Deck 7: Electrons in Atoms and Periodic Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/104
Play
Full screen (f)
Deck 7: Electrons in Atoms and Periodic Properties
1
What is the frequency (, in Hz) of the photons emitted by a He-Ne laser with a wavelength () of 632.8 nm?
A) 4.738 105 Hz
B) 1.897 102 Hz
C) 1.897 1011 Hz
D) 4.741 1014 Hz
E) 1.897 1014 Hz
A) 4.738 105 Hz
B) 1.897 102 Hz
C) 1.897 1011 Hz
D) 4.741 1014 Hz
E) 1.897 1014 Hz
4.741 1014 Hz
2
Which of the following is quantized?
A) the trajectory of a baseball hit from home plate
B) the speed of a Formula-1 race car
C) the diameter of a redwood tree
D) the number of marbles in a bag
E) your age
A) the trajectory of a baseball hit from home plate
B) the speed of a Formula-1 race car
C) the diameter of a redwood tree
D) the number of marbles in a bag
E) your age
the number of marbles in a bag
3
What is the minimum frequency of a photon that can eject a photoelectron from Ba metal? (The work function of barium is = 4.3 10-19 J.)
A) 2.8 10-52 Hz
B) 6.5 1014 Hz
C) 6.5 104 Hz
D) 6.5 1013 Hz
E) 2.8 1014 Hz
A) 2.8 10-52 Hz
B) 6.5 1014 Hz
C) 6.5 104 Hz
D) 6.5 1013 Hz
E) 2.8 1014 Hz
6.5 1014 Hz
4
Indicate which metal requires the shortest wavelength photons to eject photoelectrons based on the following graph. 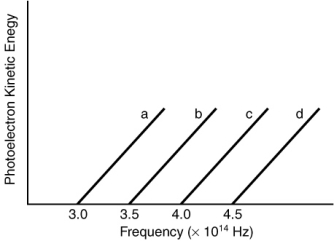
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following types of electromagnetic radiation has the longest wavelength?
A) gamma rays
B) X-rays
C) radio waves
D) infrared
E) visible
A) gamma rays
B) X-rays
C) radio waves
D) infrared
E) visible

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
6
Based on the following graph, which metal will emit the highest energy photoelectron when a photon with a frequency of 3.9 1014 Hz is incident on the metal? 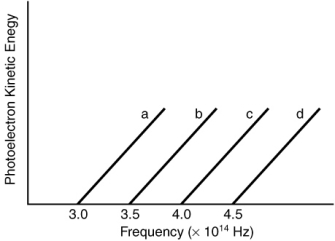
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following occurs only in discrete (quantized) increments?
A) the speed a car drives on the interstate
B) the altitude that an airplane flies
C) time
D) the level of water in a sink
E) money
A) the speed a car drives on the interstate
B) the altitude that an airplane flies
C) time
D) the level of water in a sink
E) money

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
8
In comparing the Fraunhofer lines with light emitted by elements in a flame, Bunsen and Kirchhoff demonstrated that __________
A) atoms emit and absorb electromagnetic radiation at characteristic wavelengths.
B) the solar spectrum has dark lines.
C) helium may be found in minerals that contain uranium.
D) electrons are responsible for the absorption and emission of electromagnetic radiation.
E) the solar spectrum has bright lines.
A) atoms emit and absorb electromagnetic radiation at characteristic wavelengths.
B) the solar spectrum has dark lines.
C) helium may be found in minerals that contain uranium.
D) electrons are responsible for the absorption and emission of electromagnetic radiation.
E) the solar spectrum has bright lines.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
9
Indicate which of the following photons can cause emission of photoelectrons from a surface of gallium ( = 6.7 10-19 J).
A) 350 nm
B) 400 nm
C) 200 nm
D) 650 nm
E) 500 nm
A) 350 nm
B) 400 nm
C) 200 nm
D) 650 nm
E) 500 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
10
What is the wavelength (, in m) of a radio station operating at a frequency of 99.6 MHz?
A) 3.01 106 m
B) 3.01 m
C) 3.32 10-7 m
D) 0.332 m
E) 3.32 m
A) 3.01 106 m
B) 3.01 m
C) 3.32 10-7 m
D) 0.332 m
E) 3.32 m

