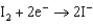 0.535
0.535 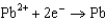 0.124
0.124 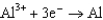 1.66
1.66 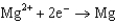 2.37
2.37 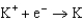 2.93
2.93
A)Pb4
B)Pb2
C)K
D)K
E)Al
Potentials (volts) in Aqueous Solution
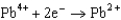 1.80
1.80 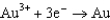 1.50
1.50 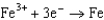 0.771
0.771 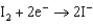 0.535
0.535 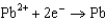 0.124
0.124 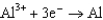 1.66
1.66 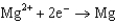 2.37
2.37 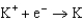 2.93
2.93A)Pb4
B)Pb2
C)K
D)K
E)Al

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
42
This is a true story; can you explain what happened? Prior to a really important dinner party, the hostess discovered that her silverware was very tarnished. She needed a quick fix. She remembered reading that the tarnish (Ag2S) would be removed if you immersed the silverware in a hot solution of baking soda (NaHCO3) in a pan lined with aluminum foil. So she did, and so it was, but she noticed a bit of a rotten egg smell (H2S) being produced. Metal/Metal ion 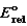 silver/silver(I)
silver/silver(I)
0)799
Aluminum/aluminum(III)1.677
A)Aluminum ions react with S2, form an aluminum sulfide precipitate, and gaseous carbon dioxide is released.
B)Silver ions in the presence of the baking soda, (NaHCO3), oxidize the sulfide to elemental sulfur that attacks the aluminum foil, which produces the smelly aluminum sulfide.
C)The aluminum acts as a reducing agent for the silver(I) in the silver sulfide; then the bicarbonate ion protonates the sulfide ion that is released.
D)Aluminum is plated onto the silver surface, making it shiny again, and then the reaction of the bicarbonate with the aluminum oxide releases CO2.
E)Silver in Ag2S reduces the aluminum, becomes metallic silver in the process, and releases hydrogen sulfide, H2S.
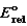 silver/silver(I)
silver/silver(I)0)799
Aluminum/aluminum(III)1.677
A)Aluminum ions react with S2, form an aluminum sulfide precipitate, and gaseous carbon dioxide is released.
B)Silver ions in the presence of the baking soda, (NaHCO3), oxidize the sulfide to elemental sulfur that attacks the aluminum foil, which produces the smelly aluminum sulfide.
C)The aluminum acts as a reducing agent for the silver(I) in the silver sulfide; then the bicarbonate ion protonates the sulfide ion that is released.
D)Aluminum is plated onto the silver surface, making it shiny again, and then the reaction of the bicarbonate with the aluminum oxide releases CO2.
E)Silver in Ag2S reduces the aluminum, becomes metallic silver in the process, and releases hydrogen sulfide, H2S.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
43
Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest oxidizing agent. Standard Reduction
Potentials (volts) in Aqueous Solution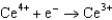 1.70
1.70 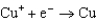 0.520
0.520 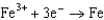 0.036
0.036 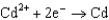 0.400
0.400 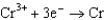 0.730
0.730 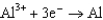 1.66
1.66
A)Cu
B)Cr3
C)Cd2
D)Ce3
E)Al
Potentials (volts) in Aqueous Solution
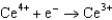 1.70
1.70 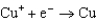 0.520
0.520 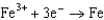 0.036
0.036 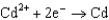 0.400
0.400 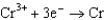 0.730
0.730 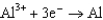 1.66
1.66A)Cu
B)Cr3
C)Cd2
D)Ce3
E)Al

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
44
Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution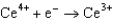 1.70
1.70 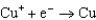 0.520
0.520 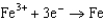 0.036
0.036 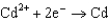 0.400
0.400 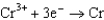 0.73
0.73 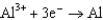 1.66
1.66
A)Cr
B)Fe
C)Cu
D)Ce4
E)Al3
Potentials (volts) in Aqueous Solution
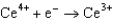 1.70
1.70 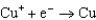 0.520
0.520 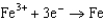 0.036
0.036 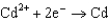 0.400
0.400 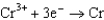 0.73
0.73 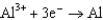 1.66
1.66A)Cr
B)Fe
C)Cu
D)Ce4
E)Al3

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following standard reduction potentials. Reduction Half-Reaction 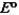 (volts)
(volts) 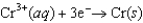 0.74
0.74 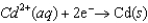 0.40
0.40 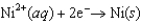 0.23
0.23
The Cr/Cr3 half-reaction can be paired with the other two to produce voltaic cells because ________
A)Cr is a powerful oxidizing agent.
