Deck 2: Adaptations to Aquatic Environments
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 2: Adaptations to Aquatic Environments
1
Which is the most basic?
A) human blood
B) acid rain
C) carbonated beverages
D) pure water
A) human blood
B) acid rain
C) carbonated beverages
D) pure water
A
2
Adding dissolved compounds such as salt to water _____ the boiling point and _____ the freezing point.
A) increases; increases
B) increases; decreases
C) decrease; increases
D) decreases; decreases
A) increases; increases
B) increases; decreases
C) decrease; increases
D) decreases; decreases
B
3
The low density of ice
A) makes it ineffective at insulating water from the cold.
B) allows aquatic plants to survive the winter.
C) is due to the high viscosity of water.
D) prevents it from moving in water.
A) makes it ineffective at insulating water from the cold.
B) allows aquatic plants to survive the winter.
C) is due to the high viscosity of water.
D) prevents it from moving in water.
B
4
Which part of an organism is less dense than water?
A) bone
B) protein
C) muscle
D) fat
A) bone
B) protein
C) muscle
D) fat

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Limestone deposits are due to
A) the low pH of ocean water.
B) the solubility of calcium carbonate.
C) the polar nature of water.
D) acid deposition.
A) the low pH of ocean water.
B) the solubility of calcium carbonate.
C) the polar nature of water.
D) acid deposition.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
At what temperature does water reach its maximum density?
A) 32ºC
B) 0ºC
C) 4ºC
D) −12ºC
E) 100ºC
A) 32ºC
B) 0ºC
C) 4ºC
D) −12ºC
E) 100ºC

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
Why is liquid water important for the formation of life on Earth?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
What is unusual about the physical properties of water?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
Water's polar nature
A) explains its high density.
B) makes it a good solvent.
C) causes it to freeze at 0ºC.
D) limits the amount of dissolved nutrients it can hold.
A) explains its high density.
B) makes it a good solvent.
C) causes it to freeze at 0ºC.
D) limits the amount of dissolved nutrients it can hold.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
A liquid with low pH would have
A) high OH− concentration.
B) low NO2 concentration.
C) low CaCO3 concentration.
D) high H+ concentration.
A) high OH− concentration.
B) low NO2 concentration.
C) low CaCO3 concentration.
D) high H+ concentration.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
You are studying a small stream and find that its pH is 4.5. What does this tell you about the stream, and what might be the cause?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
The high specific heat of water
A) means large amounts of heat are needed to change the temperature of water.
B) requires significant heat energy to make the transition from solid to liquid.
C) requires significant heat energy to make the transition from liquid to gas.
D) makes it difficult to increase the temperature of liquid water above 100ºC.
A) means large amounts of heat are needed to change the temperature of water.
B) requires significant heat energy to make the transition from solid to liquid.
C) requires significant heat energy to make the transition from liquid to gas.
D) makes it difficult to increase the temperature of liquid water above 100ºC.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Which is NOT an adaptation that exploits the density of water?
A) a gas-filled swim bladder
B) droplets of oil on algae
C) long, filamentous appendages
D) high percentages of fat
A) a gas-filled swim bladder
B) droplets of oil on algae
C) long, filamentous appendages
D) high percentages of fat

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
The limit to the amount of minerals water can hold is called
A) the dissolution limit.
B) the solvent point.
C) deposition.
D) saturation.
A) the dissolution limit.
B) the solvent point.
C) deposition.
D) saturation.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
Which causes acid rain?
A) CO2
B) SO2
C) HCO3
D) NaOH
A) CO2
B) SO2
C) HCO3
D) NaOH

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
How does a low pH harm aquatic environments?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Aquatic organisms have developed streamlined shapes to adapt to
A) the density of water.
B) the viscosity of water.
C) the polar nature of water.
D) the basic nature of water.
A) the density of water.
B) the viscosity of water.
C) the polar nature of water.
D) the basic nature of water.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the nutrients listed below is NOT required by all organisms?
A) nitrogen
B) phosphorus
C) potassium
D) sulfur
E) silicon
A) nitrogen
B) phosphorus
C) potassium
D) sulfur
E) silicon