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
11
In refraction, polychromatic light is dispersed into its component wavelengths by __________
A) constructive and destructive interference of light waves in air.
B) internal reflections within a prism.
C) differences in the bending of different wavelengths of light by a prism.
D) constructive and destructive interference of light waves in a prism.
E) the reduction in the intensity of white light to reveal the colors.
A) constructive and destructive interference of light waves in air.
B) internal reflections within a prism.
C) differences in the bending of different wavelengths of light by a prism.
D) constructive and destructive interference of light waves in a prism.
E) the reduction in the intensity of white light to reveal the colors.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
12
Which statement about electromagnetic radiation is not correct?
A) Electromagnetic radiation consists of oscillating electric and magnetic fields.
B) Electromagnetic radiation is emitted by all stars.
C) All electromagnetic radiation is visible to the eye.
D) Electromagnetic radiation spans a very wide range of wavelengths from gamma rays to radio waves.
E) The frequency and wavelength of electromagnetic radiation are related to each other.
A) Electromagnetic radiation consists of oscillating electric and magnetic fields.
B) Electromagnetic radiation is emitted by all stars.
C) All electromagnetic radiation is visible to the eye.
D) Electromagnetic radiation spans a very wide range of wavelengths from gamma rays to radio waves.
E) The frequency and wavelength of electromagnetic radiation are related to each other.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following types of electromagnetic radiation has the shortest wavelength?
A) gamma rays
B) X-rays
C) radio waves
D) infrared
E) visible
A) gamma rays
B) X-rays
C) radio waves
D) infrared
E) visible

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
14
Fraunhofer lines are due to __________
A) emission of radiation.
B) absorption of radiation.
C) the photoelectric effect.
D) constructive interference.
E) destructive interference.
A) emission of radiation.
B) absorption of radiation.
C) the photoelectric effect.
D) constructive interference.
E) destructive interference.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
15
The Fraunhofer lines are evidence that __________
A) atoms contain nuclei.
B) atoms contain electrons.
C) waves interfere constructively.
D) elements absorb light.
E) elements emit light.
A) atoms contain nuclei.
B) atoms contain electrons.
C) waves interfere constructively.
D) elements absorb light.
E) elements emit light.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
16
The fact that the absorption and emission spectra of atoms and their ions are different is evidence that the spectra are due to __________
A) electrons.
B) protons.
C) neutrons.
D) the nuclei.
E) electromagnetic interactions.
A) electrons.
B) protons.
C) neutrons.
D) the nuclei.
E) electromagnetic interactions.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
17
The studies of light emitted by hot objects (Max Planck) and the photoelectric effect (Albert Einstein) revolutionized physics because they revealed __________
A) that energy is quantized.
B) that light intensity does not affect the number of electrons ejected from a metal.
C) that atomic spectra are due to the quantized transitions of electrons.
D) that blackbody radiation is continuous.
E) that atomic spectra are continuous.
A) that energy is quantized.
B) that light intensity does not affect the number of electrons ejected from a metal.
C) that atomic spectra are due to the quantized transitions of electrons.
D) that blackbody radiation is continuous.
E) that atomic spectra are continuous.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
18
The work function of sodium is = 2.9 10-19 J. What is the maximum wavelength that can cause ejection of photoelectrons from a sodium surface?
A) 151 nm
B) 222 nm
C) 45.1 nm
D) 451 nm
E) 685 nm
A) 151 nm
B) 222 nm
C) 45.1 nm
D) 451 nm
E) 685 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
19
Atomic spectra are due to the changes in the energy of __________
A) protons.
B) neutrons.
C) nuclei.
D) electrons.
E) electromagnetic radiation.
A) protons.
B) neutrons.
C) nuclei.
D) electrons.
E) electromagnetic radiation.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
20
The emission spectra of Na and Na+ are different because __________
A) Na+ has fewer electrons.
B) Na has fewer electrons.
C) Na+ has more protons.
D) Na has fewer neutrons.
E) Na has more protons.
A) Na+ has fewer electrons.
B) Na has fewer electrons.
C) Na+ has more protons.
D) Na has fewer neutrons.
E) Na has more protons.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
21
Indicate which of the following sources produces the highest energy photons.
A) a radio station with = 106.7 MHz
B) a dentist's X-ray source with = 100 pm
C) the laser in a CD player with = 650 nm
D) a microwave oven with = 6.0 1010 Hz
E) your cell phone with = 1.750 GHz
A) a radio station with = 106.7 MHz
B) a dentist's X-ray source with = 100 pm
C) the laser in a CD player with = 650 nm
D) a microwave oven with = 6.0 1010 Hz
E) your cell phone with = 1.750 GHz

