Deck 4: Reactions of Alkenes and Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/43
Play
Full screen (f)
Deck 4: Reactions of Alkenes and Alkynes
1
What alkene would you use to prepare the following alkyl halide?
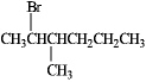 Only one alkene would be possible:
Only one alkene would be possible: 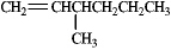
2
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate:
Predict and indicate:

3
Instructions: Predict the structure of the alkene you would use to prepare the folloiwng compounds. There may be more than one answer in some cases.
Predict.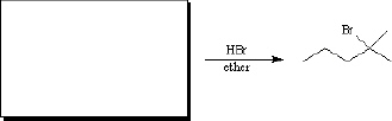
Predict.

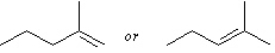
4
Instructions: Predict the structure of the alkene you would use to prepare the folloiwng compounds. There may be more than one answer in some cases.
Predict.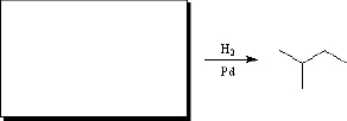
Predict.


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
5
Instructions: The reaction of 2-methylpropene with HBr in ether gives one of the two products below. Answer the following question(s) about this reaction. 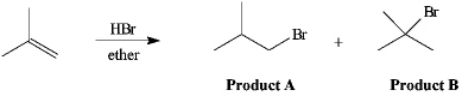 Refer to instructions. Which product is the Markovnikov product?
Refer to instructions. Which product is the Markovnikov product?
 Refer to instructions. Which product is the Markovnikov product?
Refer to instructions. Which product is the Markovnikov product?
Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
6
Predict the major organic product(s) in the reaction below. If more than one major organic product is expected, draw each one. 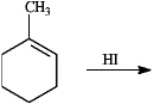


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
7
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate.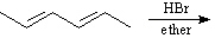
Predict and indicate.


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
8
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate.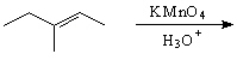
Predict and indicate.


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
9
The product(s) of the reaction when carried out in an organic solvent 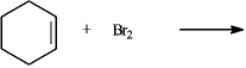 would be:
would be:
A) cis-1,2-dibromocyclohexane only
B) trans-1,2-dibromocyclohexane only
C) 50/50 mixture of cis-1,2-dibromocyclohexane and trans-1,2-dibromocyclohexane only
D) mixture with > 50% being trans-1,2-dibromocyclohexane only
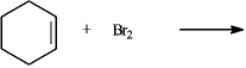 would be:
would be:A) cis-1,2-dibromocyclohexane only
B) trans-1,2-dibromocyclohexane only
C) 50/50 mixture of cis-1,2-dibromocyclohexane and trans-1,2-dibromocyclohexane only
D) mixture with > 50% being trans-1,2-dibromocyclohexane only

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
10
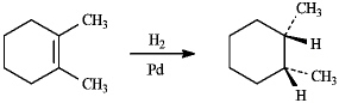
The following reaction is carried out in cyclohexane with the application of heat. Write the complete equation for the reaction below. If more than one major organic product is expected, draw each one. 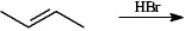
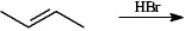

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
11
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate.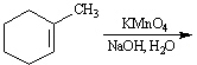
Predict and indicate.


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
12
Predict the major organic product(s) in the reaction below. If more than one major organic product is expected, draw each one. Explain the significance of "35 C" and "ether" shown in the reaction. 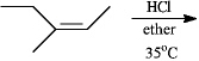
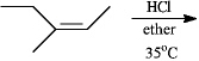

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
13
Alkene chemistry is dominated by what type of reaction?
A) substitution
B) electrophilic addition
C) nucleophilic addition
D) elimination
E) both b and c
A) substitution
B) electrophilic addition
C) nucleophilic addition
D) elimination
E) both b and c

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
14
Instructions: Predict the structure of the alkene you would use to prepare the folloiwng compounds. There may be more than one answer in some cases.
Predict: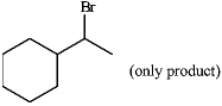
Predict:


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
15
Instructions: To answer the question(s) below, consider the following reaction:
When cyclohexene reacts with chlorine in tetrachloromethane, the following dihalide is formed.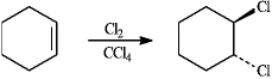 Refer to instructions. Since the two chlorine atoms add to opposite faces of the cyclohexene double bond, we say that the reaction occurs with:
Refer to instructions. Since the two chlorine atoms add to opposite faces of the cyclohexene double bond, we say that the reaction occurs with:
A) syn stereochemistry
B) cis stereochemistry
C) anti stereochemistry
D) retention of stereochemistry
When cyclohexene reacts with chlorine in tetrachloromethane, the following dihalide is formed.
 Refer to instructions. Since the two chlorine atoms add to opposite faces of the cyclohexene double bond, we say that the reaction occurs with:
Refer to instructions. Since the two chlorine atoms add to opposite faces of the cyclohexene double bond, we say that the reaction occurs with:A) syn stereochemistry
B) cis stereochemistry
C) anti stereochemistry
D) retention of stereochemistry

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
16
Instructions: To answer the question(s) below, consider the following reaction:
When cyclohexene reacts with chlorine in tetrachloromethane, the following dihalide is formed.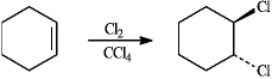 Refer to instructions. Provide the IUPAC name for the product of this reaction.
Refer to instructions. Provide the IUPAC name for the product of this reaction.
When cyclohexene reacts with chlorine in tetrachloromethane, the following dihalide is formed.
 Refer to instructions. Provide the IUPAC name for the product of this reaction.
Refer to instructions. Provide the IUPAC name for the product of this reaction.
Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
17
Instructions: The reaction of 2-methylpropene with HBr in ether gives one of the two products below. Answer the following question(s) about this reaction. 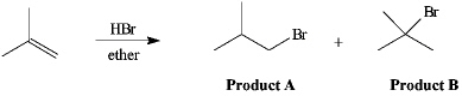 Refer to instructions. The reaction mixture would contain a majority of which isomeric product?
Refer to instructions. The reaction mixture would contain a majority of which isomeric product?
 Refer to instructions. The reaction mixture would contain a majority of which isomeric product?
Refer to instructions. The reaction mixture would contain a majority of which isomeric product?
Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate.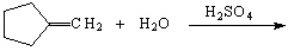
Predict and indicate.
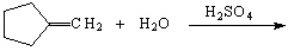

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the major product of the following reaction. 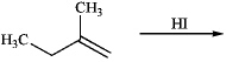


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
20
Instructions: Predict the structure of the alkene you would use to prepare the folloiwng compounds. There may be more than one answer in some cases.
Predict.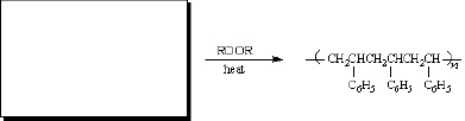
Predict.


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
21
Circle whichever of the following would be classified as a conjugated diene. 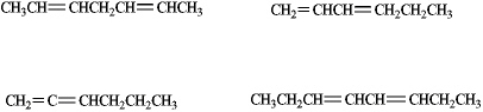
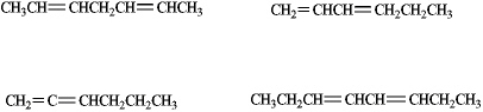

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
22
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate.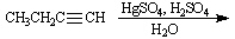
Predict and indicate.
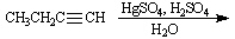

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
23
MATCH each definition to the term it describes. Place the letter of the term in the blank to the left of the definition.
A. Cahn-Ingold-Prelog Rules
B. Markovnikov's Rule
Refer to Instructions. _____Assigns priorities to substituent groups on a carbon.
A. Cahn-Ingold-Prelog Rules
B. Markovnikov's Rule
Refer to Instructions. _____Assigns priorities to substituent groups on a carbon.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
24
What type of reactive intermediate is formed in the reaction of an alkene with HBr to give a bromoalkane?
A) carbocation
B) carbanion
C) radical
D) cyclic bromonium ion
A) carbocation
B) carbanion
C) radical
D) cyclic bromonium ion