B)Cr is a powerful reducing agent.
C)Cr3 is a powerful oxidizing agent.
D)Cr3 is a powerful reducing agent.
E)Cd and Ni are readily oxidized.
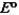 (volts)
(volts) 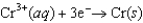 0.74
0.74 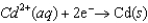 0.40
0.40 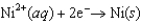 0.23
0.23The Cr/Cr3 half-reaction can be paired with the other two to produce voltaic cells because ________
A)Cr is a powerful oxidizing agent.
B)Cr is a powerful reducing agent.
C)Cr3 is a powerful oxidizing agent.
D)Cr3 is a powerful reducing agent.
E)Cd and Ni are readily oxidized.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the following standard reduction potentials. Reduction Half-Reaction 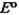 (volts)
(volts) 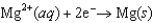 2.38
2.38 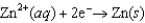 0.76
0.76 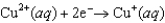 0.16
0.16
Which reaction AD will proceed spontaneously as written from left to right?
A)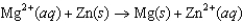
B)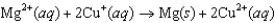
C)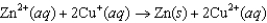
D)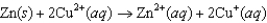
E)None of these will proceed spontaneously.
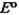 (volts)
(volts) 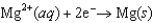 2.38
2.38 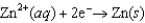 0.76
0.76 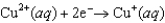 0.16
0.16Which reaction AD will proceed spontaneously as written from left to right?
A)
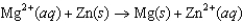
B)
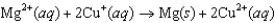
C)
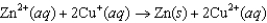
D)
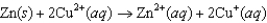
E)None of these will proceed spontaneously.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
47
Using the following data, determine the standard cell potential 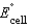 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 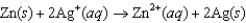 Half-reaction Standard Reduction Potential (V )
Half-reaction Standard Reduction Potential (V ) 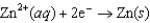 0.763
0.763 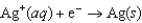 0.337
0.337
A)(1.100 V)
B)(1.100 V)
C)(0.426 V)
D)(0.426 V)
E)(1.437 V)
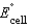 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 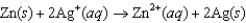 Half-reaction Standard Reduction Potential (V )
Half-reaction Standard Reduction Potential (V ) 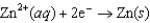 0.763
0.763 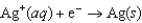 0.337
0.337A)(1.100 V)
B)(1.100 V)
C)(0.426 V)
D)(0.426 V)
E)(1.437 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
48
What is the standard cell potential for a voltaic cell using the Pb2/Pb and Mg2/Mg half-reactions? Which metal is the cathode? Standard Reduction
Potentials (volts) in Aqueous Solution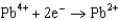 1.80
1.80 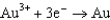 1.50
1.50 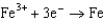 0.771
0.771 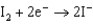 0.535
0.535 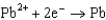 0.124
0.124 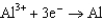 1.66
1.66 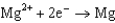 2.37
2.37 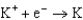 2.93
2.93
A)(2.25 V, Pb is the cathode)
B)(2.25 V, Mg is the cathode)
C)(2.25 V, Mg is the cathode)
D)(2.25 V, Pb is the cathode)
E)(2.49 V, Mg is the cathode)
Potentials (volts) in Aqueous Solution
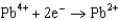 1.80
1.80 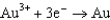 1.50
1.50 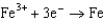 0.771
0.771 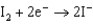 0.535
0.535 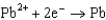 0.124
0.124 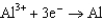 1.66
1.66 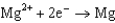 2.37
2.37 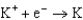 2.93
2.93A)(2.25 V, Pb is the cathode)
B)(2.25 V, Mg is the cathode)
C)(2.25 V, Mg is the cathode)
D)(2.25 V, Pb is the cathode)
E)(2.49 V, Mg is the cathode)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
49
The diagram below represents a voltaic cell. In this cell, which species is reduced? Zn(s)|Zn2(1.0 M)||Cu2(1.0 M)|Cu(s)
A)Zn(s)
B)Zn2(aq)
C)Cu2(aq)
D)Cu(s)
E)C(s)
A)Zn(s)
B)Zn2(aq)
C)Cu2(aq)
D)Cu(s)
E)C(s)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the following standard reduction potentials. Reduction Half-Reaction 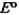 (volts)
(volts) 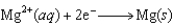 2.38
2.38 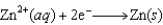 0.76
0.76 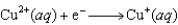 0.16
0.16
The Mg/Mg2 half-reaction can be paired with the other two to produce voltaic cells because ________
A)Mg is a powerful oxidizing agent.