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following photons has the lowest frequency?
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following photons has the highest energy?
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
24
The study of light emitted by the hydrogen atom by Johann Balmer, Johannes Rydberg, Niels Bohr and others revolutionized physics because it revealed that __________
A) energy is quantized.
B) light intensity does not affect the number of atoms excited.
C) the emitted light is produced by the electron making a transition between quantized energy levels.
D) blackbody radiation is continuous.
E) atomic spectra are continuous.
A) energy is quantized.
B) light intensity does not affect the number of atoms excited.
C) the emitted light is produced by the electron making a transition between quantized energy levels.
D) blackbody radiation is continuous.
E) atomic spectra are continuous.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
25
If each of the following metals is exposed to light with a wavelength of 240 nm, which will emit photoelectrons with the greatest kinetic energy?
A) iron ( = 7.2 10-19 J)
B) platinum ( = 9.1 10-19 J)
C) nickel ( = 8.3 10-19 J)
D) palladium ( = 8.2 10-19 J)
E) sodium ( = 4.4 10-19 J)
A) iron ( = 7.2 10-19 J)
B) platinum ( = 9.1 10-19 J)
C) nickel ( = 8.3 10-19 J)
D) palladium ( = 8.2 10-19 J)
E) sodium ( = 4.4 10-19 J)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following sources produces the lowest energy photons?
A) a doctor's X-ray source
B) the heat lamp in your bathroom
C) a microwave oven
D) gamma rays from a star
E) radio waves from your local station
A) a doctor's X-ray source
B) the heat lamp in your bathroom
C) a microwave oven
D) gamma rays from a star
E) radio waves from your local station

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following will lead to an increase in the kinetic energies of photoelectrons emitted when light is incident on a metal surface?
A) decrease in the light's wavelength
B) increase in the light's intensity
C) increase in the light's wavelength
D) decrease in the light's frequency
E) decrease in the light's intensity
A) decrease in the light's wavelength
B) increase in the light's intensity
C) increase in the light's wavelength
D) decrease in the light's frequency
E) decrease in the light's intensity

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
28
What is the energy (E, in J) of the photons emitted by an Ar+ laser with a wavelength of = 488 nm?
A) 2.46 1018 J
B) 9.69 10-23 J
C) 1.36 10-36 J
D) 4.07 10-19 J
E) 2.46 10-18 J
A) 2.46 1018 J
B) 9.69 10-23 J
C) 1.36 10-36 J
D) 4.07 10-19 J
E) 2.46 10-18 J

