Deck 9: Molecular Geometry and Bonding Theories
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 9: Molecular Geometry and Bonding Theories
1
Which of the following molecules is T-shaped?
A) NCl3
B) PCl3
C) SCl3
D) ICl3
E) COCl2
A) NCl3
B) PCl3
C) SCl3
D) ICl3
E) COCl2
ICl3
2
In VSEPR theory, molecular geometry is determined by __________
A) electron-proton attractive forces.
B) electron-electron attractive forces.
C) electron-electron repulsive forces.
D) proton-proton repulsive forces.
E) electron-nucleus attractive forces.
A) electron-proton attractive forces.
B) electron-electron attractive forces.
C) electron-electron repulsive forces.
D) proton-proton repulsive forces.
E) electron-nucleus attractive forces.
electron-electron repulsive forces.
3
Which of the following compounds has a trigonal bipyramidal shape?
A) ICl3
B) BrF5
C) PCl5
D) PH3
E) SiF4
A) ICl3
B) BrF5
C) PCl5
D) PH3
E) SiF4
PCl5
4
What is the geometry of the ClF4- ion?
A) square planar
B) square pyramidal
C) trigonal pyramidal
D) tetrahedral
E) seesaw
A) square planar
B) square pyramidal
C) trigonal pyramidal
D) tetrahedral
E) seesaw

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following has the largest bond angle?
A) CF4
B) NF3
C) F2O
D) NF2-
E) NH3
A) CF4
B) NF3
C) F2O
D) NF2-
E) NH3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the molecular geometry of CF2Cl2.
A) linear
B) bent
C) trigonal bipyramidal
D) tetrahedral
E) trigonal pyramidal
A) linear
B) bent
C) trigonal bipyramidal
D) tetrahedral
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds has the same molecular shape as CCl3H?
A) SiF4
B) KrF4
C) SF4
D) C2H4
E) C2H2
A) SiF4
B) KrF4
C) SF4
D) C2H4
E) C2H2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
What is the geometry of the triiodide ion (I3-)?
A) linear
B) bent
C) tetrahedral
D) trigonal pyramidal
E) trigonal planar
A) linear
B) bent
C) tetrahedral
D) trigonal pyramidal
E) trigonal planar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
Which electron-pair geometry has the lowest electron-electron repulsive forces?
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
A) linear
B) trigonal planar
C) tetrahedral
D) trigonal bipyramidal
E) octahedral

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following molecules has a trigonal pyramid shape?
A) KrF4
B) COCl2
C) SeO32-
D) NH4+
E) O3
A) KrF4
B) COCl2
C) SeO32-
D) NH4+
E) O3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
Which bond angle is the smallest?
A) H - C - H in CH4
B) H - C - H in C2H4
C) H - C - H in H2CO
D) H - C - C in C2H2
E) H - C - O in H2CO
A) H - C - H in CH4
B) H - C - H in C2H4
C) H - C - H in H2CO
D) H - C - C in C2H2
E) H - C - O in H2CO

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following molecules is linear?
A) H2O
B) H2S
C) ICl3
D) I3-
E) SO2
A) H2O
B) H2S
C) ICl3
D) I3-
E) SO2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
For the series methane, ammonia, and water, the bond angle increases in the following order: H2O < NH3 < CH4. This trend is due to __________
A) a decreasing effective nuclear charge.
B) a decrease in the number of lone pairs.
C) an increase in atomic radius.
D) an increase in the polarity of the molecules.
E) an increasing effective nuclear charge.
A) a decreasing effective nuclear charge.
B) a decrease in the number of lone pairs.
C) an increase in atomic radius.
D) an increase in the polarity of the molecules.
E) an increasing effective nuclear charge.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following compounds has a square pyramid shape?
A) ICl3
B) BrF5
C) PCl5
D) PH3
E) SiF4
A) ICl3
B) BrF5
C) PCl5
D) PH3
E) SiF4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following ions is linear?
A) SCN-
B) NO2-
C) SO32-
D) NH4+
E) SO22-
A) SCN-
B) NO2-
C) SO32-
D) NH4+
E) SO22-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
Arrange the interactions between pairs of electrons in order of increasing strength.
A) bonding pair-bonding pair < lone pair-bonding pair < lone pair-lone pair
B) bonding pair-bonding pair < lone pair-lone pair < lone pair-bonding pair
C) lone pair-lone pair < lone pair-bonding pair < bonding pair-bonding pair
D) lone pair-lone pair < bonding pair-bonding pair < lone pair-bonding pair
E) lone pair-bonding pair < bonding pair-bonding pair < lone pair-lone pair
A) bonding pair-bonding pair < lone pair-bonding pair < lone pair-lone pair
B) bonding pair-bonding pair < lone pair-lone pair < lone pair-bonding pair
C) lone pair-lone pair < lone pair-bonding pair < bonding pair-bonding pair
D) lone pair-lone pair < bonding pair-bonding pair < lone pair-bonding pair
E) lone pair-bonding pair < bonding pair-bonding pair < lone pair-lone pair