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
25
On the structures provided, draw arrows showing the electron flow in the reaction mechanism for the electrophilic addition of hydrogen bromide to hex-1-yne. 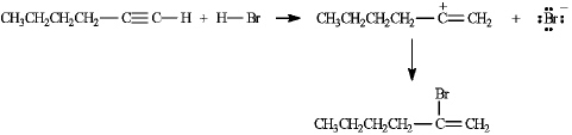


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
26
What type of reaction mechanism accounts for the reaction of an alkene with HBr to give an alkyl bromide?
A) nucleophilic addition
B) electrophilic addition
C) radical addition
D) elimination
A) nucleophilic addition
B) electrophilic addition
C) radical addition
D) elimination

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
27
Draw the structure of the monomer used to prepare the polymer shown below.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
28
What type of reactive intermediate is involved in both of the following general reaction types? 1) reaction of an alkene with in the presence of water to give a bromohydrin
2) two-step hydroxylation of an alkene
A) carbocation
B) carbanion
C) radical
D) cyclic bromonium ion
E) cyclic ion
2) two-step hydroxylation of an alkene
A) carbocation
B) carbanion
C) radical
D) cyclic bromonium ion
E) cyclic ion

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
29
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate.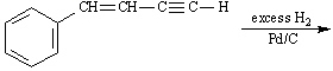
Predict and indicate.
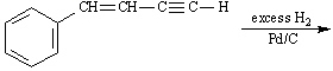

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
30
MATCH each definition to the term it describes. Place the letter of the term in the blank to the left of the definition.
A. Cahn-Ingold-Prelog Rules
B. Markovnikov's Rule
Refer to Instructions. _____Predicts that in electrophilic additions to alkenes the more stable carbocation intermediate is formed.
A. Cahn-Ingold-Prelog Rules
B. Markovnikov's Rule
Refer to Instructions. _____Predicts that in electrophilic additions to alkenes the more stable carbocation intermediate is formed.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
31
MATCH each definition to the term it describes. Place the letter of the term in the blank to the left of the definition.
A. Cahn-Ingold-Prelog Rules
B. Markovnikov's Rule
Refer to Instructions. _____Predicts that in addition of HX to alkenes, the H adds to the less substituted alkene carbon and the X adds to the more substituted alkene carbon.
A. Cahn-Ingold-Prelog Rules
B. Markovnikov's Rule
Refer to Instructions. _____Predicts that in addition of HX to alkenes, the H adds to the less substituted alkene carbon and the X adds to the more substituted alkene carbon.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
32
To answer the questions below, consider the following information:
In an abandoned laboratory has been found a flammable liquid, A, in a bottle bearing only the label "Compound A: C7H12. Government agents have offered you a considerable sum to determine the structure of this compound. After verifying the molecular formula by elemental analysis, you find that Compound A reacts with 1 mol equiv of hydrogen and, after treatment with acidic KMnO4, gives the dicarboxylic acid C (see below). Another bottle from the same laboratory is labeled "Compound B (isomer of A)." Compound B also reacts with 1 mol equiv of hydrogen, but yields cyclohexanone after treatment with acidic KMnO4.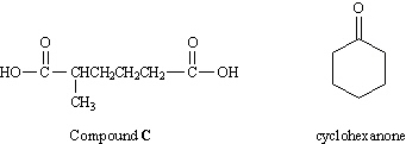 Refer to Instructions. Suggest structures for A and
Refer to Instructions. Suggest structures for A and
B.
In an abandoned laboratory has been found a flammable liquid, A, in a bottle bearing only the label "Compound A: C7H12. Government agents have offered you a considerable sum to determine the structure of this compound. After verifying the molecular formula by elemental analysis, you find that Compound A reacts with 1 mol equiv of hydrogen and, after treatment with acidic KMnO4, gives the dicarboxylic acid C (see below). Another bottle from the same laboratory is labeled "Compound B (isomer of A)." Compound B also reacts with 1 mol equiv of hydrogen, but yields cyclohexanone after treatment with acidic KMnO4.
 Refer to Instructions. Suggest structures for A and
Refer to Instructions. Suggest structures for A and B.