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
29
Based on the following graph, which metal will emit the highest energy photoelectron when a photon with a wavelength of 650 nm is incident on the metal? 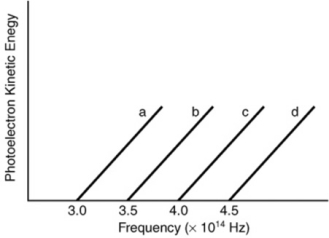
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following photons has the lowest energy?
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following photons has the highest frequency?
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm
A) a photon from a Nd:YAG laser with = 1064 nm
B) a photon from an Ar+ laser with = 514.5 nm
C) a photon from a Kr+ laser with = 647 nm
D) a photon from an ArF laser with = 193 nm
E) a photon from a He-Ne laser with = 633 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
32
What wavelength of light is required to cause the ejection of photoelectrons with kinetic energies of 5.0 10-20 J from a calcium surface ( = 4.60 10-19 J)?
A) 485 nm
B) 390 nm
C) 308 nm
D) 632 nm
E) 480 nm
A) 485 nm
B) 390 nm
C) 308 nm
D) 632 nm
E) 480 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following metals would be an appropriate choice for the construction of a photon detector for 550 nm light?
A) sodium ( = 4.4 10-19 J)
B) rubidium ( = 3.5 10-19J)
C) barium ( = 4.3 10-19 J)
D) gold ( = 8.2 10-19 J)
E) platinum ( = 9.1 10-19 J)
A) sodium ( = 4.4 10-19 J)
B) rubidium ( = 3.5 10-19J)
C) barium ( = 4.3 10-19 J)
D) gold ( = 8.2 10-19 J)
E) platinum ( = 9.1 10-19 J)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
34
What is the energy (E, in J) of a photon from a microwave oven with a frequency of = 6.0 1010 Hz?
A) 3.98 10-30 J
B) 3.98 10-39 J
C) 3.98 10-23 J
D) 3.98 10-17 J
E) 3.98 10-13 J
A) 3.98 10-30 J
B) 3.98 10-39 J
C) 3.98 10-23 J
D) 3.98 10-17 J
E) 3.98 10-13 J

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
35
Electromagnetic radiation with a frequency of 8.6 1014 Hz incident on an unknown metal surface causes ejection of photoelectrons with kinetic energies of 1.3 10-19 J. What is the unknown metal?
A) rubidium ( = 3.5 10-19 J)
B) gold ( = 8.2 10-19 J)
C) nickel ( = 8.3 10-19 J)
D) sodium ( = 4.4 10-19 J)
E) platinum ( = 9.1 10-19 J)
A) rubidium ( = 3.5 10-19 J)
B) gold ( = 8.2 10-19 J)
C) nickel ( = 8.3 10-19 J)
D) sodium ( = 4.4 10-19 J)
E) platinum ( = 9.1 10-19 J)

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following sources produces the highest energy photons?
A) a doctor's X-ray source
B) the heat lamp in your bathroom
C) a microwave oven
D) gamma rays from a star
E) radio waves from your local station
A) a doctor's X-ray source
B) the heat lamp in your bathroom
C) a microwave oven
D) gamma rays from a star
E) radio waves from your local station

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
37
What is the kinetic energy of the photoelectrons emitted from a sodium surface ( = 2.9 10-19 J) when it is irradiated by photons with a wavelength of 350 nm?
A) 2.9 10-19 J
B) 5.7 10-19 J
C) 0 J
D) 2.8 10-19 J
E) 3.6 10-19 J
A) 2.9 10-19 J
B) 5.7 10-19 J
C) 0 J
D) 2.8 10-19 J
E) 3.6 10-19 J

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
38
Which transition in a hydrogen atom requires the largest change in energy?
A) n1 = 2 to n2 = 3
B) n1 = 2 to n2 = 4
C) n1 = 1 to n2 = 3
D) n1 = 1 to n2 = 2
E) n1 = 9 to n2 = 10
A) n1 = 2 to n2 = 3
B) n1 = 2 to n2 = 4
C) n1 = 1 to n2 = 3
D) n1 = 1 to n2 = 2
E) n1 = 9 to n2 = 10

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
39
Indicate which of the following sources produces the lowest energy photons.
A) a radio station with = 106.7 MHz
B) a dentist's X-ray source with = 100 pm
C) the laser in a CD player with = 650 nm
D) a microwave oven with = 6.0 1010 Hz
E) your cell phone with = 1.750 GHz
A) a radio station with = 106.7 MHz
B) a dentist's X-ray source with = 100 pm
C) the laser in a CD player with = 650 nm
D) a microwave oven with = 6.0 1010 Hz
E) your cell phone with = 1.750 GHz