B)Mg is a powerful reducing agent.
C)Mg2 is a powerful reducing agent.
D)Mg2 is a powerful oxidizing agent.
E)Zn and Cu are readily oxidized.
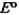 (volts)
(volts) 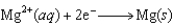 2.38
2.38 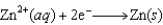 0.76
0.76 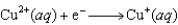 0.16
0.16The Mg/Mg2 half-reaction can be paired with the other two to produce voltaic cells because ________
A)Mg is a powerful oxidizing agent.
B)Mg is a powerful reducing agent.
C)Mg2 is a powerful reducing agent.
D)Mg2 is a powerful oxidizing agent.
E)Zn and Cu are readily oxidized.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the strongest reducing agent in the following half-reactions. The standard reduction potentials are listed.0.95 V 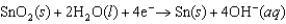 0.61 V
0.61 V 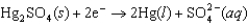 1.48 V
1.48 V 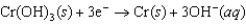 1.22 V
1.22 V 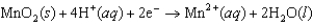
A)Cr
B)MnO2
C)Hg2SO4
D)Sn
E)Hg
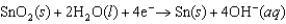 0.61 V
0.61 V 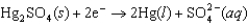 1.48 V
1.48 V 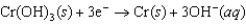 1.22 V
1.22 V 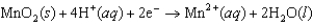
A)Cr
B)MnO2
C)Hg2SO4
D)Sn
E)Hg

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
52
Using the following data, determine the standard cell potential E for an electrochemical cell with zinc as the anode, lead as the cathode, and solutions of the respective ions. 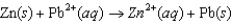 Half-reaction Standard Reduction Potential
Half-reaction Standard Reduction Potential 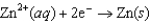 0.763
0.763 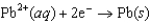 0.126
0.126
A)(1.274 V)
B)(0.637 V)
C)(0.889 V)
D)(0.889 V)
E)(0.637 V)
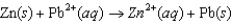 Half-reaction Standard Reduction Potential
Half-reaction Standard Reduction Potential 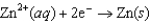 0.763
0.763 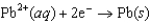 0.126
0.126A)(1.274 V)
B)(0.637 V)
C)(0.889 V)
D)(0.889 V)
E)(0.637 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
53
Which statement about a voltaic cell is not correct?
A)The standard reduction potential is a measure of the propensity for a reduction half-reaction to occur when concentrations are 1 M, the pressure is 1 atm, and the temperature is 298 K.
B)A reduction half-reaction with a standard reduction potential,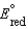 , when reversed becomes an oxidation half-reaction with
, when reversed becomes an oxidation half-reaction with 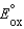
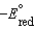 .
.
C)The standard cell potential,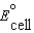 , is a measure of the electromotive force (emf) generated by the cell reactions at 298 K when concentrations are 1 M and the pressure is 1 atm.
, is a measure of the electromotive force (emf) generated by the cell reactions at 298 K when concentrations are 1 M and the pressure is 1 atm.
