Deck 1: Matter, Energy, and the Origins of the Universe
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 1: Matter, Energy, and the Origins of the Universe
1
The density of an object that weighs 10.0 g and occupies a volume of 2.5 cm3 is __________
A) 4.0 g/cm3.
B) 4.0 cm3/g.
C) 0.25 g/cm3.
D) 0.25 cm3/g.
E) dependent on the temperature.
A) 4.0 g/cm3.
B) 4.0 cm3/g.
C) 0.25 g/cm3.
D) 0.25 cm3/g.
E) dependent on the temperature.
4.0 g/cm3.
2
Distillation may be used to separate components in a mixture based on differences in __________
A) solubilities.
B) boiling points.
C) melting points.
D) masses.
E) color.
A) solubilities.
B) boiling points.
C) melting points.
D) masses.
E) color.
boiling points.
3
Which of the following is not a pure substance?
A) air
B) nitrogen gas
C) oxygen gas
D) argon gas
E) table salt (sodium chloride)
A) air
B) nitrogen gas
C) oxygen gas
D) argon gas
E) table salt (sodium chloride)
air
4
An example of a chemical property of formaldehyde (CH2O) is __________
A) it is flammable.
B) it has a density of 1.09 g/mL.
C) it is colorless.
D) it dissolves in water.
E) it is a gas at room temperature.
A) it is flammable.
B) it has a density of 1.09 g/mL.
C) it is colorless.
D) it dissolves in water.
E) it is a gas at room temperature.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
When you place a piece of dry ice (solid carbon dioxide) on a plate, you notice that no liquid forms, unlike ice that melts to form liquid water. This is because dry ice __________
A) as a liquid quickly evaporates.
B) undergoes deposition instead of melting.
C) sublimes instead of melting.
D) in the liquid form does not exist.
E) contains no water.
A) as a liquid quickly evaporates.
B) undergoes deposition instead of melting.
C) sublimes instead of melting.
D) in the liquid form does not exist.
E) contains no water.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
The law of constant composition states that __________
A) compounds such as NO2 and SO2 have identical chemical properties.
B) for a given compound, the elements forming the compound always react in the same proportions.
C) nitrogen and oxygen can combine to form NO or NO2.
D) elements do not always combine in the same proportion to give the same compound.
E) only one compound can be produced when two elements combine.
A) compounds such as NO2 and SO2 have identical chemical properties.
B) for a given compound, the elements forming the compound always react in the same proportions.
C) nitrogen and oxygen can combine to form NO or NO2.
D) elements do not always combine in the same proportion to give the same compound.
E) only one compound can be produced when two elements combine.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
Extensive properties are __________
A) physical properties and not chemical properties.
B) identical for all substances.
C) independent of the volume of substance present.
D) dependent on the amount of substance.
E) dependent on factors external to the substance itself.
A) physical properties and not chemical properties.
B) identical for all substances.
C) independent of the volume of substance present.
D) dependent on the amount of substance.
E) dependent on factors external to the substance itself.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
An element __________
A) can be separated into its components by physical methods.
B) may have different chemical properties depending on its source.
C) cannot be separated into simpler substances by chemical methods.
D) can also be a compound.
E) exists only as atoms and not as molecules.
A) can be separated into its components by physical methods.
B) may have different chemical properties depending on its source.
C) cannot be separated into simpler substances by chemical methods.
D) can also be a compound.
E) exists only as atoms and not as molecules.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
A structural formula __________
A) always shows correct bond distances and angles in a molecule.
B) is the same as a chemical formula.
C) shows how the molecule can be synthesized.
D) shows how atoms are connected in a chemical species.
E) is the same as a molecular formula.
A) always shows correct bond distances and angles in a molecule.
B) is the same as a chemical formula.
C) shows how the molecule can be synthesized.
D) shows how atoms are connected in a chemical species.
E) is the same as a molecular formula.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a chemical property?
A) Hydrogen is flammable.
B) Hydrogen is a gas.
C) Hydrogen gas has mass.
D) The boiling point of hydrogen is 20 K.
E) Hydrogen gas exerts pressure on the walls of a container.
A) Hydrogen is flammable.
B) Hydrogen is a gas.
C) Hydrogen gas has mass.
D) The boiling point of hydrogen is 20 K.
E) Hydrogen gas exerts pressure on the walls of a container.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a pure substance?
A) mineral water
B) blood
C) brass (an alloy of copper and zinc)
D) sucrose (table sugar)
E) beer
A) mineral water
B) blood
C) brass (an alloy of copper and zinc)
D) sucrose (table sugar)
E) beer