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the transitions in the hydrogen atom energy-level diagram shown here is not possible? 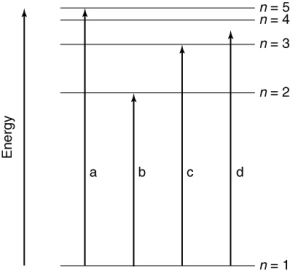
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is not an allowed value for the principal quantum number?
A) 1
B) 3
C) 10
D) 0
E) 2
A) 1
B) 3
C) 10
D) 0
E) 2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
42
The change in energy of a one-electron atom or ion for an electronic transition from the initial energy level ni to the final energy level nf is given by E = -(2.18 10-18 J) Z2 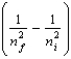 Which of the following species will have the longest wavelength emission line for the transition between the ni = 2 and nf = 1 levels?
Which of the following species will have the longest wavelength emission line for the transition between the ni = 2 and nf = 1 levels?
A) H
B) He+
C) Li2+
D) Be3+
E) B4+
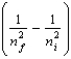 Which of the following species will have the longest wavelength emission line for the transition between the ni = 2 and nf = 1 levels?
Which of the following species will have the longest wavelength emission line for the transition between the ni = 2 and nf = 1 levels?A) H
B) He+
C) Li2+
D) Be3+
E) B4+

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
43
What  quantum numbers are possible when n = 3?
quantum numbers are possible when n = 3?
A) 1 and 2
B) 1, 2, and 3
C) 0, 1, 2, and 3
D) 0, 1, and 2
E) any integer
 quantum numbers are possible when n = 3?
quantum numbers are possible when n = 3?A) 1 and 2
B) 1, 2, and 3
C) 0, 1, 2, and 3
D) 0, 1, and 2
E) any integer

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the transitions in the hydrogen atom energy-level diagram shown here requires the longest wavelength photon? 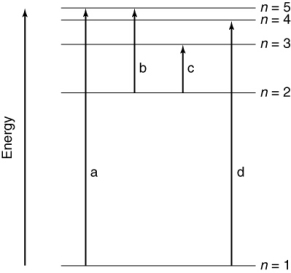
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the transitions in the following hydrogen atom energy-level diagram involves the shortest wavelength photon? 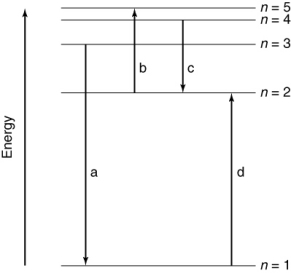
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
46
The energy of a one-electron atom is given by E = -(2.18 10-18 J) 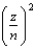 where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
A) Li2+
B) Be3+
C) He+
D) B4+
E) C5+
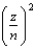 where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?
where Z is the atomic number of the element. Which of the following one-electron ions has the largest ionization energy?A) Li2+
B) Be3+
C) He+
D) B4+
E) C5+

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
47
A shell is defined by __________
A) the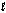 quantum number.
quantum number.
B) the ml quantum number.
C) the ms quantum number.
D) the n quantum number.
E) both the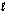 and ml quantum numbers.
and ml quantum numbers.
A) the
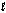 quantum number.
quantum number.B) the ml quantum number.
C) the ms quantum number.
D) the n quantum number.
E) both the
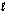 and ml quantum numbers.
and ml quantum numbers.
Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
48
In quantum mechanics, an atomic orbital __________
A) provides the position of an electron at any instant of time in the space around an atomic nucleus.
B) locates all the electrons in an atom.
C) is identical to the orbits Bohr used in his analysis of the hydrogen atom.
D) identifies the most probable position of an atomic nucleus.
E) provides the probability of finding an electron at any point in the space around an atomic nucleus.
A) provides the position of an electron at any instant of time in the space around an atomic nucleus.
B) locates all the electrons in an atom.
C) is identical to the orbits Bohr used in his analysis of the hydrogen atom.
D) identifies the most probable position of an atomic nucleus.
E) provides the probability of finding an electron at any point in the space around an atomic nucleus.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
49
What is the speed of an argon atom that has a de Broglie wavelength of 5.2 pm?
A) 0.52 m/s
B) 5.2 103 m/s
C) 1.9 103 m/s
D) 1.9 m/s
E) 25 m/s
A) 0.52 m/s
B) 5.2 103 m/s
C) 1.9 103 m/s
D) 1.9 m/s
E) 25 m/s

