Deck 18: The Colorful Chemistry of Metals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/114
Play
Full screen (f)
Deck 18: The Colorful Chemistry of Metals
1
Identify the Lewis acid in the following reaction: PH3(g) + H+(g) PH4+(g)
A) PH3
B) H+
C) PH4+
D) None of these is an acid.
E) All of these are acids.
A) PH3
B) H+
C) PH4+
D) None of these is an acid.
E) All of these are acids.
H+
2
What do you predict for the geometry of Ti(H2O)63+?
A) octahedral
B) tetrahedral
C) square planar
D) trigonal bipyramidal
E) square pyramidal
A) octahedral
B) tetrahedral
C) square planar
D) trigonal bipyramidal
E) square pyramidal
octahedral
3
A Lewis base is any species capable of __________ an electron pair.
A) accepting
B) donating
C) creating
D) neutralizing
E) gaining
A) accepting
B) donating
C) creating
D) neutralizing
E) gaining
donating
4
The complex ion Fe(H2O)63+ has 5 unpaired electrons in 3d-orbitals. Given this information, which iron orbitals would you expect to be hybridized and used to form the coordinate covalent bonds with water?
A) 4s, 4p, and 4d
B) 3s and 3p
C) 3s, 3p, and 3d
D) 4s, 4p, and 3d
E) 4s and 4p
A) 4s, 4p, and 4d
B) 3s and 3p
C) 3s, 3p, and 3d
D) 4s, 4p, and 3d
E) 4s and 4p

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
5
A coordinate covalent bond forms when __________
A) a chemical species donates one electron to another species to form a covalent bond.
B) a chemical species donates a pair of electrons to another species to form a covalent bond.
C) a chemical species accepts one electron from another species to form a covalent bond.
D) a chemical species accepts an electron and becomes an anion.
E) a chemical species accepts a pair of electrons and becomes an anion.
A) a chemical species donates one electron to another species to form a covalent bond.
B) a chemical species donates a pair of electrons to another species to form a covalent bond.
C) a chemical species accepts one electron from another species to form a covalent bond.
D) a chemical species accepts an electron and becomes an anion.
E) a chemical species accepts a pair of electrons and becomes an anion.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the molar concentration of Cl- ions in a 1.00 M solution of AgCl2- with no excess silver ions. Given: Kf = 2.5 105 for AgCl2-.
A) 0.020 M
B) 0.0040 M
C) 3.2 10-2 M
D) 2.5 10-2 M
E) 2.5 105 M
A) 0.020 M
B) 0.0040 M
C) 3.2 10-2 M
D) 2.5 10-2 M
E) 2.5 105 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
7
What is the shape of Zn(OH)42-?
A) triangular pyramid
B) tetrahedral
C) octahedral
D) square pyramid
E) T-shaped
A) triangular pyramid
B) tetrahedral
C) octahedral
D) square pyramid
E) T-shaped

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
8
What is the shape of the complex ion CoF63-?
A) square pyramidal
B) octahedral
C) tetrahedral
D) trigonal bipyramidal
E) square planar
A) square pyramidal
B) octahedral
C) tetrahedral
D) trigonal bipyramidal
E) square planar

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
9
A ligand is any __________ forming a coordinate bond to a metal cation.
A) Lewis acid
B) ion
C) Lewis base
D) organic compound
E) species
A) Lewis acid
B) ion
C) Lewis base
D) organic compound
E) species

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
10
In the following reaction, which species is the Lewis base? Cu2+ + NH3 Cu(NH3)2+
A) Cu2+
B) NH3
C) [Cu(NH3)]2+
D) None of these is a base.
E) All of these are bases.
A) Cu2+
B) NH3
C) [Cu(NH3)]2+
D) None of these is a base.
E) All of these are bases.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
11
When NF3 reacts with BF3 to form F3NBF3,
A) a coordinate covalent bond is formed.
B) an ionic bond is formed.
C) the bond is formed by sharing an electron from NF3 and an electron from BF3.
D) a complex ion is formed.
E) a covalent bond is formed.
A) a coordinate covalent bond is formed.
B) an ionic bond is formed.
C) the bond is formed by sharing an electron from NF3 and an electron from BF3.
D) a complex ion is formed.
E) a covalent bond is formed.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
12
A Lewis acid is any species capable of __________ an electron pair.
A) accepting
B) donating
C) creating
D) neutralizing
E) losing
A) accepting
B) donating
C) creating
D) neutralizing
E) losing