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
The correct H - N - H bond angle in NH3 is __________
A) the same as the H - O - H angle in water.
B) exactly 109.5.
C) greater than 109.5.
D) less than 109.5°.
E) 120°.
A) the same as the H - O - H angle in water.
B) exactly 109.5.
C) greater than 109.5.
D) less than 109.5°.
E) 120°.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
What is the molecular geometry of SF4?
A) tetrahedral
B) square pyramidal
C) seesaw
D) square planar
E) T-shaped
A) tetrahedral
B) square pyramidal
C) seesaw
D) square planar
E) T-shaped

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds has the same shape as SO2?
A) H2O
B) HCN
C) ICl3
D) OCS
E) CO2
A) H2O
B) HCN
C) ICl3
D) OCS
E) CO2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a planar molecule?
A) NH3
B) PCl5
C) KrF4
D) CH3COOH
E) SiH4
A) NH3
B) PCl5
C) KrF4
D) CH3COOH
E) SiH4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
Benzene (C6H6) is a cyclic, nonpolar molecule. By removing one of the hydrogens and replacing it with another atom or group, this substituted benzene becomes polar. Which of the following substituted benzenes is the most polar?
A) C6H5F (fluorobenzene)
B) C6H5Br (bromobenzene)
C) C6H5Cl (chlorobenzene)
D) C6H5I (iodobenzene)
E) C6H5CH3 (methylbenzene)
A) C6H5F (fluorobenzene)
B) C6H5Br (bromobenzene)
C) C6H5Cl (chlorobenzene)
D) C6H5I (iodobenzene)
E) C6H5CH3 (methylbenzene)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following molecules has a carbon atom that is sp3 hybridized?
A) C2H2
B) H2CO
C) CH3Cl
D) C2H4
E) C2H2Cl2
A) C2H2
B) H2CO
C) CH3Cl
D) C2H4
E) C2H2Cl2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
All homonuclear diatomic molecules __________
A) have polar bonds.
B) are polar.
C) are nonpolar.
D) cannot vibrate.
E) are liquids.
A) have polar bonds.
B) are polar.
C) are nonpolar.
D) cannot vibrate.
E) are liquids.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following has a central atom with the same hybridization as the oxygen in water?
A) SO2
B) OCS
C) CS2
D) NH3
E) CO2
A) SO2
B) OCS
C) CS2
D) NH3
E) CO2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following diagrams shows the correct orientation of the dipole in sulfur dioxide?
A)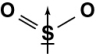
B)
C)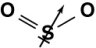
D)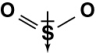
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
What is the hybridization of the bromine atom in BrF5?
A) sp3
B) sp3d
C) sp3d2
D) sp
E) sp2
A) sp3
B) sp3d
C) sp3d2
D) sp
E) sp2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following molecules has a central atom that is sp3 hybridized?
A) C2H2
B) H2CO
C) H2S
D) C2H4
E) C2H2Cl2
A) C2H2
B) H2CO
C) H2S
D) C2H4
E) C2H2Cl2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
What is the hybridization of the oxygen in the H3O+ ion?
A) sp2
B) sp3
C) sp3d
D) sp
E) sp3d2
A) sp2
B) sp3
C) sp3d
D) sp
E) sp3d2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the polar molecule.
A) CF4
B) SiH4
C) CHCl3
D) CS2
E) CO2
A) CF4
B) SiH4
C) CHCl3
D) CS2
E) CO2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following has a central atom with the same hybridization as the carbon in formaldehyde (H2CO)?
A) SO2
B) OCS
C) ICl3
D) I3-
E) C2H2
A) SO2
B) OCS
C) ICl3
D) I3-
E) C2H2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
What is the hybridization of the central iodine atom in I3-?
A) sp
B) sp2
C) sp3d
D) sp3d2
E) sp3
A) sp
B) sp2
C) sp3d
D) sp3d2
E) sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
What type of hybridization is needed to describe the bonding in a T-shaped molecule?
A) sp2
B) sp3
C) sp3d
D) sp
E) sp3d2
A) sp2
B) sp3
C) sp3d
D) sp
E) sp3d2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following shows the orientation of the net dipole for OCS?
A)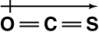
B)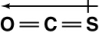
C)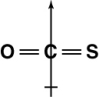
D)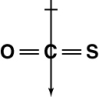
A)
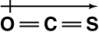
B)
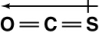
C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
What is the molecular geometry of SF4O?
A) pentagonal
B) tetrahedral
C) trigonal bipyramidal
D) trigonal planar
E) square pyramidal
A) pentagonal
B) tetrahedral
C) trigonal bipyramidal
D) trigonal planar
E) square pyramidal