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
50
Which transition in a hydrogen atom will cause emission of the shortest wavelength photon?
A) n1 = 4 to n2 = 3
B) n1 = 4 to n2 = 2
C) n1 = 3 to n2 = 2
D) n1 = 3 to n2 = 1
E) n1 = 10 to n2 = 9
A) n1 = 4 to n2 = 3
B) n1 = 4 to n2 = 2
C) n1 = 3 to n2 = 2
D) n1 = 3 to n2 = 1
E) n1 = 10 to n2 = 9

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
51
De Broglie reasoned that for the electron in the hydrogen atom to behave as a stable circular wave, the circumference of the electron's orbit must be __________
A) equal to the wavelength of the electron.
B) an integer multiple of the electron's wavelength.
C) a half integer multiple of the electron's wavelength.
D) twice the wavelength of the electron.
E) twice the diameter of the orbit.
A) equal to the wavelength of the electron.
B) an integer multiple of the electron's wavelength.
C) a half integer multiple of the electron's wavelength.
D) twice the wavelength of the electron.
E) twice the diameter of the orbit.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
52
Recently, buckyballs (C60) became the largest object with a measured de Broglie wavelength. If the mass of a C60 molecule is 1.20 10-24 kg, what will be its de Broglie wavelength if it is moving at a speed of 220 m/sec?
A) 2.52 pm
B) 2.52 fm
C) 2.52 m
D) 1.20 pm
E) 5.22 m
A) 2.52 pm
B) 2.52 fm
C) 2.52 m
D) 1.20 pm
E) 5.22 m

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following electrons will have the smallest de Broglie wavelength?
A) an electron moving 220 m/sec
B) an electron moving 20 mi/hr
C) an electron moving 75 km/hr
D) an electron moving at 25% the speed of light
E) an electron at rest with no velocity
A) an electron moving 220 m/sec
B) an electron moving 20 mi/hr
C) an electron moving 75 km/hr
D) an electron moving at 25% the speed of light
E) an electron at rest with no velocity

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
54
How much energy is required to ionize a He+ ion in its ground state?
A) 2.18 10-18 J
B) 4.36 10-18 J
C) 8.72 10-18 J
D) 1.74 10-18 J
A) 2.18 10-18 J
B) 4.36 10-18 J
C) 8.72 10-18 J
D) 1.74 10-18 J

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
55
Subshells are __________
A) orbitals with the same principal and angular momentum quantum numbers.
B) orbitals with the same principal quantum numbers.
C) orbitals with the same angular momentum quantum numbers.
D) orbitals with the same angular momentum and magnetic quantum numbers.
E) orbitals with the same spin quantum number.
A) orbitals with the same principal and angular momentum quantum numbers.
B) orbitals with the same principal quantum numbers.
C) orbitals with the same angular momentum quantum numbers.
D) orbitals with the same angular momentum and magnetic quantum numbers.
E) orbitals with the same spin quantum number.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
56
According to de Broglie, if the circumference of the electron's orbit in the hydrogen atom is twice the electron's wavelength, the orbit will __________
A) decay until the electron falls into the nucleus.
B) diverge, allowing the electron to escape.
C) be stable.
D) cause the electron to emit a photon.
E) not be circular.
A) decay until the electron falls into the nucleus.
B) diverge, allowing the electron to escape.
C) be stable.
D) cause the electron to emit a photon.
E) not be circular.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
57
A shell consists of all __________
A) electrons with the same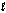 quantum number.
quantum number.
B) orbitals with the same quantum numbers.
C) orbitals with the same principal quantum number.
D) electrons with the same magnetic quantum number.
E) orbitals with the same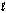 and ml quantum numbers.
and ml quantum numbers.
A) electrons with the same
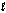 quantum number.
quantum number.B) orbitals with the same quantum numbers.
C) orbitals with the same principal quantum number.
D) electrons with the same magnetic quantum number.
E) orbitals with the same
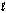 and ml quantum numbers.
and ml quantum numbers.
Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following objects, all moving at the same speed, will have the largest de Broglie wavelength?
A) a proton
B) an electron
C) a bowling ball
D) a neon atom
E) a neutron
A) a proton
B) an electron
C) a bowling ball
D) a neon atom
E) a neutron