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
13
A Lewis base is __________
A) an electron-pair acceptor.
B) an electron-pair donor.
C) a proton donor
D) a proton acceptor.
E) never viewed also as a Brønsted-Lowry base.
A) an electron-pair acceptor.
B) an electron-pair donor.
C) a proton donor
D) a proton acceptor.
E) never viewed also as a Brønsted-Lowry base.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
14
In the following reaction, which species is the Lewis acid? Cu2+ + NH3 Cu(NH3)2+
A) Cu2+
B) NH3
C) [Cu(NH3)]2+
D) None of these is an acid.
E) All of these are acids.
A) Cu2+
B) NH3
C) [Cu(NH3)]2+
D) None of these is an acid.
E) All of these are acids.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
15
A Lewis acid is __________
A) a proton donor.
B) a proton acceptor.
C) an electron-pair donor.
D) an electron-pair acceptor.
E) is never viewed also as a Brønsted-Lowry acid.
A) a proton donor.
B) a proton acceptor.
C) an electron-pair donor.
D) an electron-pair acceptor.
E) is never viewed also as a Brønsted-Lowry acid.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following species is a Lewis base?
A) H+
B) Cs+
C) NF3
D) NF4+
E) NH4+
A) H+
B) Cs+
C) NF3
D) NF4+
E) NH4+

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
17
What would you predict for the hybridization of the Zn orbitals, based on the tetrahedral geometry of Zn(OH)42-?
A) sp3d2
B) sp3
C) sp2d
D) sp3d
E) p2d2
A) sp3d2
B) sp3
C) sp2d
D) sp3d
E) p2d2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
18
What is the molar concentration of Ag+(aq) in a 1.00 M solution of Ag(NH3)2+ with no excess ammonia? Given: Kf = 1.70 107 for Ag(NH3)2+.
A) 2.45 10-3 M
B) 3.09 10-3 M
C) 2.42 10-4 M
D) 1.47 10-8 M
E) 1.70 10-8 M
A) 2.45 10-3 M
B) 3.09 10-3 M
C) 2.42 10-4 M
D) 1.47 10-8 M
E) 1.70 10-8 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
19
Identify the Lewis base in the following reaction: PH3(g) + H+(g) PH4+(g)
A) PH3
B) H+
C) PH4+
D) None of these is a base.
E) All of these are bases.
A) PH3
B) H+
C) PH4+
D) None of these is a base.
E) All of these are bases.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
20
Determine the molar concentration of Ag+(aq) ions in a 1.00 M solution of AgCl2+ with no excess chloride. Given: Kf = 2.50 105 for AgCl2-.
A) 2.52 10-2 M
B) 1.26 10-2 M
C) 0.0100 M
D) 0.0020 M
E) 2.50 105 M
A) 2.52 10-2 M
B) 1.26 10-2 M
C) 0.0100 M
D) 0.0020 M
E) 2.50 105 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
21
Oxalic acid is a dicarboxylic acid with the structure HOOCCOOH. How many coordination sites does the ion (OOCCOO)2- have?
A) 2
B) 3
C) 4
D) 1
E) 5
A) 2
B) 3
C) 4
D) 1
E) 5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
22
Complex ions with different ligands have different colors because the ligands __________
A) are different colors.
B) affect the energy levels of the lone-pair electrons on the metal.
C) have different energies for their bonding electrons.
D) affect the energy levels of the metal d orbitals.
E) have different energies for their lone-pair electrons.
A) are different colors.
B) affect the energy levels of the lone-pair electrons on the metal.
C) have different energies for their bonding electrons.
D) affect the energy levels of the metal d orbitals.
E) have different energies for their lone-pair electrons.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a chelation agent?
A) EDTA
B) Cl-
C) NH2
D) SCN-
E) CN-
A) EDTA
B) Cl-
C) NH2
D) SCN-
E) CN-

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
24
The reaction Cr(NH3)63+(aq) + 3 en(aq) Cr(en)33+(aq) + 6 NH3(aq)
Where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the equilibrium constant for this reaction is large because __________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) the equilibrium constant does not depend on the enthalpy change.
Where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the equilibrium constant for this reaction is large because __________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) the equilibrium constant does not depend on the enthalpy change.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements is/are correct? A large negative free-energy change for a reaction means that __________
(I) the equilibrium constant for the reaction is large.
(II) the equilibrium constant for the reaction is small.
(III) the reaction greatly favors formation of the products.
(IV) only a small amount of product is produced at equilibrium.
A) I only
B) II only
C) both I and III
D) both I and IV
E) both II and IV
(I) the equilibrium constant for the reaction is large.
(II) the equilibrium constant for the reaction is small.
(III) the reaction greatly favors formation of the products.
(IV) only a small amount of product is produced at equilibrium.
A) I only
B) II only
C) both I and III
D) both I and IV
E) both II and IV