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a homogeneous mixture?
A) filtered water
B) chicken noodle soup
C) clouds
D) trail mix snack
E) fruit salad
A) filtered water
B) chicken noodle soup
C) clouds
D) trail mix snack
E) fruit salad

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
A pure substance __________
A) cannot be separated into simpler substances by physical means.
B) can have a composition that varies from sample to sample.
C) must be an element.
D) has different chemical and physical properties depending on its source.
E) must be a compound.
A) cannot be separated into simpler substances by physical means.
B) can have a composition that varies from sample to sample.
C) must be an element.
D) has different chemical and physical properties depending on its source.
E) must be a compound.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Which represents an intensive property?
A) Hydrogen gas has mass.
B) Hydrogen gas has a given density.
C) A balloon filled with hydrogen gas has a given volume.
D) Hydrogen releases a given amount of energy when it reacts with oxygen.
E) Hydrogen gas in a steel tank exerts a given pressure.
A) Hydrogen gas has mass.
B) Hydrogen gas has a given density.
C) A balloon filled with hydrogen gas has a given volume.
D) Hydrogen releases a given amount of energy when it reacts with oxygen.
E) Hydrogen gas in a steel tank exerts a given pressure.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is a heterogeneous mixture?
A) air
B) sugar dissolved in water
C) muddy river water
D) brass
E) table salt (sodium chloride)
A) air
B) sugar dissolved in water
C) muddy river water
D) brass
E) table salt (sodium chloride)

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following represents a physical property of water?
A) Water boils at 100°C.
B) An electrical current decomposes water into hydrogen gas and oxygen gas.
C) Water reacts with iron metal and oxygen to form rust.
D) Water reacts with carbon monoxide to form carbon dioxide and hydrogen gas.
E) Water is used in photosynthesis.
A) Water boils at 100°C.
B) An electrical current decomposes water into hydrogen gas and oxygen gas.
C) Water reacts with iron metal and oxygen to form rust.
D) Water reacts with carbon monoxide to form carbon dioxide and hydrogen gas.
E) Water is used in photosynthesis.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following mixtures can be separated by filtration?
A) sugar dissolved in coffee
B) sand and water
C) gasoline
D) alcohol dissolved in water
E) air
A) sugar dissolved in coffee
B) sand and water
C) gasoline
D) alcohol dissolved in water
E) air

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following is a mixture?
A) an aqueous solution of sugar
B) pure water
C) nitrogen gas
D) copper metal
E) table salt (sodium chloride)
A) an aqueous solution of sugar
B) pure water
C) nitrogen gas
D) copper metal
E) table salt (sodium chloride)

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following represents a chemical property of copper metal?
A) Copper metal conducts heat.
B) Copper metal reacts with nitric acid to produce copper(II) nitrate.
C) Copper metal melts at 1085°C.
D) Copper metal conducts electricity.
E) Copper metal has an orange color.
A) Copper metal conducts heat.
B) Copper metal reacts with nitric acid to produce copper(II) nitrate.
C) Copper metal melts at 1085°C.
D) Copper metal conducts electricity.
E) Copper metal has an orange color.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an element?
A) Cl2
B) H2O
C) NaCl
D) MgO
E) HCl
A) Cl2
B) H2O
C) NaCl
D) MgO
E) HCl

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a derived SI unit?
A) m
B) kg
C) cm3
D) m3
E) lb
A) m
B) kg
C) cm3
D) m3
E) lb

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
The concentration (in % by volume) of methyl tert-butyl ether (MTBE) was determined in four samples of the same gasoline. What is the average value, and which measurement was the most accurate, compared to the average? Sample % (v/v) MTBE
1 5.01
2 4.95
3 5.10
4 5.15
A) 5.05, sample 1
B) 5.05, sample 2
C) 5.05, sample 3
D) 5.05, sample 4
E) 5.0525, sample 3
1 5.01
2 4.95
3 5.10
4 5.15
A) 5.05, sample 1
B) 5.05, sample 2
C) 5.05, sample 3
D) 5.05, sample 4
E) 5.0525, sample 3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
As a summer intern at the National Institute of Standards and Technology, a student performed three measurements to determine the density of water at 25°C to four significant figures. She obtained the following results. The known density of water at 25°C to three significant figures is 0.958 g/mL. Trial Density (g/mL)
1 0)9345
2 0)9523
3 0)9107
The measurements were __________
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.
1 0)9345
2 0)9523
3 0)9107
The measurements were __________
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
An irregularly shaped metal object with a mass of 25.43 g was placed in a graduated cylinder with water. The before and after volumes are shown below. What is the density of the metal? 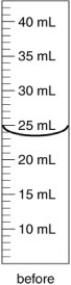