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
59
The mathematical description of an electron as a wave was developed by __________
A) Bohr.
B) Heisenberg.
C) Einstein.
D) De Broglie.
E) Schrödinger.
A) Bohr.
B) Heisenberg.
C) Einstein.
D) De Broglie.
E) Schrödinger.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
60
Determine the wavelength of the line in the hydrogen atom spectrum corresponding to the n1 = 3 to n2 = 4 transition.
A) 1094 nm
B) 1875 nm
C) 656 nm
D) 335 nm
E) 109 nm
A) 1094 nm
B) 1875 nm
C) 656 nm
D) 335 nm
E) 109 nm

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
61
Which combination of quantum numbers is possible for an atom with five orbitals in one subshell?
A) n = 1,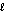 = 0
= 0
B)n = 2,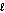 = 4
= 4
C) n = 3,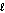 = 2
= 2
D) n = 4,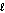 = 4
= 4
E) n = 5,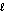 = 0
= 0
A) n = 1,
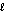 = 0
= 0B)n = 2,
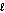 = 4
= 4C) n = 3,
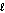 = 2
= 2D) n = 4,
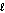 = 4
= 4E) n = 5,
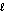 = 0
= 0
Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following represents an s orbital?
A)
B)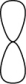
C)
D)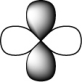
A)

B)
C)

D)


Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
63
How many orbitals exist for the quantum numbers n = 4, 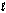 = 1?
= 1?
A) 1
B) 2
C) 3
D) 4
E) 7
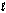 = 1?
= 1?A) 1
B) 2
C) 3
D) 4
E) 7

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
64
What are the principal and angular momentum quantum numbers for a 4f orbital?
A) n = 4,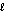 = 2
= 2
B) n = 4,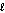 = 3
= 3
C) n = 4,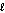 = 4
= 4
D) n = 3,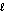 = 4
= 4
E) n = 4,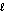 = 1
= 1
A) n = 4,
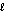 = 2
= 2B) n = 4,
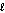 = 3
= 3C) n = 4,
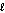 = 4
= 4D) n = 3,
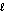 = 4
= 4E) n = 4,
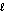 = 1
= 1
Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
65
The size of a typical atomic orbital is on the order of __________
A) femtometers.
B) picometers.
C) nanometers.
D) micrometers.
E) millimeters.
A) femtometers.
B) picometers.
C) nanometers.
D) micrometers.
E) millimeters.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is the ground-state electron configuration of the Mg2+ ion?
A) 1s22s22p63s2
B) [Ne]3s2
C) [Ne]3s23p2
D) [He]2s22p6
E) [He]2s22p8
A) 1s22s22p63s2
B) [Ne]3s2
C) [Ne]3s23p2
D) [He]2s22p6
E) [He]2s22p8

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
67
Which subshell only has five orbitals?
A)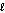 = 2
= 2
B)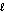 = 1
= 1
C)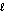 = 5
= 5
D)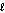 = 3
= 3
E)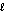 = 4
= 4
A)
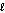 = 2
= 2B)
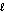 = 1
= 1C)
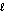 = 5
= 5D)
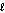 = 3
= 3E)
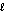 = 4
= 4
Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following orbitals is the lowest in energy (assuming they are all in the same shell)?
A)
B)
C)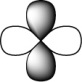
D)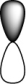
A)

B)

C)

D)
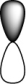

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
69
The atomic radius of germanium (Z = 32) is smaller than the atomic radius of potassium (Z = 19) because of __________
A) a change in the n quantum number.
B) an increase in the effective nuclear charge.
C) the fact that p and d orbitals have the same orbital penetration.
D) a decrease in the effective nuclear charge.
E) germanium having 32 rather than 19 electrons.
A) a change in the n quantum number.
B) an increase in the effective nuclear charge.
C) the fact that p and d orbitals have the same orbital penetration.
D) a decrease in the effective nuclear charge.
E) germanium having 32 rather than 19 electrons.

