Deck 2: Chemical Aspects of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 2: Chemical Aspects of Life
1
Steroids are a type of
A)protein.
B)lipid.
C)sugar.
D)nucleic acid.
A)protein.
B)lipid.
C)sugar.
D)nucleic acid.
B
2
Which of the following is NOT an example of a lipid?
A)fats.
B)amino acids.
C)steroids.
D)phospholipids.
A)fats.
B)amino acids.
C)steroids.
D)phospholipids.
B
3
Covalent bonds form when
A)two or more atoms share electrons equally.
B)a positive ion and a negative ion attract.
C)two or more molecules share electrons unequally.
D)two or more atoms share electrons equally and two or more molecules share electrons unequally.
A)two or more atoms share electrons equally.
B)a positive ion and a negative ion attract.
C)two or more molecules share electrons unequally.
D)two or more atoms share electrons equally and two or more molecules share electrons unequally.
D
4
Enzymes directly act to
A)maintain cell structure.
B)slow down chemical reactions.
C)speed up chemical reactions.
D)supply energy.
A)maintain cell structure.
B)slow down chemical reactions.
C)speed up chemical reactions.
D)supply energy.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
A saturated fat will have rev: 09_24_2013_QC_34141
A)significant numbers of carbon-carbon double bonds.
B)very few hydrogen atoms.
C)no carbon to carbon double bonds.
D)excessive nutrients.
A)significant numbers of carbon-carbon double bonds.
B)very few hydrogen atoms.
C)no carbon to carbon double bonds.
D)excessive nutrients.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
If an atom has 8 protons and 8 neutrons in its nucleus, and 8 orbiting electrons, its atomic number would be
A)24.
B)16.
C)8.
D)12.
A)24.
B)16.
C)8.
D)12.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
There are ____ naturally occurring elements of which ______ are involved in maintaining life.
A)96; 22
B)104; 28
C)92; 24
D)58; 34
A)96; 22
B)104; 28
C)92; 24
D)58; 34

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
To be considered an organic molecule a substance must contain
A)carbon and nitrogen.
B)carbon and hydrogen.
C)carbon and oxygen.
D)oxygen and hydrogen.
A)carbon and nitrogen.
B)carbon and hydrogen.
C)carbon and oxygen.
D)oxygen and hydrogen.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
To form an ion, an atom must either donate or receive a(n) ________ .
A)electron
B)proton
C)neutron
D)electrons and neutron
A)electron
B)proton
C)neutron
D)electrons and neutron

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
Nucleic acids are made up of
A)fats.
B)amino acids.
C)nucleotides.
D)sugars.
A)fats.
B)amino acids.
C)nucleotides.
D)sugars.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
About 96% of the body consists of what four elements?
A)oxygen, hydrogen, glucose, and carbon
B)oxygen, hydrogen, carbon, and copper
C)oxygen, hydrogen, carbon, and sodium
D)oxygen, hydrogen, carbon, and nitrogen
A)oxygen, hydrogen, glucose, and carbon
B)oxygen, hydrogen, carbon, and copper
C)oxygen, hydrogen, carbon, and sodium
D)oxygen, hydrogen, carbon, and nitrogen

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
The valence electrons are those
A)active in chemical bonds.
B)close to the nucleus of the atom.
C)located in the outermost shell.
D)located in the innermost shell.
A)active in chemical bonds.
B)close to the nucleus of the atom.
C)located in the outermost shell.
D)located in the innermost shell.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
Proteins are made up of
A)fats.
B)amino acids.
C)nucleotides.
D)sugars.
A)fats.
B)amino acids.
C)nucleotides.
D)sugars.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
The difference between DNA and RNA is that
A)each contains different sugars.
B)each has different bases.
C)each has a difference in the number of strands.
D)there are differences in sugars, bases, and the number of strands.
A)each contains different sugars.
B)each has different bases.
C)each has a difference in the number of strands.
D)there are differences in sugars, bases, and the number of strands.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
Anything that has weight and occupies space can be described as
A)an atom.
B)matter.
C)a compound.
D)a molecule.
A)an atom.
B)matter.
C)a compound.
D)a molecule.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
This would be the general representation of 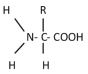
A)an amino acid.
B)a fatty acid.
C)a nucleic acid.
D)glycerol.