D)The emf of a cell is a measure of the force with which the cell pumps electrons from the cathode to the anode.
E)The cell voltage measured with a voltmeter is not necessarily the standard cell potential.
A)The standard reduction potential is a measure of the propensity for a reduction half-reaction to occur when concentrations are 1 M, the pressure is 1 atm, and the temperature is 298 K.
B)A reduction half-reaction with a standard reduction potential,
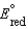 , when reversed becomes an oxidation half-reaction with
, when reversed becomes an oxidation half-reaction with 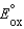
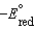 .
.C)The standard cell potential,
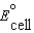 , is a measure of the electromotive force (emf) generated by the cell reactions at 298 K when concentrations are 1 M and the pressure is 1 atm.
, is a measure of the electromotive force (emf) generated by the cell reactions at 298 K when concentrations are 1 M and the pressure is 1 atm.D)The emf of a cell is a measure of the force with which the cell pumps electrons from the cathode to the anode.
E)The cell voltage measured with a voltmeter is not necessarily the standard cell potential.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
54
Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has chromium, Cr, and cadmium, Cd, electrodes? Also, identify the anode. Standard Reduction
Potentials (volts) in Aqueous Solution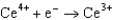 1.70
1.70 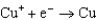 0.520
0.520 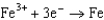 0.036
0.036 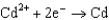 0.400
0.400 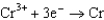 0.73
0.73 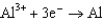 1.66
1.66
A)(0.33 V with Cr as the anode)
B)(0.33 V with Cr as the anode)
C)(0.26 V with Cd as the anode)
D)(0.33 V with Cd as the anode)
E)(0.33 V with Cd as the anode)
Potentials (volts) in Aqueous Solution
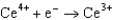 1.70
1.70 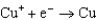 0.520
0.520 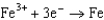 0.036
0.036 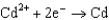 0.400
0.400 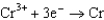 0.73
0.73 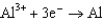 1.66
1.66A)(0.33 V with Cr as the anode)
B)(0.33 V with Cr as the anode)
C)(0.26 V with Cd as the anode)
D)(0.33 V with Cd as the anode)
E)(0.33 V with Cd as the anode)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the following standard reduction potentials. Reduction Half-Reaction 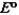 (volts)
(volts) 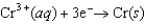 0.74
0.74 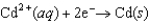 0.40
0.40 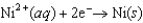 0.23
0.23
Which reaction A-D will proceed spontaneously as written from left to right?
A)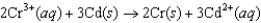
B)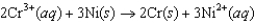
C)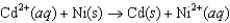
D)
E)None of these will proceed spontaneously.
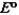 (volts)
(volts) 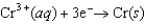 0.74
0.74 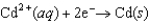 0.40
0.40 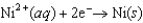 0.23
0.23Which reaction A-D will proceed spontaneously as written from left to right?