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following atoms has no unpaired electrons?
A) B
B) C
C) O
D) Mg
E) Na
A) B
B) C
C) O
D) Mg
E) Na

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
71
How many unpaired electrons does the nitride (N3-) ion have?
A) 0
B) 1
C) 2
D) 3
E) 6
A) 0
B) 1
C) 2
D) 3
E) 6

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
72
If the principal quantum number is seven (n = 7) and the angular momentum quantum number is three ( 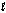 = 3), which of the following values is not an allowed value of the magnetic quantum number (ml)?
= 3), which of the following values is not an allowed value of the magnetic quantum number (ml)?
A) 0
B) 1
C) 2
D) -3
E) 4
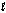 = 3), which of the following values is not an allowed value of the magnetic quantum number (ml)?
= 3), which of the following values is not an allowed value of the magnetic quantum number (ml)?A) 0
B) 1
C) 2
D) -3
E) 4

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following elements has the ground-state electron configuration 1s22s22p63s23p4?
A) Al
B) Cl
C) S
D) P
E) Ar
A) Al
B) Cl
C) S
D) P
E) Ar

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following atoms or ions has a single unpaired electron?
A) He
B) Sr
C) Na+
D) Al
E) Be
A) He
B) Sr
C) Na+
D) Al
E) Be

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is a possible set of quantum numbers for a 3d orbital?
A) n = 3,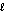 = 1, m
= 1, m 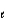
= -1
B) n = 3,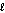 = 0, m
= 0, m 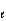
= 0
C) n = 3,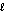 = 2, m
= 2, m 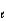
= 3
D) n = 3,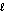 = 2, m
= 2, m 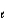
= 0
E) n = 3,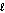 = 1, m
= 1, m 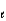
= 2
A) n = 3,
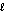 = 1, m
= 1, m 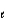
= -1
B) n = 3,
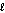 = 0, m
= 0, m 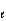
= 0
C) n = 3,
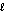 = 2, m
= 2, m 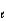
= 3
D) n = 3,
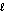 = 2, m
= 2, m 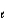
= 0
E) n = 3,
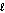 = 1, m
= 1, m 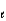
= 2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
76
A certain shell is known to have a total of 16 orbitals. Which shell is it?
A) n = 4
B) n = 16
C) n = 8
D) n = 2
A) n = 4
B) n = 16
C) n = 8
D) n = 2

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
77
What is the ground-state electron configuration of a Cl- ion?
A) 1s22s22p63s23p4
B) 1s22s22p63s23p5
C) 1s22s22p63s23p6
D) 1s22s22p63s23p2
E) 1s22s22p63s23p8
A) 1s22s22p63s23p4
B) 1s22s22p63s23p5
C) 1s22s22p63s23p6
D) 1s22s22p63s23p2
E) 1s22s22p63s23p8

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
78
Which arrangement is correct for increasing atomic radius?
A) C < N < F
B) Rb < K < Li
C) Sc < Ge < Br
D) Se < Cu < Ca
E) H < He
A) C < N < F
B) Rb < K < Li
C) Sc < Ge < Br
D) Se < Cu < Ca
E) H < He

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
79
What is the orbital designation for an electron with the quantum numbers n = 3, 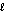 = 2?
= 2?
A) 3s
B) 3d
C) 2p
D) 2f
E) 3e
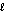 = 2?
= 2?A) 3s
B) 3d
C) 2p
D) 2f
E) 3e

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck
80
How many orbitals are possible for the n = 2 shell?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 104 flashcards in this deck.
Unlock Deck
k this deck