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
26
Which atoms on EDTA bond to metal ions?
A) carbon and oxygen
B) carbon and nitrogen
C) oxygen and nitrogen
D) nitrogen and hydrogen
E) oxygen and hydrogen
A) carbon and oxygen
B) carbon and nitrogen
C) oxygen and nitrogen
D) nitrogen and hydrogen
E) oxygen and hydrogen

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is/are correct? A large equilibrium constant means that __________
(I) the free-energy change for the reaction is large and negative.
(II) the free-energy change for the reaction is large and positive.
(III) the reaction greatly favors formation of the products.
(IV) only a small amount of product is produced at equilibrium.
A) I only
B) II only
C) both I and III
D) both II and IV
E) both II and III
(I) the free-energy change for the reaction is large and negative.
(II) the free-energy change for the reaction is large and positive.
(III) the reaction greatly favors formation of the products.
(IV) only a small amount of product is produced at equilibrium.
A) I only
B) II only
C) both I and III
D) both II and IV
E) both II and III

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
28
Ethylenediamine is an example of a __________ ligand.
A) tridentate
B) hexadentate
C) bidentate
D) pentadentate
E) hydrocarbon
A) tridentate
B) hexadentate
C) bidentate
D) pentadentate
E) hydrocarbon

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
29
What is the equilibrium concentration of Cu+(aq) in a solution that is 0.0200 M solution in CuNO3 and 0.450 M HCl(aq)? Given: Cu+(aq) + 3Cl-(aq) CuCl32- Kf = 5 105
A) 1.0 10-7 M
B) 6.7 10-7 M
C) 4.4 10-7 M
D) 5.0 10-7 M
E) 5.0 105 M
A) 1.0 10-7 M
B) 6.7 10-7 M
C) 4.4 10-7 M
D) 5.0 10-7 M
E) 5.0 105 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
30
How many chelation sites and donor groups does EDTA have?
A) 2
B) 10
C) 6
D) 4
E) 5
A) 2
B) 10
C) 6
D) 4
E) 5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
31
Hydrated transition metal ions produce solutions that are __________
A) acidic.
B) basic.
C) neutral.
D) strongly basic.
E) strongly acidic.
A) acidic.
B) basic.
C) neutral.
D) strongly basic.
E) strongly acidic.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
32
The reaction Cr(NH3)63+(aq) + 3en(aq) Cr(en)33+(aq) + 6 NH3(aq)
Where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because __________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) ethylene diamine has amino groups that are stronger bases than ammonia.
Where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because __________
A) the reaction rate is fast.
B) the entropy change is large and positive.
C) the enthalpy change is large enough to matter.
D) the entropy change is large and negative.
E) ethylene diamine has amino groups that are stronger bases than ammonia.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
33
When a molecule of ethylenediamine replaces two molecules of NH3 in Co(NH3)63+, the entropy of the system __________
A) increases.
B) decreases.
C) remains the same.
D) cannot be determined.
E) is irrelevant.
A) increases.
B) decreases.
C) remains the same.
D) cannot be determined.
E) is irrelevant.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
34
What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag+ and 0.500 M 1,10-phenanthroline?
Ag+(aq) + 2phen [Ag(phen)2]+ Kf = 1.2 1012
[Ag (phen)2]+![<strong>What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag<sup>+</sup> and 0.500 M 1,10-phenanthroline? Ag<sup>+</sup>(aq) + 2phen <font style=font-family: 'Wingdings 3'></font> [Ag(phen)<sub>2</sub>]<sup>+ </sup> K<sub>f</sub> = 1.2 <font face=symbol></font> 10<sup>12</sup> [Ag (phen)<sub>2</sub>]<sup>+</sup> </strong> A) 9 <font face=symbol></font> 10<sup>-</sup><sup>7</sup> M B) 8 <font face=symbol></font> 10<sup>-</sup><sup>13</sup> M C) 6 <font face=symbol></font> 10<sup>-</sup><sup>6</sup> M D) 3.4 <font face=symbol></font> 10<sup>-</sup><sup>15</sup> M E) 1.2 <font face=symbol></font> 10<sup>-</sup><sup>12</sup> M](https://storage.examlex.com/TB3833/11eaae02_0885_deee_95d8_1f22e896588f_TB3833_00.jpg)