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Solutes
A) are membranes through which nutrients pass into cells.
B) reduce the acidity of water.
C) are particles that can pass through cell membranes.
D) are substances dissolved in water.
A) are membranes through which nutrients pass into cells.
B) reduce the acidity of water.
C) are particles that can pass through cell membranes.
D) are substances dissolved in water.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
A freshwater fish with a high concentration of dissolved nutrients will
A) have high osmotic pressure.
B) have low osmotic pressure.
C) be hyposmotic.
D) actively secrete solutes.
A) have high osmotic pressure.
B) have low osmotic pressure.
C) be hyposmotic.
D) actively secrete solutes.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
How does water in a bog differ from water in other locations?
A) More bicarbonate and more carbon dioxide are available.
B) More bicarbonate and less carbon dioxide are available.
C) Less bicarbonate and more carbon dioxide are available.
D) Less bicarbonate and less carbon dioxide are available.
A) More bicarbonate and more carbon dioxide are available.
B) More bicarbonate and less carbon dioxide are available.
C) Less bicarbonate and more carbon dioxide are available.
D) Less bicarbonate and less carbon dioxide are available.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is NOT a part of carbon equilibrium in water?
A) bicarbonate
B) ammonia
C) hydrogen ions
D) carbonic acid
A) bicarbonate
B) ammonia
C) hydrogen ions
D) carbonic acid

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Ammonia is a byproduct of
A) digesting proteins.
B) absorbing excess salts.
C) excreting urea.
D) active uptake in gills.
A) digesting proteins.
B) absorbing excess salts.
C) excreting urea.
D) active uptake in gills.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
In which location would you expect to find the highest levels of dissolved oxygen?
A) deep ocean water
B) a freshwater bog
C) a landlocked lake
D) a fast, shallow river
A) deep ocean water
B) a freshwater bog
C) a landlocked lake
D) a fast, shallow river

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Mangroves grow on salt-laden coastal mudflats that are inundated daily by high tides. How do these plants address problems of water acquisition and elimination of excess salts?
I) by maintaining high concentrations of organic solutes in their roots
II) by excluding salts from their roots by active transport
III) by actively excreting salt from glands on the surfaces of their leaves
A) I and II only
B) I and III only
C) II and III only
D) III only
E) I, II, and III
I) by maintaining high concentrations of organic solutes in their roots
II) by excluding salts from their roots by active transport
III) by actively excreting salt from glands on the surfaces of their leaves
A) I and II only
B) I and III only
C) II and III only
D) III only
E) I, II, and III

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Anaerobic conditions
A) decrease photosynthesis.
B) are due to increased pH.
C) decrease the diffusion of oxygen.
D) are more common in deep water than in the shallows.
A) decrease photosynthesis.
B) are due to increased pH.
C) decrease the diffusion of oxygen.
D) are more common in deep water than in the shallows.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
How does the concentration of bicarbonate in water compare with the concentration of carbon dioxide in the air?
A) one tenth
B) about the same
C) twice as much
D) about 30 times more
E) over 100 times more
A) one tenth
B) about the same
C) twice as much
D) about 30 times more
E) over 100 times more

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
The dissolved oxygen levels in water did NOT require adaptation for
A) dolphins.
B) sharks.
C) squid.
D) zooplankton.
A) dolphins.
B) sharks.
C) squid.
D) zooplankton.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Hyperosmotic conditions
A) cause low osmotic pressure.
B) occur in freshwater organisms.
C) cause active secretion in gills.
D) occur in arid landlocked lakes.
A) cause low osmotic pressure.
B) occur in freshwater organisms.
C) cause active secretion in gills.
D) occur in arid landlocked lakes.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Which does NOT limit the ability of aquatic plants to photosynthesize?
A) slow diffusion of carbon dioxide in water
B) boundary layers
C) the size of bicarbonate molecules
D) high levels of carbonic acid
A) slow diffusion of carbon dioxide in water
B) boundary layers
C) the size of bicarbonate molecules
D) high levels of carbonic acid