A)
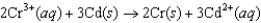
B)
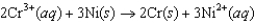
C)
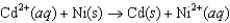
D)

E)None of these will proceed spontaneously.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
56
The diagram below represents a voltaic cell. In this cell, which species is the oxidizing agent? Al(s)|Al3(1.0 M)||Cu2(1.0 M)|Cu(s)
A)Cu2(aq)
B)Cu(s)
C)Al(s)
D)Al3(aq)
E)Pt(s)
A)Cu2(aq)
B)Cu(s)
C)Al(s)
D)Al3(aq)
E)Pt(s)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
57
Use the table of standard reduction potentials below to identify the metal or metal ion that is the strongest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution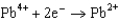 1.80
1.80 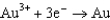 1.50
1.50 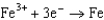 0.771
0.771 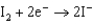 0.535
0.535 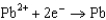 0.124
0.124 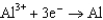 1.66
1.66 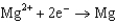 2.37
2.37 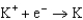 2.93
2.93
A)Pb4
B)Pb2
C)K
D)K
E)Al
Potentials (volts) in Aqueous Solution
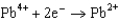 1.80
1.80 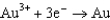 1.50
1.50 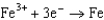 0.771
0.771 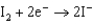 0.535
0.535 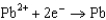 0.124
0.124 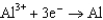 1.66
1.66 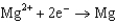 2.37
2.37 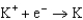 2.93
2.93A)Pb4
B)Pb2
C)K
D)K
E)Al

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
58
Using the following data, determine the standard cell potential 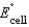 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 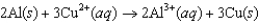 Half-reaction Standard Reduction Potential (V)
Half-reaction Standard Reduction Potential (V) 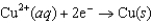 0.34
0.34 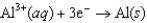 1.66
1.66
A)(1.32 V)
B)(1.32 V)
C)(2.00 V)
D)(2.00 V)
E)(2.30 V)
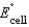 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 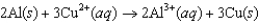 Half-reaction Standard Reduction Potential (V)
Half-reaction Standard Reduction Potential (V) 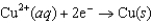 0.34
0.34 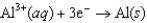 1.66
1.66A)(1.32 V)
B)(1.32 V)
C)(2.00 V)
D)(2.00 V)
E)(2.30 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
59
Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has iron, Fe, and magnesium, Mg, electrodes? Also, identify the cathode. Standard Reduction
Potentials (volts) in Aqueous Solution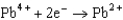 1.80
1.80 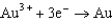 1.50
1.50 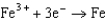 0.771
0.771 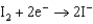 0.535
0.535 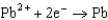 0.124
0.124 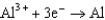 1.66
1.66 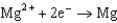 2.37
2.37 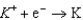 2.93
2.93
A)(3.14 V with Fe as the cathode)
B)(3.14 V with Mg as the cathode)
C)(3.14 V with Fe as the cathode)
D)(3.14 V with Mg as the cathode)
E)(1.60 V with Fe as the cathode)
Potentials (volts) in Aqueous Solution
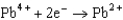 1.80
1.80 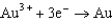 1.50
1.50 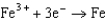 0.771
0.771 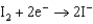 0.535
0.535 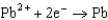 0.124
0.124 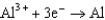 1.66
1.66 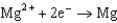 2.37
2.37 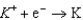 2.93
2.93A)(3.14 V with Fe as the cathode)
B)(3.14 V with Mg as the cathode)
C)(3.14 V with Fe as the cathode)
D)(3.14 V with Mg as the cathode)
E)(1.60 V with Fe as the cathode)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
60
The diagram below represents a voltaic cell. What is the balanced electrochemical reaction represented by this cell? Al(s)|Al3(1.0 M)||Cu2(1.0 M)|Cu(s)
A)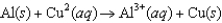
B)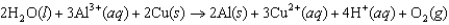
C)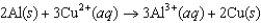
D)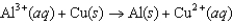
E)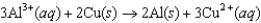
A)
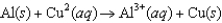
B)
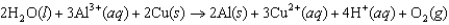
C)
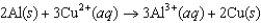
D)
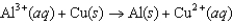
E)
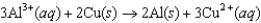

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
61
If the free-energy change of the following voltaic cell 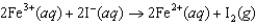 is 46.3 kJ, what is the standard potential of the cell?
is 46.3 kJ, what is the standard potential of the cell?
A)(0.080 V)
B)(0.080 V)
C)(0.240 V)
D)(0.240 V)
E)(0.480 V)
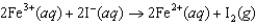 is 46.3 kJ, what is the standard potential of the cell?
is 46.3 kJ, what is the standard potential of the cell?A)(0.080 V)
B)(0.080 V)
C)(0.240 V)
D)(0.240 V)
E)(0.480 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
62
The magnitude of the charge on a mole of electrons is ________
A)1C.
B)9.65 C.
C)9.65 10 4 C.
D)6.02 10 23 C.
E)9,650 C.
A)1C.
B)9.65 C.
C)9.65 10 4 C.
D)6.02 10 23 C.