A) 2.8 g/cm3
B) 2.906 g/cm3
C) 0.782 g/cm3
D) 0.344 g/cm3
E) 2.734 g/cm3


A) 2.8 g/cm3
B) 2.906 g/cm3
C) 0.782 g/cm3
D) 0.344 g/cm3
E) 2.734 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following quantities has two significant figures?
A) 0.4
B) 101
C) 1.10 103
D) 0.0092
E) 0.520
A) 0.4
B) 101
C) 1.10 103
D) 0.0092
E) 0.520

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
The density of iron is 7.9 g/cm3. What is the volume of a 4.5 kg iron block?
A) 570 cm3
B) 0.570 cm3
C) 3.56 104 cm3
D) 35.6 cm3
E) 1.76 cm3
A) 570 cm3
B) 0.570 cm3
C) 3.56 104 cm3
D) 35.6 cm3
E) 1.76 cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
John Dalton postulated that all matter is composed of small particles called atoms. For this proposition to be considered a valid scientific theory, __________
A) it must be continually supported by experimental evidence and testing.
B) it must be impossible to prove wrong by experiment.
C) all possible experiments must never find an exception to it.
D) some, but only a few, experiments may find exceptions to it.
E) it must be voted on by the scientific community and accepted by all.
A) it must be continually supported by experimental evidence and testing.
B) it must be impossible to prove wrong by experiment.
C) all possible experiments must never find an exception to it.
D) some, but only a few, experiments may find exceptions to it.
E) it must be voted on by the scientific community and accepted by all.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the most massive?
A) 2.5 kg of oxygen gas
B) 0.25 kg of iron
C) 2.5 g of sodium chloride (table salt)
D) 250 g of helium gas
E) 250 mg of aluminum
A) 2.5 kg of oxygen gas
B) 0.25 kg of iron
C) 2.5 g of sodium chloride (table salt)
D) 250 g of helium gas
E) 250 mg of aluminum

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the SI base unit for mass?
A) g
B) kg
C) mg
D) lb
E) m
A) g
B) kg
C) mg
D) lb
E) m

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
Indicate which of the following common laboratory devices will deliver 25 mL of a solution with the greatest precision.
A) a 50 mL Erlenmeyer flask (without volume divisions)
B) a 50 mL beaker (with volume divisions every 10 mL)
C) a 50 mL graduated cylinder (with volume divisions every 2 mL)
D) a 25 mL Erlenmeyer flask (without volume divisions)
E) a 25 mL volumetric pipet (with a to deliver error of 0.01 mL at 25°C)
A) a 50 mL Erlenmeyer flask (without volume divisions)
B) a 50 mL beaker (with volume divisions every 10 mL)
C) a 50 mL graduated cylinder (with volume divisions every 2 mL)
D) a 25 mL Erlenmeyer flask (without volume divisions)
E) a 25 mL volumetric pipet (with a to deliver error of 0.01 mL at 25°C)

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
A buret (shown below) was used to add dilute hydrochloric acid (HCl) to a solution containing sodium hydroxide (NaOH). If the buret initially was read as 0.00 mL, how much HCl has been delivered according to the reading in the figure? 
A) 5.4 mL
B) 5.40 mL
C) 4.60 mL
D) 4.3 mL
E) 4.30 mL

A) 5.4 mL
B) 5.40 mL
C) 4.60 mL
D) 4.3 mL
E) 4.30 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
Electromagnetic radiation in the mid-infrared region of the spectrum has wavelengths around 10.6 m. Express this wavelength in meters using exponential notation (1 m = 10-6 m).
A) 1.06 10-6 m
B) 1.06 10-5 m
C) 1.06 m
D) 1.06 107 m
E) 1.06 105 m
A) 1.06 10-6 m
B) 1.06 10-5 m
C) 1.06 m
D) 1.06 107 m
E) 1.06 105 m