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following compounds is the most polar?
A) NH3
B) PH3
C) AsH3
D) SbH3
E) BiH3
A) NH3
B) PH3
C) AsH3
D) SbH3
E) BiH3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
What type of hybridization is needed to describe the bonding in a seesaw-shaped molecule?
A) sp2
B) sp3
C) sp3d
D) sp
E) sp3d2
A) sp2
B) sp3
C) sp3d
D) sp
E) sp3d2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds has the smallest dipole moment?
A) CF2Cl2
B) CF3Cl
C) CF4
D) CFCl3
E) CHFCl2
A) CF2Cl2
B) CF3Cl
C) CF4
D) CFCl3
E) CHFCl2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules or ions is not polar?
A) O3
B) H2O
C) SO2
D) I3-
E) S3
A) O3
B) H2O
C) SO2
D) I3-
E) S3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
What hybridization is needed to describe the square planar molecular geometry of KrF4?
A) sp3
B) sp3d
C) sp3d2
D) sp2
E) sp
A) sp3
B) sp3d
C) sp3d2
D) sp2
E) sp

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following compounds has the largest dipole moment?
A) CH2Cl2
B) CH3Cl
C) CHCl3
D) CCl4
E) CH4
A) CH2Cl2
B) CH3Cl
C) CHCl3
D) CCl4
E) CH4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
Which statement regarding a pi bond between two carbon atoms is correct?
A) The region of high electron density lies along the bond axis connecting the nuclei of the two atoms.
B) Can be described by the overlap of sp hybrid orbitals from each atom.
C) Can be described by the overlap of sp2 hybrid orbitals from each atom.
D) Can be described by the overlap of p atomic orbitals from each atom.
E) Can be described by the overlap of a p atomic orbital from one atom with an sp2 hybrid orbital from the other atom.
A) The region of high electron density lies along the bond axis connecting the nuclei of the two atoms.
B) Can be described by the overlap of sp hybrid orbitals from each atom.
C) Can be described by the overlap of sp2 hybrid orbitals from each atom.
D) Can be described by the overlap of p atomic orbitals from each atom.
E) Can be described by the overlap of a p atomic orbital from one atom with an sp2 hybrid orbital from the other atom.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement regarding a sigma bond between two carbon atoms is not correct?
A) The region of electron density lies along the bond axis connecting the nuclei of the two atoms.
B) The bond can be described by the overlap of sp hybrid orbitals from each atom.
C) The bond can be described by the overlap of sp2 hybrid orbitals from each atom.
D) The bond can be described by the overlap of sp3d hybrid orbitals from each atom.
E) The bond can be described by the overlap of an sp2 hybrid orbital from one atom with an sp3 hybrid orbital from the other atom.
A) The region of electron density lies along the bond axis connecting the nuclei of the two atoms.
B) The bond can be described by the overlap of sp hybrid orbitals from each atom.
C) The bond can be described by the overlap of sp2 hybrid orbitals from each atom.
D) The bond can be described by the overlap of sp3d hybrid orbitals from each atom.
E) The bond can be described by the overlap of an sp2 hybrid orbital from one atom with an sp3 hybrid orbital from the other atom.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
Both cyclohexane (C6H12) and benzene (C6H6) have the carbon atoms forming a six-member ring. In a valence bond picture of the C - C bonds, _____ hybrid orbitals would overlap for cyclohexane, and _____ hybrid orbitals would overlap for benzene.
A) sp; sp
B) sp; sp2
C) sp2; sp3
D) sp3; sp2
E) sp3; sp
A) sp; sp
B) sp; sp2
C) sp2; sp3
D) sp3; sp2
E) sp3; sp