E)9,650 C.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
63
A concentration cell is constructed by using the same half-reaction for both the cathode and anode. What is the value of standard cell potential, 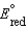 , for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? (
, for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? ( 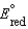 0.80 V for Ag/Ag)
0.80 V for Ag/Ag)
A)(0.21 V)
B)0.00 V
C)(0.80 V)
D)(0.80 V)
E)(0.21 V)
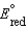 , for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? (
, for a concentration cell that combines a silver anode in contact with 0.10 M silver nitrate and a silver cathode in contact with 0.00003 M silver nitrate? ( 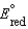 0.80 V for Ag/Ag)
0.80 V for Ag/Ag)A)(0.21 V)
B)0.00 V
C)(0.80 V)
D)(0.80 V)
E)(0.21 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
64
An electrochemical cell with a standard hydrogen electrode and a cathode consisting of a metallic chromium electrode, Cr(s), in contact with a 1.00 M chromium solution, Cr3(aq), was constructed. The voltage produced by this cell was measured at 25C. Which statements describe the results of this measurement, assuming the conditions are ideal? The cell voltage with the appropriate sign equals ________
I. the cell potential.
II. the electromotive force.
III. the standard cell potential.
IV. the standard reduction potential for Cr/Cr3.
A)I only
B)I and II
C)I, II, and III
D)I, II, III, and IV
E)III only
I. the cell potential.
II. the electromotive force.
III. the standard cell potential.
IV. the standard reduction potential for Cr/Cr3.
A)I only
B)I and II
C)I, II, and III
D)I, II, III, and IV
E)III only

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
65
The spontaneous redox reaction in a voltaic cell has ________
A)a negative value of Ecell and a negative value of G.
B)a positive value of Ecell and a positive value of G.
C)a negative value of Ecell and a positive value of G.
D)a positive value of Ecell and a negative value of G.
E)a positive value of Ecell and a value of zero for G.
A)a negative value of Ecell and a negative value of G.
B)a positive value of Ecell and a positive value of G.
C)a negative value of Ecell and a positive value of G.
D)a positive value of Ecell and a negative value of G.
E)a positive value of Ecell and a value of zero for G.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
66
The work involved in moving exactly 1 mole of electrons through a potential difference of exactly 1 V is ________
A)1 J.
B)1 kJ.
C)96.5 J.
D)6.02 kJ.
E)96.5 kJ.
A)1 J.
B)1 kJ.
C)96.5 J.
D)6.02 kJ.
E)96.5 kJ.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
67
If the potential of a voltaic cell is 0.837 V, what is the free-energy change when two moles of electrons are transferred in the oxidation-reduction reaction?
A)(162 kJ)
B)(162 kJ)
C)(82.0 kJ)
D)(82.0 kJ)
E)(41.0 kJ)
A)(162 kJ)
B)(162 kJ)
C)(82.0 kJ)
D)(82.0 kJ)
E)(41.0 kJ)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
68
Copper is oxidized by nitric acid. If this property were used in an electrochemical cell, what would the standard cell potential be? The relevant reduction reactions and standard reduction potentials are given below.0.34 V 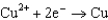 0.96 V
0.96 V 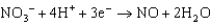
A)(0.62 V)
B)(0.62 V)
C)(1.30 V)
D)(1.30 V)
E)(0.68 V)
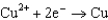 0.96 V
0.96 V 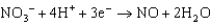
A)(0.62 V)
B)(0.62 V)
C)(1.30 V)
D)(1.30 V)
E)(0.68 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
69
If the free-energy change of the following voltaic cell 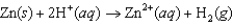 is 147.2 kJ, what is the standard potential of the cell?
is 147.2 kJ, what is the standard potential of the cell?
A)(0.763 V)
B)(0.763 V)
C)(1.53 V)
D)(1.53 V)
E)(2.12 V)
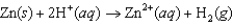 is 147.2 kJ, what is the standard potential of the cell?
is 147.2 kJ, what is the standard potential of the cell?A)(0.763 V)
B)(0.763 V)
C)(1.53 V)
D)(1.53 V)
E)(2.12 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
70
The change in free energy for a reaction, G, depends on the stoichiometric coefficients used in writing the reaction, but cell potentials, E, do not depend on these coefficients. Which statement accounts for this difference?