![<strong>What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag<sup>+</sup> and 0.500 M 1,10-phenanthroline? Ag<sup>+</sup>(aq) + 2phen <font style=font-family: 'Wingdings 3'></font> [Ag(phen)<sub>2</sub>]<sup>+ </sup> K<sub>f</sub> = 1.2 <font face=symbol></font> 10<sup>12</sup> [Ag (phen)<sub>2</sub>]<sup>+</sup> </strong> A) 9 <font face=symbol></font> 10<sup>-</sup><sup>7</sup> M B) 8 <font face=symbol></font> 10<sup>-</sup><sup>13</sup> M C) 6 <font face=symbol></font> 10<sup>-</sup><sup>6</sup> M D) 3.4 <font face=symbol></font> 10<sup>-</sup><sup>15</sup> M E) 1.2 <font face=symbol></font> 10<sup>-</sup><sup>12</sup> M](https://storage.examlex.com/TB3833/11eaae02_0885_deef_95d8_15e895cb0039_TB3833_00.jpg)
A) 9 10-7 M
B) 8 10-13 M
C) 6 10-6 M
D) 3.4 10-15 M
E) 1.2 10-12 M
Ag+(aq) + 2phen [Ag(phen)2]+ Kf = 1.2 1012
[Ag (phen)2]+
![<strong>What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag<sup>+</sup> and 0.500 M 1,10-phenanthroline? Ag<sup>+</sup>(aq) + 2phen <font style=font-family: 'Wingdings 3'></font> [Ag(phen)<sub>2</sub>]<sup>+ </sup> K<sub>f</sub> = 1.2 <font face=symbol></font> 10<sup>12</sup> [Ag (phen)<sub>2</sub>]<sup>+</sup> </strong> A) 9 <font face=symbol></font> 10<sup>-</sup><sup>7</sup> M B) 8 <font face=symbol></font> 10<sup>-</sup><sup>13</sup> M C) 6 <font face=symbol></font> 10<sup>-</sup><sup>6</sup> M D) 3.4 <font face=symbol></font> 10<sup>-</sup><sup>15</sup> M E) 1.2 <font face=symbol></font> 10<sup>-</sup><sup>12</sup> M](https://storage.examlex.com/TB3833/11eaae02_0885_deee_95d8_1f22e896588f_TB3833_00.jpg)
![<strong>What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag<sup>+</sup> and 0.500 M 1,10-phenanthroline? Ag<sup>+</sup>(aq) + 2phen <font style=font-family: 'Wingdings 3'></font> [Ag(phen)<sub>2</sub>]<sup>+ </sup> K<sub>f</sub> = 1.2 <font face=symbol></font> 10<sup>12</sup> [Ag (phen)<sub>2</sub>]<sup>+</sup> </strong> A) 9 <font face=symbol></font> 10<sup>-</sup><sup>7</sup> M B) 8 <font face=symbol></font> 10<sup>-</sup><sup>13</sup> M C) 6 <font face=symbol></font> 10<sup>-</sup><sup>6</sup> M D) 3.4 <font face=symbol></font> 10<sup>-</sup><sup>15</sup> M E) 1.2 <font face=symbol></font> 10<sup>-</sup><sup>12</sup> M](https://storage.examlex.com/TB3833/11eaae02_0885_deef_95d8_15e895cb0039_TB3833_00.jpg)
A) 9 10-7 M
B) 8 10-13 M
C) 6 10-6 M
D) 3.4 10-15 M
E) 1.2 10-12 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the equilibrium concentration of Zn2+(aq) in a solution that is 0.0125 M Zn(NO3)2 and 0.600 M NH3. Given: Zn2+(aq) + 4 NH3(aq) Zn(NH3)42+(aq) Kf = 2.9 109
A) 7.8 10-12 M
B) 4.7 10-11 M
C) 1.5 10-8 M
D) 2.5 10-9 M
E) 2.9 109 M
A) 7.8 10-12 M
B) 4.7 10-11 M
C) 1.5 10-8 M
D) 2.5 10-9 M
E) 2.9 109 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
36
For the reaction: Fe(H2O)63+ + H2O( 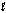 ) Fe(H2O)5(OH)2+ + H3O+(aq)
) Fe(H2O)5(OH)2+ + H3O+(aq)
Fe(H2O)63+ is a(n) __________ and water is a(n) __________
A) acid; base.
B) base; acid.
C) acid; catalyst.
D) catalyst; base.
E) Lewis base, Lewis acid.
 ) Fe(H2O)5(OH)2+ + H3O+(aq)
) Fe(H2O)5(OH)2+ + H3O+(aq)Fe(H2O)63+ is a(n) __________ and water is a(n) __________
A) acid; base.
B) base; acid.
C) acid; catalyst.
D) catalyst; base.
E) Lewis base, Lewis acid.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is a polydentate ligand?
A) NH3
B) OH-
C) H2O
D) diethylenetriamine
E) CN-
A) NH3
B) OH-
C) H2O
D) diethylenetriamine
E) CN-

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
38
EDTA is an example of a __________ ligand.
A) tridentate
B) hexadentate
C) tetradentate
D) pentadentate
E) hydrocarbon
A) tridentate
B) hexadentate
C) tetradentate
D) pentadentate
E) hydrocarbon