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
For the molecule CH3CHCHCH3, the local molecular geometry around the second carbon atom from the end and its hybridization are ___________
A) trigonal bipyramid and sp.
B) trigonal planar and sp3.
C) trigonal planar and sp2.
D) tetrahedral and sp3.
E) linear and sp.
A) trigonal bipyramid and sp.
B) trigonal planar and sp3.
C) trigonal planar and sp2.
D) tetrahedral and sp3.
E) linear and sp.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
What is the valence electron molecular orbital electron configuration of C2?
A)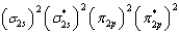
B)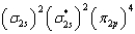
C)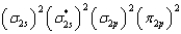
D)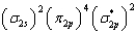
E)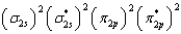
A)
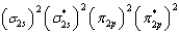
B)
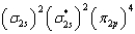
C)
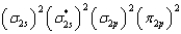
D)
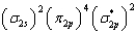
E)
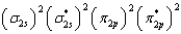

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
Which type of molecular orbital is used to describe electron density building up above and below the internuclear axis to form a bond?
A) ""
B) ""
C) "*"
D) "*"
E) s
A) ""
B) ""
C) "*"
D) "*"
E) s

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
Which type of molecular orbital contains a node perpendicular to the axis connecting two atomic nuclei?
A) ""
B) ""
C) "*"
D) s
E) p
A) ""
B) ""
C) "*"
D) s
E) p

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
Which type of molecular orbital has maximum electron density above and below the internuclear axis but zero density in a plane perpendicular to the internuclear axis?
A) ""
B) ""
C) "*"
D) "*"
E) p
A) ""
B) ""
C) "*"
D) "*"
E) p

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
Which type of molecular orbital has only one nodal plane, which contains the atomic nuclei?
A) ""
B) ""
C) "*"
D) "*"
E) s
A) ""
B) ""
C) "*"
D) "*"
E) s

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
Which statement regarding a pi bond between two carbon atoms is correct?
A) Regions of high electron density lie above and below the bond axis connecting the nuclei of the two atoms.
B) Can be described by the overlap of sp hybrid orbitals from each atom.
C) Can be described by the overlap of sp2 hybrid orbitals from each atom.
D) Can be described by the overlap of sp3 atomic orbitals from each atom.
E) Can be described by the overlap of a p atomic orbital from one atom with an sp2 hybrid orbital from the other atom.
A) Regions of high electron density lie above and below the bond axis connecting the nuclei of the two atoms.
B) Can be described by the overlap of sp hybrid orbitals from each atom.
C) Can be described by the overlap of sp2 hybrid orbitals from each atom.
D) Can be described by the overlap of sp3 atomic orbitals from each atom.
E) Can be described by the overlap of a p atomic orbital from one atom with an sp2 hybrid orbital from the other atom.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
Ethanol has the formula CH3CH2OH. The central carbon atom has a _____ local molecular geometry and _____ hybridization.
A) trigonal planar; sp
B) tetrahedral; sp3
C) tetrahedral; sp2
D) seesaw; sp2
E) seesaw, sp3
A) trigonal planar; sp
B) tetrahedral; sp3
C) tetrahedral; sp2
D) seesaw; sp2
E) seesaw, sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
Ethanol has the formula CH3CH2OH. The end carbon atom has a _____ local molecular geometry and _____ hybridization.
A) trigonal planar; sp
B) tetrahedral; sp3
C) tetrahedral; sp2
D) seesaw; sp2
E) seesaw; sp3
A) trigonal planar; sp
B) tetrahedral; sp3
C) tetrahedral; sp2
D) seesaw; sp2
E) seesaw; sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following compounds has a central atom with the same hybridization as PCl5?
A) IF6+
B) SiF4
C) BrF5
D) SbF5
E) XeF4
A) IF6+
B) SiF4
C) BrF5
D) SbF5
E) XeF4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
What are the hybridizations of the carbon atoms in CH3CH2COH, in order from left to right?
A) sp3, sp3, sp2
B) sp3, sp3, sp3
C) sp3, sp2, sp3
D) sp2, sp2, sp3
E) sp3, sp2, sp2
A) sp3, sp3, sp2
B) sp3, sp3, sp3
C) sp3, sp2, sp3
D) sp2, sp2, sp3
E) sp3, sp2, sp2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
Ethanol has the formula CH3CH2OH. The oxygen atom has a _____ local molecular geometry and _____ hybridization.
A) trigonal planar; sp
B) tetrahedral; sp3
C) tetrahedral; sp2
D) seesaw; sp2
E) bent; sp3
A) trigonal planar; sp
B) tetrahedral; sp3
C) tetrahedral; sp2
D) seesaw; sp2
E) bent; sp3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
Which type of molecular orbital is used to describe a buildup of electron density along the axis connecting two atomic nuclei to form a bond?
A) ""
B) ""
C) "*"
D) "*"
E) py
A) ""
B) ""
C) "*"
D) "*"
E) py