A)These quantities (G and E) are not related, so this difference is not an issue.
B)The free-energy change is defined for general reactions, and the electromotive force is defined for electrochemical reactions, so this difference is not an issue.
C)The difference is not relevant because the units differ: kJ/mol for G, and V for E.
D)The change in free energy depends on both the reaction and the amount of material reacting, while the cell potential depends only on the cell composition.
E)The statement is false.G does not depend on the stoichiometric coefficients.
A)These quantities (G and E) are not related, so this difference is not an issue.
B)The free-energy change is defined for general reactions, and the electromotive force is defined for electrochemical reactions, so this difference is not an issue.
C)The difference is not relevant because the units differ: kJ/mol for G, and V for E.
D)The change in free energy depends on both the reaction and the amount of material reacting, while the cell potential depends only on the cell composition.
E)The statement is false.G does not depend on the stoichiometric coefficients.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
71
Using the following data, determine the standard cell potential 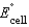 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 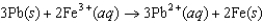 Half-reaction Standard Reduction Potential (V )
Half-reaction Standard Reduction Potential (V ) 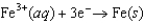 0.771
0.771 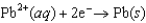 0.124
0.124
A)(0.647 V)
B)(0.647 V)
C)(0.895 V)
D)(0.895 V)
E)(1.17 V)
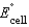 for the electrochemical cell constructed using the following reaction.
for the electrochemical cell constructed using the following reaction. 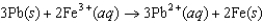 Half-reaction Standard Reduction Potential (V )
Half-reaction Standard Reduction Potential (V ) 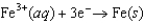 0.771
0.771 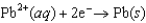 0.124
0.124A)(0.647 V)
B)(0.647 V)
C)(0.895 V)
D)(0.895 V)
E)(1.17 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
72
The Nernst equation can be used to calculate ________
A)standard cell potentials from standard reduction potentials.
B)the change in standard Gibbs free energy from standard cell potentials.
C)cell potentials from standard cell potentials when the conditions of concentration and temperature are not standard.
D)cell potentials given the temperature and reactant concentrations.
E)cell potentials from standard oxidation potentials.
A)standard cell potentials from standard reduction potentials.
B)the change in standard Gibbs free energy from standard cell potentials.
C)cell potentials from standard cell potentials when the conditions of concentration and temperature are not standard.
D)cell potentials given the temperature and reactant concentrations.
E)cell potentials from standard oxidation potentials.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
73
If the free-energy change of a voltaic cell is 258 kJ, what is the potential of the cell if three moles of electrons are transferred in the oxidation-reduction reaction?
A)(0.890 V)
B)(0.890 V)
C)(2.67 V)
D)(2.67 V)
E)(1.53 V)
A)(0.890 V)
B)(0.890 V)
C)(2.67 V)
D)(2.67 V)
E)(1.53 V)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
74
Which statement does not correctly describe a standard hydrogen electrode (SHE)?
A)The SHE is assigned a standard reduction potential of exactly 1 V.
B)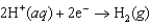
C)Pt|H2(g, 1atm)|H(aq, 1 M )||
D)||H(aq, 1 M )|H2(g, 1atm)|Pt
E)The SHE consists of a platinum electrode immersed in an acid solution and a stream of hydrogen gas.
A)The SHE is assigned a standard reduction potential of exactly 1 V.
B)
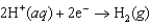
C)Pt|H2(g, 1atm)|H(aq, 1 M )||
D)||H(aq, 1 M )|H2(g, 1atm)|Pt
E)The SHE consists of a platinum electrode immersed in an acid solution and a stream of hydrogen gas.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
75
Does pH have an effect on the cell potential (Ecell) for the following oxidation-reduction reaction? 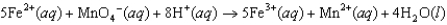
A)Yes, because Ecell values for all redox reactions depend on the pH.