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Why is it important for organisms to osmoregulate?
A) Organisms cannot survive in hyposmotic conditions.
B) Active transport requires large amounts of energy.
C) An imbalance in solutes disrupts cell functions.
D) High osmotic pressure can burst cell walls.
A) Organisms cannot survive in hyposmotic conditions.
B) Active transport requires large amounts of energy.
C) An imbalance in solutes disrupts cell functions.
D) High osmotic pressure can burst cell walls.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
What is the osmotic potential of ocean water?
A) −10 MPa
B) −1.2 MPa
C) −0.4 MPa
D) 0 MPa
E) 2 MPa
A) −10 MPa
B) −1.2 MPa
C) −0.4 MPa
D) 0 MPa
E) 2 MPa

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Why do sharks retain urea rather than excrete it? How does this influence their fitness?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
How does the permeable nature of cells affect evolution in aquatic animals?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Which is a byproduct of anaerobic respiration?
A) CO2
B) H2CO3
C) H2S
D) HCl
A) CO2
B) H2CO3
C) H2S
D) HCl

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Which is used to increase oxygen extraction?
A) hydrogen ions
B) boundary layers
C) countercurrent circulation
D) concurrent circulation
A) hydrogen ions
B) boundary layers
C) countercurrent circulation
D) concurrent circulation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
The use of salt on roads in winter has led to
A) adaptation of roadside plants to increased salt levels.
B) decreased survival of freshwater organisms in nearby ponds.
C) hyperosmotic conditions.
D) increased acid deposition.
A) adaptation of roadside plants to increased salt levels.
B) decreased survival of freshwater organisms in nearby ponds.
C) hyperosmotic conditions.
D) increased acid deposition.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Why are both carbon dioxide and oxygen limited in aquatic environments?
A) They are not very soluble.
B) They change to different chemical forms in water.
C) They cannot diffuse across cell membranes.
D) They are rare in the atmosphere and therefore limited in water.
A) They are not very soluble.
B) They change to different chemical forms in water.
C) They cannot diffuse across cell membranes.
D) They are rare in the atmosphere and therefore limited in water.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
Which is NOT an adaptation to low-oxygen aquatic environments?
A) increased hemoglobin
B) breathing air
C) increased metabolic activity
D) symbiotic relationship with algae
A) increased hemoglobin
B) breathing air
C) increased metabolic activity
D) symbiotic relationship with algae

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Explain why freshwater fish do not need to drink water.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Organisms that survive in varied temperatures adapt to changes by using
A) supercooling.
B) hyperosmotic molecules.
C) countercurrent circulation.
D) isozymes.
A) supercooling.
B) hyperosmotic molecules.
C) countercurrent circulation.
D) isozymes.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
What prevents ice formation in blood and tissues of marine animals?
A) an increased concentration of glycerol
B) an increased concentration of oxygen
C) a decreased concentration of salt
D) a decreased concentration of trimethylamine oxide
A) an increased concentration of glycerol
B) an increased concentration of oxygen
C) a decreased concentration of salt
D) a decreased concentration of trimethylamine oxide

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
The rate of biological processes increases two to four times for each _____ increase in temperature
A) 12ºC
B) 10ºC
C) 8ºC
D) 5ºC
A) 12ºC
B) 10ºC
C) 8ºC
D) 5ºC