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
What is the hybridization of the iodine atom in ICl3?
A) sp2
B) sp3d2
C) sp3d
D) sp3
E) sp
A) sp2
B) sp3d2
C) sp3d
D) sp3
E) sp

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following has a local molecular geometry about a carbon atom that is trigonal planar?
A) CH3CH2OH
B) CH3OH
C) C2H4
D) CH3OCH3
E) C2H2
A) CH3CH2OH
B) CH3OH
C) C2H4
D) CH3OCH3
E) C2H2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
For the molecule CH3CHCHCH3, the local molecular geometry around a carbon atom at the end and its hybridization are ___________
A) trigonal bipyramid and sp.
B) trigonal planar and sp3.
C) trigonal planar and sp2.
D) tetrahedral and sp3.
E) linear and sp.
A) trigonal bipyramid and sp.
B) trigonal planar and sp3.
C) trigonal planar and sp2.
D) tetrahedral and sp3.
E) linear and sp.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
The local molecular geometry and the hybridization around each carbon atom in benzene (C6H6 with a hexagonal ring structure) is _______
A) square planar and sp.
B) trigonal planar and sp.
C) tetrahedral and sp3.
D) trigonal planar and sp2.
E) T-shaped and sp2.
A) square planar and sp.
B) trigonal planar and sp.
C) tetrahedral and sp3.
D) trigonal planar and sp2.
E) T-shaped and sp2.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
Using the energy level diagram below, determine the bond order of the PO molecule? 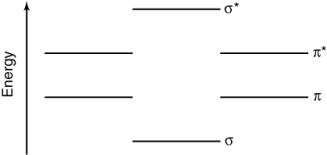
A) 2
B) 2.5
C) 1.5
D) 1
E) 0.5

A) 2
B) 2.5
C) 1.5
D) 1
E) 0.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
Which one of the following statements regarding molecular orbitals for diatomic molecules is not correct?
A) An atomic orbital on one atom combines with an atomic orbital on the other atom to form two molecular orbitals.
B) 2p atomic orbitals only form and * molecular orbitals.
C) 2s atomic orbitals only form and * atomic orbitals.
D) "" orbitals are symmetric with respect to rotation around the internuclear axis; orbitals do not have this symmetry.
E) A bonding molecular orbital describes the build-up of negatively charged electrons between the nuclei.
A) An atomic orbital on one atom combines with an atomic orbital on the other atom to form two molecular orbitals.
B) 2p atomic orbitals only form and * molecular orbitals.
C) 2s atomic orbitals only form and * atomic orbitals.
D) "" orbitals are symmetric with respect to rotation around the internuclear axis; orbitals do not have this symmetry.
E) A bonding molecular orbital describes the build-up of negatively charged electrons between the nuclei.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
Which statement concerning benzene is not correct? 
A) A second Lewis structure is needed to describe benzene.
B) All the bonds originate from p atomic orbitals.
C) Benzene does not have a dipole moment.
D) The carbon atomic orbitals are sp2 hybridized.
E) Benzene has both long and short carbon-carbon bonds.