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
39
The greater affinity of metal ions for polydentate ligands than for monodentate ligands is known as the __________ effect.
A) dentate
B) ligand
C) chelate
D) Lewis base
E) Lewis acid
A) dentate
B) ligand
C) chelate
D) Lewis base
E) Lewis acid

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
40
What is the equilibrium concentration of Cl-(aq) in a solution that is 0.0200 M solution in CuNO3 and 0.450 M HCl(aq)? Given: Cu+(aq) + 3 Cl-(aq) CuCl32- Kf = 5 105
A) 0.450 M
B) 2.0 10-6 M
C) 0.390 M
D) 0.430 M
E) 5 105 M
A) 0.450 M
B) 2.0 10-6 M
C) 0.390 M
D) 0.430 M
E) 5 105 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
41
As the value of the crystal field splitting, o, increases from one complex ion to another, __________.
A) the wavelength of absorbed light increases.
B) the wavelength of absorbed light decreases.
C) the number of d electrons increases.
D) the number of d electrons decreases.
E) the color of the solution shifts from red towards blue.
A) the wavelength of absorbed light increases.
B) the wavelength of absorbed light decreases.
C) the number of d electrons increases.
D) the number of d electrons decreases.
E) the color of the solution shifts from red towards blue.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
42
Co(NH3)63+(aq) absorbs light in the blue region of the spectrum. The color of a solution containing this ion is __________
A) blue.
B) blue-violet.
C) violet.
D) yellow-orange.
E) red.
A) blue.
B) blue-violet.
C) violet.
D) yellow-orange.
E) red.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following complexes has the largest crystal field splitting of the d orbitals?
A) CoF63-
B) Co(CN)63-
C) CoBr63-
D) Co(H2O)63+
E) CoI63-
A) CoF63-
B) Co(CN)63-
C) CoBr63-
D) Co(H2O)63+
E) CoI63-

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
44
When the five 3d orbitals on a metal ion experience an octahedral field in a complex, they split into __________ energy levels.
A) two
B) three
C) four
D) five
E) six
A) two
B) three
C) four
D) five
E) six

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
45
Which d orbital(s) is/are highest in energy in a tetrahedral complex?
A) dz2
B) dxy, dxz, and dyz
C) dz2 and dx2 - y2
D) dx2 - y2
E) dxy and dxz
A) dz2
B) dxy, dxz, and dyz
C) dz2 and dx2 - y2
D) dx2 - y2
E) dxy and dxz

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
46
In a tetrahedral crystal field, __________
A) dxy, dxz, and dyz are lower in energy than dx2 - y2 and dz2.
B) dx2 - y2 and dz2 are lower in energy than dxy, dxz, and dyz.
C) dxy, dxz, dyz, dx2 - y2, and dz2 all have different energies.
D) dxy, dxz, dyz, dx2 - y2, and dz2 all have the same energy.
E) dxy, dxz, and dyz have the same energy, and dx2 - y2 and dz2 have different energies.
A) dxy, dxz, and dyz are lower in energy than dx2 - y2 and dz2.
B) dx2 - y2 and dz2 are lower in energy than dxy, dxz, and dyz.
C) dxy, dxz, dyz, dx2 - y2, and dz2 all have different energies.
D) dxy, dxz, dyz, dx2 - y2, and dz2 all have the same energy.
E) dxy, dxz, and dyz have the same energy, and dx2 - y2 and dz2 have different energies.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following ligands will cause the smallest crystal field splitting of the d orbitals in an octahedral complex?
A) H2O
B) Br-
C) NH3
D) CN-
E) I-
A) H2O
B) Br-
C) NH3
D) CN-
E) I-

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
48
Transition metal ions often absorb visible light in promoting electrons from a ground state to an excited state. Why doesn't aluminum(III) ion have this property?
A) It does, and this fact accounts for the color of some gemstones.
B) Aluminum(III) has a noble gas configuration-that of argon.
C) Aluminum(III) has a noble gas configuration-that of neon.
D) Aluminum(III) has a filled d subshell.
E) The crystal field splitting of the d orbitals in aluminum(III) is very large.
A) It does, and this fact accounts for the color of some gemstones.
B) Aluminum(III) has a noble gas configuration-that of argon.
C) Aluminum(III) has a noble gas configuration-that of neon.
D) Aluminum(III) has a filled d subshell.
E) The crystal field splitting of the d orbitals in aluminum(III) is very large.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
49
The dxy, dxz, and dyz orbitals are lower in energy than the dz2 and dx2 - y2 orbitals in an octahedral complex because these three orbitals __________
A) do not point directly at ligands.
B) point directly at ligands.
C) occupy larger volumes than the other two orbitals.
D) are in the same plane and repel one another.
E) occupy smaller volumes than the other two orbitals.
A) do not point directly at ligands.
B) point directly at ligands.
C) occupy larger volumes than the other two orbitals.
D) are in the same plane and repel one another.
E) occupy smaller volumes than the other two orbitals.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
50
A transition metal complex ion with octahedral geometry has a value of 4.4 10-19 J for the crystal field splitting, o. What is the wavelength for the absorption maximum, max for this complex ion?
A) 5.0 10-15 nm
B) 452 nm
C) 4.5 10-16 nm
D) 524 mm
E) 637 nm
A) 5.0 10-15 nm
B) 452 nm
C) 4.5 10-16 nm
D) 524 mm
E) 637 nm