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
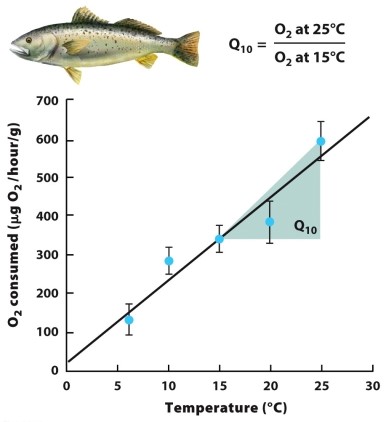
What concept does the nearby graph illustrate? Explain why it is important.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Samples of ocean water are taken from 25 locations, and the mean salt concentration is 36 ppt, with a sample variance of 1 ppt. What is the standard error of the sample?
A) 0.2
B) 0.5
C) 1
D) 1.2
E) 5
A) 0.2
B) 0.5
C) 1
D) 1.2
E) 5

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Coral bleaching
A) occurs when coral exoskeletons begin to break down.
B) is due to a lack of dissolved nutrients from the surrounding water.
C) is a temporary process that is usually reversed within days.
D) occurs when algae are expelled from coral.
A) occurs when coral exoskeletons begin to break down.
B) is due to a lack of dissolved nutrients from the surrounding water.
C) is a temporary process that is usually reversed within days.
D) occurs when algae are expelled from coral.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
Why is it surprising that some organisms are able to live at temperatures above 75ºC?
A) High temperatures decrease the efficiency of glycoproteins.
B) High temperatures denature proteins.
C) High temperatures evaporate water in cells.
D) High temperatures increase the permeability of cell membranes.
A) High temperatures decrease the efficiency of glycoproteins.
B) High temperatures denature proteins.
C) High temperatures evaporate water in cells.
D) High temperatures increase the permeability of cell membranes.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Explain why thermal pollution is relatively rare in oceans.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
What are some ways to prevent an aquarium from becoming anoxic?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
What percentage of a normal distribution is within one standard deviation of the mean?
A) 34%
B) 50%
C) 68%
D) 76%
E) 95%
A) 34%
B) 50%
C) 68%
D) 76%
E) 95%

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Glycoproteins coat ice crystals that begin to form in blood and prevent freezing in a process called
A) antifreeze accumulation.
B) supercooling.
C) isozymal coating.
D) osmoregulation.
A) antifreeze accumulation.
B) supercooling.
C) isozymal coating.
D) osmoregulation.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
The primary cause of coral bleaching is
A) decreased water temperature.
B) increased water temperature.
C) decreased salt concentrations.
D) increased water pH.
E) decreased water pH.
A) decreased water temperature.
B) increased water temperature.
C) decreased salt concentrations.
D) increased water pH.
E) decreased water pH.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
What unique low-temperature challenge are marine organisms likely to encounter?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
What enable thermophilic bacteria to withstand very high temperatures?
A) high glycerol concentrations that protect cell membranes
B) low concentrations of isozymes that change form at high temperatures
C) cell materials that reduce heat transfer
D) high proportions of particular amino acids that form strong bonds
A) high glycerol concentrations that protect cell membranes
B) low concentrations of isozymes that change form at high temperatures
C) cell materials that reduce heat transfer
D) high proportions of particular amino acids that form strong bonds

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
Nine ponds are sampled, and the mean salt concentration is 121 ppm, with a sample variance of 25 ppm. What is the standard deviation of the sample?
A) 0.6
B) 2.2
C) 3
D) 5
E) 11
A) 0.6
B) 2.2
C) 3
D) 5
E) 11

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
Explain why there are relatively few plant species in mangrove forests.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Explain how increased atmospheric carbon dioxide can affect the ability of coral to build their exoskeletons.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Suppose organisms in a lake are limited by oxygen. How might the lake's ability to support organisms change if the temperature increased by 10ºC, assuming that all the organisms were still within their thermal optima?
A) It could support about twice as many organisms.
B) It could support about half as many organisms.
C) It could support about the same number of organisms.
D) More information is required to determine whether it would change.
A) It could support about twice as many organisms.
B) It could support about half as many organisms.
C) It could support about the same number of organisms.
D) More information is required to determine whether it would change.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
Explain how osmotic regulation might make marine organisms better at surviving low temperatures than freshwater organisms.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
What do a large standard deviation and small standard error tell you about the data from several samples?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