A) A second Lewis structure is needed to describe benzene.
B) All the bonds originate from p atomic orbitals.
C) Benzene does not have a dipole moment.
D) The carbon atomic orbitals are sp2 hybridized.
E) Benzene has both long and short carbon-carbon bonds.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
Use energy levels of diatomic molecules derived from molecular orbital theory to predict the magnetic properties of the oxygen molecule O2 and the peroxide anion O22-.
A) oxygen (paramagnetic); peroxide (paramagnetic)
B) oxygen (paramagnetic); peroxide (diamagnetic)
C) oxygen (diamagnetic); peroxide (paramagnetic)
D) oxygen (diamagnetic); peroxide (diamagnetic)
E) Neither have magnetic properties, only metals have magnetic properties.
A) oxygen (paramagnetic); peroxide (paramagnetic)
B) oxygen (paramagnetic); peroxide (diamagnetic)
C) oxygen (diamagnetic); peroxide (paramagnetic)
D) oxygen (diamagnetic); peroxide (diamagnetic)
E) Neither have magnetic properties, only metals have magnetic properties.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
Use MO theory to predict the bond orders of the following molecular ions. Which one do you predict does not exist?
A) N2+
B) O2+
C) C2+
D) F22-
E) O22-
A) N2+
B) O2+
C) C2+
D) F22-
E) O22-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
Boron nitride is being investigated in frontier research directed at producing novel electronic devices. If you used the following energy level diagram for the molecular orbitals of boron nitride, BN, what would you predict? 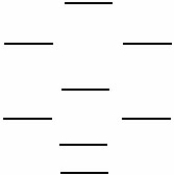
(I) Boron nitride is diamagnetic.
(II) Boron nitride has a bond order of 2.
(III) Boron nitride is paramagnetic.
(IV) The bond in BN- is stronger than the bond in BN.
A) I and II
B) II and III
C) I, II, and IV
D) III
E) IV

(I) Boron nitride is diamagnetic.
(II) Boron nitride has a bond order of 2.
(III) Boron nitride is paramagnetic.
(IV) The bond in BN- is stronger than the bond in BN.
A) I and II
B) II and III
C) I, II, and IV
D) III
E) IV

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Boron nitride, BN, is a new high tech material. According to the molecular orbital energy level diagram below, which one of the following statements is not correct about boron nitride? 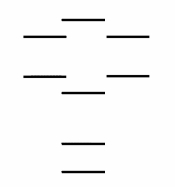
A) The bond order in BN is 2.0.
B) The cation BN+ has a longer bond than BN.
C) BN is paramagnetic.
D) The bond order in the cation BN+ is 1.5.
E) The anion BN- has a weaker bond than BN.