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
33
Draw the structure of the polymer formed from the monomer given below, showing four repeating units. 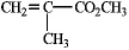
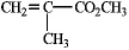

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
34
Draw all the isomeric products formed from the addition of HCl to the following compound. 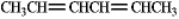
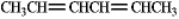

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
35
Complete the following reaction 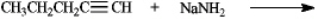
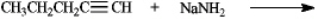

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
36
Draw two resonance structures for the species below. 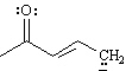


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
37
Draw two resonance structures for the species below. 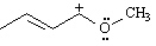


Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
38
Instructions: Predict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate.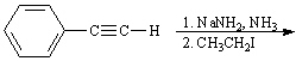
Predict and indicate.
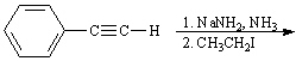

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
39
What type of reaction mechanism accounts for the reaction of an alkyne with HBr to give an alkyl bromide?
A) nucleophilic addition
B) electrophilic addition
C) radical addition
D) elimination
A) nucleophilic addition
B) electrophilic addition
C) radical addition
D) elimination

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
40
What type of reactive intermediate is formed in the reaction of an alkene with aqueous acid to give an alcohol?
A) carbocation
B) carbanion
C) radical
D) carbene
A) carbocation
B) carbanion
C) radical
D) carbene

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
41
Povidone is produced commercially as a series of products having mean molecular weights ranging from about 10,000 to 700,000. Complexed with iodine, povidone yields an iodophor, marketed under the tradename Betadine, which is used as a topical anti-infective. 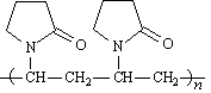 Identify the monomer unit(s) in povidone.
Identify the monomer unit(s) in povidone.
 Identify the monomer unit(s) in povidone.
Identify the monomer unit(s) in povidone.
Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
42
To answer the questions below, consider the following information:
In an abandoned laboratory has been found a flammable liquid, A, in a bottle bearing only the label "Compound A: C7H12. Government agents have offered you a considerable sum to determine the structure of this compound. After verifying the molecular formula by elemental analysis, you find that Compound A reacts with 1 mol equiv of hydrogen and, after treatment with acidic KMnO4, gives the dicarboxylic acid C (see below). Another bottle from the same laboratory is labeled "Compound B (isomer of A)." Compound B also reacts with 1 mol equiv of hydrogen, but yields cyclohexanone after treatment with acidic KMnO4.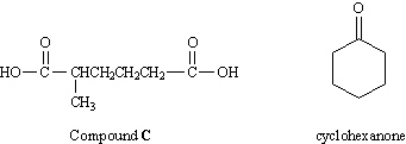 What was the other product formed in the KMnO4 oxidation of B?
What was the other product formed in the KMnO4 oxidation of B?
In an abandoned laboratory has been found a flammable liquid, A, in a bottle bearing only the label "Compound A: C7H12. Government agents have offered you a considerable sum to determine the structure of this compound. After verifying the molecular formula by elemental analysis, you find that Compound A reacts with 1 mol equiv of hydrogen and, after treatment with acidic KMnO4, gives the dicarboxylic acid C (see below). Another bottle from the same laboratory is labeled "Compound B (isomer of A)." Compound B also reacts with 1 mol equiv of hydrogen, but yields cyclohexanone after treatment with acidic KMnO4.
 What was the other product formed in the KMnO4 oxidation of B?
What was the other product formed in the KMnO4 oxidation of B?
Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck
44
Match between columns

Unlock Deck
Unlock for access to all 43 flashcards in this deck.
Unlock Deck
k this deck