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
51
A solution containing the ion CoF63- has an absorption maximum at max = 680 nm. The color of this solution is __________
A) red.
B) green.
C) blue.
D) orange.
E) violet.
A) red.
B) green.
C) blue.
D) orange.
E) violet.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
52
CoF63- absorbs light in the red region of the spectrum, while Co(NH3)63+ absorbs light on the blue region of the spectrum. This difference means that __________
A) the splitting of the d orbitals of the two complexes is nearly identical.
B) the value of the crystal field splitting is very small for both complexes.
C) the crystal field splitting is larger for the ammonia complex than for the fluoride complex.
D) the dz2 and dx2 - y2 orbitals are higher in energy than the dxy, dxz, and dyz orbitals in CoF63- and just the opposite splitting order for Co(NH3)63+.
E) the dz2 and dx2 - y2 orbitals are lower in energy than the dxy, dxz, and dyz orbitals in CoF63- and just the opposite splitting order for Co(NH3)63+.
A) the splitting of the d orbitals of the two complexes is nearly identical.
B) the value of the crystal field splitting is very small for both complexes.
C) the crystal field splitting is larger for the ammonia complex than for the fluoride complex.
D) the dz2 and dx2 - y2 orbitals are higher in energy than the dxy, dxz, and dyz orbitals in CoF63- and just the opposite splitting order for Co(NH3)63+.
E) the dz2 and dx2 - y2 orbitals are lower in energy than the dxy, dxz, and dyz orbitals in CoF63- and just the opposite splitting order for Co(NH3)63+.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
53
Which statement describing the splitting of metal d-orbitals in an octahedral field is not correct?
A) The d orbitals are split into two energy levels.
B) One energy level is three-fold degenerate.
C) One energy level is two-fold degenerate.
D) The dxy, dxz, and dyz orbitals have the lowest energy.
E) Photons cannot produce electronic transitions from one energy level to another.
A) The d orbitals are split into two energy levels.
B) One energy level is three-fold degenerate.
C) One energy level is two-fold degenerate.
D) The dxy, dxz, and dyz orbitals have the lowest energy.
E) Photons cannot produce electronic transitions from one energy level to another.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
54
Crystal field theory describes __________
A) how ligands cause the metal d orbitals to have different energies.
B) the covalent bonding in transition metal complexes.
C) how d orbitals change their shapes when ligands are present.
D) how metal s, p, and d orbitals are hybridized to bond with ligands.
E) the molecular orbitals that describe the bonding in transition metal complexes.
A) how ligands cause the metal d orbitals to have different energies.
B) the covalent bonding in transition metal complexes.
C) how d orbitals change their shapes when ligands are present.
D) how metal s, p, and d orbitals are hybridized to bond with ligands.
E) the molecular orbitals that describe the bonding in transition metal complexes.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
55
Which 3d orbitals have a higher energy in an octahedral field?
A) dxy and dxz
B) dz2 and dyz
C) dz2 and dx2 - y2
D) dx2 - y2 and dxz
E) dxy, dxz, and dyz
A) dxy and dxz
B) dz2 and dyz
C) dz2 and dx2 - y2
D) dx2 - y2 and dxz
E) dxy, dxz, and dyz

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following complexes has the smallest crystal field splitting of the d orbitals?
A) CoF63-
B) Co(CN)63-
C) CoBr63-
D) Co(H2O)63+
E) CoI63-
A) CoF63-
B) Co(CN)63-
C) CoBr63-
D) Co(H2O)63+
E) CoI63-

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
57
Transition metals with __________ tend to form square planar complexes.
A) d8 and d9 configurations
B) d1 and d2 configurations
C) half-filled d orbitals
D) high spin
E) no d electrons
A) d8 and d9 configurations
B) d1 and d2 configurations
C) half-filled d orbitals
D) high spin
E) no d electrons

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following ligands will cause the largest crystal field splitting of the d orbitals in an octahedral complex?
A) H2O
B) Br-
C) NH3
D) CN-
E) I-
A) H2O
B) Br-
C) NH3
D) CN-
E) I-