A)an amino acid.
B)a fatty acid.
C)a nucleic acid.
D)glycerol.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
Hydrogen bonds involve
A)multiple ions.
B)non-polar molecules.
C)polar molecules.
D)ions and non-polar molecules.
A)multiple ions.
B)non-polar molecules.
C)polar molecules.
D)ions and non-polar molecules.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
A chemical formula expresses
A)the chemical composition of a molecule.
B)the number of atoms for each element in the molecule.
C)the atoms involved in chemical bonding.
D)all of these choices are correct
A)the chemical composition of a molecule.
B)the number of atoms for each element in the molecule.
C)the atoms involved in chemical bonding.
D)all of these choices are correct

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
The process used to convert liquid vegetable oils to solids by changing its bonds is called
A)carbonization.
B)hydrogenation.
C)solidification.
D)oxygenation.
A)carbonization.
B)hydrogenation.
C)solidification.
D)oxygenation.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
Lactose, the sugar contained in milk, is an example of a
A)simple sugar.
B)monosaccharide.
C)disaccharide.
D)none of these choices are correct
A)simple sugar.
B)monosaccharide.
C)disaccharide.
D)none of these choices are correct

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
At a pH of 7, which of the following would be true?
A)H+ and OH- concentrations would be equal.
B)H+ concentration would be greater than OH- concentration.
C)OH- concentration would be greater than H+ concentration.
D)None of the above.
A)H+ and OH- concentrations would be equal.
B)H+ concentration would be greater than OH- concentration.
C)OH- concentration would be greater than H+ concentration.
D)None of the above.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
The molecule that provides immediate energy for cellular processes is
A)glucose.
B)glycogen.
C)starch.
D)adenosine triphosphate.
A)glucose.
B)glycogen.
C)starch.
D)adenosine triphosphate.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
On the typical pH scale, a pH of ____ indicates the lowest concentration of hydrogen ions, whereas a pH of ____ indicates the highest concentration of H+.
A)0; 14
B)7; 14
C)14; 0
D)0; 7
A)0; 14
B)7; 14
C)14; 0
D)0; 7

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
When the body has excess energy and builds molecules to store it, which molecule do we build MOST?
A)Glycogen
B)Glucose
C)Triglycerides
D)Cholesterol
A)Glycogen
B)Glucose
C)Triglycerides
D)Cholesterol

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
The number of protons plus the number of neutrons determines the __________ of an atom.
A)isotope
B)valence electrons
C)atomic number
D)atomic mass
A)isotope
B)valence electrons
C)atomic number
D)atomic mass

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
An ionic bond forms between
A)a cation and another cation.
B)a cation and an anion.
C)an anion and another anion.
D)all of the above.
A)a cation and another cation.
B)a cation and an anion.
C)an anion and another anion.
D)all of the above.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
Select the correct statement.
A)DNA and RNA are double-stranded molecules composed of nucleotides.
B)DNA and RNA are single-stranded molecules with dissimilar nucleotides.
C)DNA contains the genetic code, and RNA carries the coded information to the sites of protein synthesis.
D)DNA is double-stranded but RNA is single-stranded, although their nucleotides are identical.
A)DNA and RNA are double-stranded molecules composed of nucleotides.
B)DNA and RNA are single-stranded molecules with dissimilar nucleotides.
C)DNA contains the genetic code, and RNA carries the coded information to the sites of protein synthesis.
D)DNA is double-stranded but RNA is single-stranded, although their nucleotides are identical.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
A substance that cannot be broken down into a simpler substance by chemical means is a/an
A)element.
B)compound.
C)molecule.
D)nucleic acid.
A)element.
B)compound.
C)molecule.
D)nucleic acid.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
When placed in water, ionic compounds dissociate into
A)water molecules.
B)salts.
C)hydrogen ions.
D)electrolytes.
A)water molecules.
B)salts.
C)hydrogen ions.
D)electrolytes.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
Adding additional neutrons to an atom would form _____.
A)isotopes
B)ions
C)covalent bonds
D)iodine
A)isotopes
B)ions
C)covalent bonds
D)iodine

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is an organic compound?
A)NaHCO3
B)NaOH
C)C6H12O6
D)CO2
A)NaHCO3
B)NaOH
C)C6H12O6
D)CO2

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
The dissociation of a/an ________ releases hydrogen ions and increases the concentration of hydrogen ions in a solution.
A)acid
B)base
C)salt
D)solvent
A)acid
B)base
C)salt
D)solvent

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
Proteins are composed of subunits called __________ and functional proteins include _________, which speed up chemical reactions in the body.
A)amino acids; enzymes
B)fatty acids; enzymes
C)fatty acids; triglycerides
D)amino acids; antibodies
A)amino acids; enzymes
B)fatty acids; enzymes
C)fatty acids; triglycerides
D)amino acids; antibodies