B)Yes, because Ecell values for redox reactions involving the hydronium ion depend on the pH.
C)No, because Ecell values for redox reactions depend only on the major species in the reaction, in this case, Fe2, MnO4, Fe3, and Mn2.
D)No, because Ecell values for redox reactions depend on concentrations and temperatures but not on pH.
E)No, because Ecell values for redox reactions do not depend on the pH.
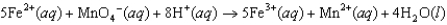
A)Yes, because Ecell values for all redox reactions depend on the pH.
B)Yes, because Ecell values for redox reactions involving the hydronium ion depend on the pH.
C)No, because Ecell values for redox reactions depend only on the major species in the reaction, in this case, Fe2, MnO4, Fe3, and Mn2.
D)No, because Ecell values for redox reactions depend on concentrations and temperatures but not on pH.
E)No, because Ecell values for redox reactions do not depend on the pH.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
76
The standard hydrogen electrode is ________
A)used to calibrate voltmeters.
B)used to produce a set of standard reduction potentials.
C)needed to activate electrochemical cells.
D)often overlooked in measuring standard reduction potentials.
E)used to produce a standard cell potential of exactly 1 V.
A)used to calibrate voltmeters.
B)used to produce a set of standard reduction potentials.
C)needed to activate electrochemical cells.
D)often overlooked in measuring standard reduction potentials.
E)used to produce a standard cell potential of exactly 1 V.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
77
The numerical value of the Faraday constant is approximately 96,500. This value represents ________
A)the coulombs of charge carried by one mole of electrons.
B)the number of electrons required to produce a charge of one coulomb.
C)the number of electrons required to produce one mole of electrical charge.
D)the number of ions produced by removing one mole of electrons.
E)the electrical current produced by transferring one mole of electrons.
A)the coulombs of charge carried by one mole of electrons.
B)the number of electrons required to produce a charge of one coulomb.
C)the number of electrons required to produce one mole of electrical charge.
D)the number of ions produced by removing one mole of electrons.
E)the electrical current produced by transferring one mole of electrons.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the voltaic cell based on the following reaction: 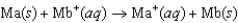 Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)
Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)
A)Yvonne is right because by definition standard cell potentials must be measured at concentrations of 1.00 M.
B)Yvonne is right because she understands the Nernst equation and what it describes.
C)Zelda is right because she understands the Nernst equation and what it describes.
D)Zelda is right because cell potentials do not depend on the concentration.
E)Both are right because cell potentials do not depend on the concentration.
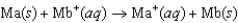 Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)
Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)A)Yvonne is right because by definition standard cell potentials must be measured at concentrations of 1.00 M.
B)Yvonne is right because she understands the Nernst equation and what it describes.
C)Zelda is right because she understands the Nernst equation and what it describes.
D)Zelda is right because cell potentials do not depend on the concentration.
E)Both are right because cell potentials do not depend on the concentration.

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
79
Which statement does not correctly describe a "dead" battery with a voltage of 0?
A)The free-energy change for the reaction now is 0.
B)All the reactants have been converted into products.
C)The products and reactants now are in equilibrium.
D)Q K
E)cell potential 0
A)The free-energy change for the reaction now is 0.
B)All the reactants have been converted into products.
C)The products and reactants now are in equilibrium.
D)Q K
E)cell potential 0

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck
80
If the potential of a voltaic cell is 1.20 V, what is the free-energy change when one mole of electrons is transferred in the oxidation-reduction reaction?
A)116 kJ
B)1.20 kJ
C)(1.20 kJ)
D)(116 kJ)
E)(602 kJ)
A)116 kJ
B)1.20 kJ
C)(1.20 kJ)
D)(116 kJ)
E)(602 kJ)

Unlock Deck
Unlock for access to all 167 flashcards in this deck.
Unlock Deck
k this deck