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
59
Given that the observed absorption maximum for the complex ion CoF63- in solution is max = 700 nm, what is the value of the crystal field splitting, o?
A) 2.8 10-19 J
B) 2.8 10-28 J
C) 7.2 1030 J
D) 3.2 10-36 J
E) 7.2 10-19 J
A) 2.8 10-19 J
B) 2.8 10-28 J
C) 7.2 1030 J
D) 3.2 10-36 J
E) 7.2 10-19 J

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
60
The dz2 and dx2 - y2 orbitals are higher in energy than the dxy, dxz, and dyz orbitals in an octahedral complex because these two orbitals __________
A) do not point directly at ligands.
B) point directly at ligands.
C) occupy larger volumes than the other three orbitals.
D) are in the same plane and repel one another.
E) occupy smaller volumes than the other three orbitals.
A) do not point directly at ligands.
B) point directly at ligands.
C) occupy larger volumes than the other three orbitals.
D) are in the same plane and repel one another.
E) occupy smaller volumes than the other three orbitals.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
61
What is the correct name for the following compound? 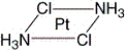
A) cis-diamminedichloroplatinum(II)
B) trans-diamminedichloroplatinum(III)
C) trans-diamminedichloroplatinum(II)
D) trans-dichlorodiammineplatinum(II)
E) cis-diamminedichloroplatinum(III)

A) cis-diamminedichloroplatinum(II)
B) trans-diamminedichloroplatinum(III)
C) trans-diamminedichloroplatinum(II)
D) trans-dichlorodiammineplatinum(II)
E) cis-diamminedichloroplatinum(III)

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
62
The correct name for the compound [Fe(NH3)5H2O](NO3)2 is __________
A) pentammineaquairon(II) nitrate.
B) pentammineaquairon(III) nitrate.
C) pentammineaquaferrate(II) nitrate.
D) aquapentammineiron(II) nitrate.
E) pentamineaquadinitroiron(III).
A) pentammineaquairon(II) nitrate.
B) pentammineaquairon(III) nitrate.
C) pentammineaquaferrate(II) nitrate.
D) aquapentammineiron(II) nitrate.
E) pentamineaquadinitroiron(III).

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
63
What is the correct formula for sodium tetracyanodihydroxovanadate(II)?
A) Na2[V(CN)4(OH)2]
B) Na3[V(CN)4(OH)2]
C) Na4[V(CN)4(OH)2]
D) Na[V(CN)4(OH)2]
E) Na3[V(CN)4(OH)]
A) Na2[V(CN)4(OH)2]
B) Na3[V(CN)4(OH)2]
C) Na4[V(CN)4(OH)2]
D) Na[V(CN)4(OH)2]
E) Na3[V(CN)4(OH)]

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
64
The correct name for K2[CuCl4] is __________
A) potassium copper chloride.
B) potassium tetrachlorocuprate(II).
C) dipotassium chlorocuprate(II).
D) potassium tetrachlorocopper(II).
E) dipotassium tetrachlorocopper(II).
A) potassium copper chloride.
B) potassium tetrachlorocuprate(II).
C) dipotassium chlorocuprate(II).
D) potassium tetrachlorocopper(II).
E) dipotassium tetrachlorocopper(II).

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
65
How many d electrons are there in the chromium(III) ion?
A) 0
B) 3
C) 4
D) 5
E) 2
A) 0
B) 3
C) 4
D) 5
E) 2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
66
The correct formula for ammonium tetracyanoplatinate(II) is __________
A) (NH3)2[Pt(CN)4].
B) (NH4)2[Pt(CN)4].
C) (NH4)2Pt(CN)4.
D) (NH4)2[Pt(CN)6].
E) NH4[Pt(CN)4].
A) (NH3)2[Pt(CN)4].
B) (NH4)2[Pt(CN)4].
C) (NH4)2Pt(CN)4.
D) (NH4)2[Pt(CN)6].
E) NH4[Pt(CN)4].

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
67
What is the correct formula for hexamminecobalt(III) nitrite?
A) [Co(NH3)6](NO3)3
B) Co(NH3)6(NO2)3
C) [Co(NH3)6](NO2)3
D) [Co(NH3)6](NO2)2
E) [Co(NH3)6](NO3)2
A) [Co(NH3)6](NO3)3
B) Co(NH3)6(NO2)3
C) [Co(NH3)6](NO2)3
D) [Co(NH3)6](NO2)2
E) [Co(NH3)6](NO3)2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following species most likely would be expected to be in a high-spin state?
A) Co(CN)63-
B) Co(H2O)63+
C) CoF63-
D) Mn(CN)64-
E) Co(NO2)63-
A) Co(CN)63-
B) Co(H2O)63+
C) CoF63-
D) Mn(CN)64-
E) Co(NO2)63-

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
69
The correct name for [Co(NH3)6][Cr(CN)6] is __________
A) cobaltammine chromiumcyanide.
B) hexamminecobalt(III) hexacyanochromate (II).
C) hexamminecobaltate(III) hexacyanochromium(III).
D) hexamminecobalt(III) hexacyanochromate(III).
E) hexamminecobalt(II) hexacyanochromate(II).
A) cobaltammine chromiumcyanide.
B) hexamminecobalt(III) hexacyanochromate (II).
C) hexamminecobaltate(III) hexacyanochromium(III).
D) hexamminecobalt(III) hexacyanochromate(III).
E) hexamminecobalt(II) hexacyanochromate(II).