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
The element that forms the backbone of organic molecules is
A)hydrogen.
B)oxygen.
C)carbon.
D)nitrogen.
A)hydrogen.
B)oxygen.
C)carbon.
D)nitrogen.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
An atom that has 6 electrons in its valence shell will be most likely to
A)donate 2 electrons.
B)donate 6 electrons.
C)receive 2 electrons.
D)receive 6 electrons.
A)donate 2 electrons.
B)donate 6 electrons.
C)receive 2 electrons.
D)receive 6 electrons.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
A carbohydrate molecule consisting of glucose combined with fructose is a
A)monosaccharide.
B)disaccharide.
C)polysaccharide.
D)starch.
A)monosaccharide.
B)disaccharide.
C)polysaccharide.
D)starch.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
The monosaccharide that is the major carbohydrate fuel for body cells is
A)sucrose.
B)fructose.
C)galactose.
D)glucose.
A)sucrose.
B)fructose.
C)galactose.
D)glucose.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
The positively charged subatomic particles located in the nucleus of an atom are the
A)electrons.
B)protons.
C)neutrons.
D)nucleons.
A)electrons.
B)protons.
C)neutrons.
D)nucleons.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
Two or more atoms of the SAME element combine to form a(n) _____.
A)isotope
B)compound
C)molecule
D)ion
A)isotope
B)compound
C)molecule
D)ion

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
When one atom donates an electron to another atom, the donating atom becomes a __________ charged ion, and the receiving atom becomes a __________ charged ion. These ions are joined together by a/an ________ chemical bond.
A)positively; negatively; ionic
B)negatively; positively; ionic
C)negatively; positively; covalent
D)positively; negatively; hydrogen
A)positively; negatively; ionic
B)negatively; positively; ionic
C)negatively; positively; covalent
D)positively; negatively; hydrogen

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
What is the most abundant substance in the human body?
A)hydrogen
B)carbon
C)glucose
D)water
A)hydrogen
B)carbon
C)glucose
D)water

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
The name for the covalent bond between two amino acids is termed
A)protein bond.
B)ionic bond.
C)enzyme bond.
D)peptide bond.
A)protein bond.
B)ionic bond.
C)enzyme bond.
D)peptide bond.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
If there are two forms of atoms for an element that differ only in the number of neutron, the various forms of the element are referred to as _____ of that element.
A)molecules
B)isotopes
C)electrolytes
D)ions
A)molecules
B)isotopes
C)electrolytes
D)ions

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is TRUE regarding enzymes?
A)They are proteins.
B)They speed up chemical reactions.
C)They are reusable.
D)All other answers are true.
A)They are proteins.
B)They speed up chemical reactions.
C)They are reusable.
D)All other answers are true.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
What is the term for a substance that causes a resistance to a change in pH?
A)electrolyte
B)base
C)buffer
D)salt
A)electrolyte
B)base
C)buffer
D)salt

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
Water molecules are special. But what makes them so special?
A)their abundance
B)polar bonds
C)the bend in their structure
D)because they are liquid
A)their abundance
B)polar bonds
C)the bend in their structure
D)because they are liquid

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
What type of reaction is shown? AB + CD AD + BC
A)decomposition
B)exchange
C)reversible
D)synthesis
A)decomposition
B)exchange
C)reversible
D)synthesis

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
What type of formula is shown? 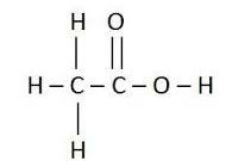
A)atomic formula
B)structural formula
C)molecular formula
D)elemental formula

A)atomic formula
B)structural formula
C)molecular formula
D)elemental formula

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
What is the notation that tells the reader the chemical composition of a molecule or compound?
A)chemical formula
B)compound formula
C)elemental notation
D)atomic structure
A)chemical formula
B)compound formula
C)elemental notation
D)atomic structure

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
What type of reaction is shown? A + B + C ABC AB + C
A)synthesis
B)exchange
C)decomposition
D)reversible
A)synthesis
B)exchange
C)decomposition
D)reversible

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
What type of reaction is shown? A + B AB
A)synthesis
B)decomposition
C)exchange
D)reversible
A)synthesis
B)decomposition
C)exchange
D)reversible

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
How does a buffer help a solution maintain pH?
A)A buffer can act like a base if pH is acidic, and it can act like an acid if pH is basic.
B)A buffer forms both cations and anions to counteract acids.
C)A buffer release base to neutralize acid.
D)A buffer releases acid to maintain proper pH.
A)A buffer can act like a base if pH is acidic, and it can act like an acid if pH is basic.
B)A buffer forms both cations and anions to counteract acids.
C)A buffer release base to neutralize acid.
D)A buffer releases acid to maintain proper pH.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
A monounsaturated fat would have
A)one carbon-carbon double bond in a fatty acid tail.
B)two fatty acid tails and a phosphate group.
C)two carbon-carbon double bonds in its fatty acid tails.
D)four carbon rings.
A)one carbon-carbon double bond in a fatty acid tail.
B)two fatty acid tails and a phosphate group.
C)two carbon-carbon double bonds in its fatty acid tails.
D)four carbon rings.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
What type of formula is shown? CH4
A)molecular formula
B)structural formula
C)atomic formula
D)elemental formula
A)molecular formula
B)structural formula
C)atomic formula
D)elemental formula

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
The form of carbohydrate our bodies use to store reserve energy is _____.
A)disaccharides
B)starches
C)glycogen
D)glucose
A)disaccharides
B)starches
C)glycogen
D)glucose

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