A) The bond order in BN is 2.0.
B) The cation BN+ has a longer bond than BN.
C) BN is paramagnetic.
D) The bond order in the cation BN+ is 1.5.
E) The anion BN- has a weaker bond than BN.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
According to the molecular orbital energy level diagram below, which one of the following statements is not correct about NO, NO+, and  ?
? 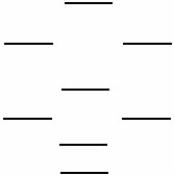
A) The bond order in NO is 2.5.
B) NO+ has the shortest bond.
C) Only one of these species is paramagnetic.
D) The bond order in is 2.0.
is 2.0.
E) has the weakest bond.
has the weakest bond.
 ?
? 
A) The bond order in NO is 2.5.
B) NO+ has the shortest bond.
C) Only one of these species is paramagnetic.
D) The bond order in
 is 2.0.
is 2.0.E)
 has the weakest bond.
has the weakest bond.
Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Use energy levels of diatomic molecules derived from molecular orbital theory to predict the bond order of the oxygen molecule O2 and the peroxide anion O22-.
A) oxygen (bond order = 2); peroxide (bond order = 3)
B) oxygen (bond order = 2); peroxide (bond order = 2)
C) oxygen (bond order = 4); peroxide (bond order = 4)
D) oxygen (bond order = 4); peroxide (bond order = 5)
E) oxygen (bond order = 2); peroxide (bond order = 1)
A) oxygen (bond order = 2); peroxide (bond order = 3)
B) oxygen (bond order = 2); peroxide (bond order = 2)
C) oxygen (bond order = 4); peroxide (bond order = 4)
D) oxygen (bond order = 4); peroxide (bond order = 5)
E) oxygen (bond order = 2); peroxide (bond order = 1)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
What is the bond order of B2?
A) 0
B) 1
C) 2
D) 3
E) 1.5
A) 0
B) 1
C) 2
D) 3
E) 1.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
Predict the bond order of the NO+ molecular ion.
A) 2
B) 2.5
C) 3
D) 1
E) 1.5
A) 2
B) 2.5
C) 3
D) 1
E) 1.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
Which of these molecules have a dipole moment? 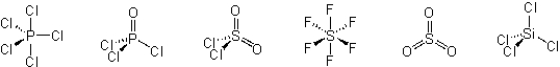
A) PCl5 and SiCl4
B) POCl3, SO2Cl2, and SO3
C) POCl3 and SO2Cl2
D) PCl5, POCl3, SOCl2, SO3, and SiCl4
E) PCl5, SF6, SO3, and SiCl4

A) PCl5 and SiCl4
B) POCl3, SO2Cl2, and SO3
C) POCl3 and SO2Cl2
D) PCl5, POCl3, SOCl2, SO3, and SiCl4
E) PCl5, SF6, SO3, and SiCl4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following molecules has a bond order that is less than one?
A) F2
B) He2-
C) Ne22+
D) N2
E) O22-
A) F2
B) He2-
C) Ne22+
D) N2
E) O22-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
Which one of the following molecules has a bond order of 3?
A) Li2
B) Be2
C) N2
D) F2
E) C2
A) Li2
B) Be2
C) N2
D) F2
E) C2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following statements about bonds between two carbon atoms is/are correct? (I) Bond strength increases as more electrons are shared between the atoms.
(II) Bond strength increases as the overlap between atomic orbitals increases.
(III) Hybrid orbitals are used to describe (account for) the geometry (bond angles) around each carbon atom.
A) I
B) II
C) III
D) Only two of these are correct statements.
E) All three are correct statements.
(II) Bond strength increases as the overlap between atomic orbitals increases.
(III) Hybrid orbitals are used to describe (account for) the geometry (bond angles) around each carbon atom.
A) I
B) II
C) III
D) Only two of these are correct statements.
E) All three are correct statements.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following molecules is paramagnetic?
A) Li2
B) C2
C) B2
D) N2
E) F2
A) Li2
B) C2
C) B2
D) N2
E) F2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
Which one of the following molecules is paramagnetic? These molecules are described by the MO energy diagram below. 
A) Li2
B) C22-
C) N22-
D) N22+
E) C2

A) Li2
B) C22-
C) N22-
D) N22+
E) C2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
Oxygen has two common molecular anions: peroxide (O22-) and superoxide (O2-). Use the MO energy level diagram below to identify which one of the following statements is not correct. 
A) The bond order of the peroxide is 1.
B) The superoxide has a shorter bond than the peroxide.
C) Like O2, the peroxide is paramagnetic.
D) The superoxide has a stronger bond than the peroxide.
E) Oxygen, O2, has a stronger bond than either of these oxides.

A) The bond order of the peroxide is 1.
B) The superoxide has a shorter bond than the peroxide.
C) Like O2, the peroxide is paramagnetic.
D) The superoxide has a stronger bond than the peroxide.
E) Oxygen, O2, has a stronger bond than either of these oxides.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
Predict the bond order of the NO- molecular ion.
A) 2
B) 2.5
C) 3
D) 1
E) 1.5
A) 2
B) 2.5
C) 3
D) 1
E) 1.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following molecules is paramagnetic?
A) F2
B) O2
C) C2
D) Be2
E) "C2"
A) F2
B) O2
C) C2
D) Be2
E) "C2"

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