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following metal ions can only have one spin state regardless of the ligand in an octahedral complex?
A) Cu2+
B) Co3+
C) Co2+
D) Mn2+
E) Mn3+
A) Cu2+
B) Co3+
C) Co2+
D) Mn2+
E) Mn3+

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
71
How many stereoisomers are there for the compound [Fe(NH3)4(Cl2)]NO3?
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
72
The correct name for [Co(NH3)5Cl]Cl2 is __________
A) pentamminechlorocobalt(III) chloride.
B) pentamminechlorocobalt(II) chloride.
C) chloropentamminecobalt(III) chloride.
D) pentamminechlorocobaltate(III) chloride.
E) pentamminedichlorocobalt(III).
A) pentamminechlorocobalt(III) chloride.
B) pentamminechlorocobalt(II) chloride.
C) chloropentamminecobalt(III) chloride.
D) pentamminechlorocobaltate(III) chloride.
E) pentamminedichlorocobalt(III).

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
73
A strong crystal field produces a large value for the crystal field splitting, o, while a weak crystal field produces a small crystal field splitting. Which would be more likely to put a metal ion in a high-spin configuration?
A) strong field
B) weak field
C) left field
D) right field
E) center field
A) strong field
B) weak field
C) left field
D) right field
E) center field

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
74
What is the correct name for the following compound? 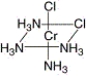
A) trans-tetramminedichlorochromium(II)
B) cis-tetramminedichlorochromium(II)
C) cis-triamminedichlorochromium(II)
D) cis-tetramminechlorochromium(II)
E) cis-tetramminedichlorochromium(III)

A) trans-tetramminedichlorochromium(II)
B) cis-tetramminedichlorochromium(II)
C) cis-triamminedichlorochromium(II)
D) cis-tetramminechlorochromium(II)
E) cis-tetramminedichlorochromium(III)

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following transition metal ions would have the greater o value when the same ligand was bound to the ions?
A) Co3+
B) Rh3+
C) Ir3+
D) They have the same o value.
A) Co3+
B) Rh3+
C) Ir3+
D) They have the same o value.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following metal ions could be found in both high-spin and low-spin configurations in octahedral complex ions?
A) Zn(II)
B) Cu(II)
C) Co(II)
D) V(II)
E) Ti(II)
A) Zn(II)
B) Cu(II)
C) Co(II)
D) V(II)
E) Ti(II)

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
77
The correct name for the complex ion [Co(NH3)5Cl]2+ is __________
A) pentamminechlorocobalt(III).
B) pentamminechlorocobalt(II).
C) chloropentamminecobalt(III).
D) pentamminechlorocobaltate(III).
E) pentamminechlorocobalt.
A) pentamminechlorocobalt(III).
B) pentamminechlorocobalt(II).
C) chloropentamminecobalt(III).
D) pentamminechlorocobaltate(III).
E) pentamminechlorocobalt.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
78
Paramagnetic substances are attracted to magnetic fields. A set of synthetic mineral crystals was prepared with different metal ions substituted into the identical octahedral site in the lattice. If each crystal contained exactly the same number of the indicated metal ion, which would have the greatest attraction to a magnetic field?
A) Mn(II), high spin
B) Fe(II), high spin
C) Co(II), high spin
D) Ni(II), high spin
E) Cu(II), high spin
A) Mn(II), high spin
B) Fe(II), high spin
C) Co(II), high spin
D) Ni(II), high spin
E) Cu(II), high spin

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
79
How many unpaired electrons are there in the octahedral complex ion, Mn(CN)64-?
A) 1
B) 2
C) 4
D) 5
E) 3
A) 1
B) 2
C) 4
D) 5
E) 3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
80
The correct formula for the complex ion tetracyanoplatinate(II) is __________
A) [Pt(CN)42+].
B) [Pt(CN)4]2-.
C) Pt(CN)4.
D) [Pt2(CN)4].
E) 4[Pt(CN)]+.
A) [Pt(CN)42+].
B) [Pt(CN)4]2-.
C) Pt(CN)4.
D) [Pt2(CN)4].
E) 4[Pt(CN)]+.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck