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
As a summer intern at the National Institute of Standards and Technology, a student performed three measurements to determine the density of water at 25°C to four significant figures. She obtained the following results. The known density of water at 25°C to three significant figures is 0.958 g/mL. Trial Density (g/mL)
1 0)9345
2 0)9346
3 0)9348
The measurements were __________
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.
1 0)9345
2 0)9346
3 0)9348
The measurements were __________
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
Given the following figure, which of the measurements listed is the best estimate of the length of the aluminum rod? 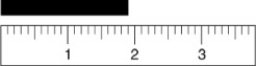
A) 1.8 cm
B) 1.81 cm
C) 1.810 cm
D) 1.9 cm
E) 2 cm

A) 1.8 cm
B) 1.81 cm
C) 1.810 cm
D) 1.9 cm
E) 2 cm

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
The following measurements of the mass of an aspirin tablet were made by different students in a lab. Which set is the most precise?
A) 1.513 g, 1.503 g, 1.522 g
B) 1.513 g, 1.511 g, 1.450 g
C) 1.513 g, 1.459 g, 1.533 g
D) 1.513 g, 1.517 g, 1.512 g
E) 1.513 mg, 1.510 mg, 1.523 mg
A) 1.513 g, 1.503 g, 1.522 g
B) 1.513 g, 1.511 g, 1.450 g
C) 1.513 g, 1.459 g, 1.533 g
D) 1.513 g, 1.517 g, 1.512 g
E) 1.513 mg, 1.510 mg, 1.523 mg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
The diameter of the sun is 1,390,000 km. In scientific notation this is
A) 1.39 10-6 km
B) 1.39 10-3 km
C) 1.39 106 km
D) 1.39 103 km
E) 1.39 108 m
A) 1.39 10-6 km
B) 1.39 10-3 km
C) 1.39 106 km
D) 1.39 103 km
E) 1.39 108 m

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
Which statement correctly describes the properties of a gas?
A) A gas does not occupy the entire volume of the container and is not highly compressible.
B) A gas occupies the entire volume of the container and is highly compressible.
C) A gas is highly ordered, and the molecules do not move about in the container.
D) A gas has a definite volume and shape.
E) A gas takes the shape of the container but is not highly compressible.
A) A gas does not occupy the entire volume of the container and is not highly compressible.
B) A gas occupies the entire volume of the container and is highly compressible.
C) A gas is highly ordered, and the molecules do not move about in the container.
D) A gas has a definite volume and shape.
E) A gas takes the shape of the container but is not highly compressible.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
Jupiter's mass is estimated to be 1.90 1027 kg, and it has a diameter of 142,984 km. Assuming that Jupiter is spherical, estimate its density (the volume of a sphere is 4r3/3).
A) 0.620 g/cm3
B) 1.61 g/cm3
C) 1.24 g/cm3
D) 0.810 g/cm3
E) 0.155 g/cm3
A) 0.620 g/cm3
B) 1.61 g/cm3
C) 1.24 g/cm3
D) 0.810 g/cm3
E) 0.155 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
A hypothesis is __________
A) supported by experimental evidence.
B) a scientific theory used to explain observations.
C) an explanation of observed processes that needs to be tested.
D) the entire process through which scientific phenomena are explained.
E) one side of a right triangle.
A) supported by experimental evidence.
B) a scientific theory used to explain observations.
C) an explanation of observed processes that needs to be tested.
D) the entire process through which scientific phenomena are explained.
E) one side of a right triangle.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is not a base SI unit?
A) cm
B) m
C) kg
D) sec
E) mol
A) cm
B) m
C) kg
D) sec
E) mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
Which one of the following statements is not correct?
A) Helium is an element.
B) Table salt (sodium chloride) is a compound.
C) Water is a pure substance.
D) Air is a solution.
E) Elements occur only in the form of atoms.
A) Helium is an element.
B) Table salt (sodium chloride) is a compound.
C) Water is a pure substance.
D) Air is a solution.
E) Elements occur only in the form of atoms.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
In 1 second, light can travel 2.998 108 m. How many inches does light travel in 1 femtosecond (1 fs = 10-15 s, 1 inch = 2.54 cm)?
A) 118 in
B) 11.8 in
C) 1.18 in
D) 1.18 10-5 in
E) 1.18 10-7 in
A) 118 in
B) 11.8 in
C) 1.18 in
D) 1.18 10-5 in
E) 1.18 10-7 in

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
Deposition is the process in which a __________ is converted into a __________.
A) liquid; solid
B) gas; liquid
C) gas; solid
D) liquid; gas
E) solid; liquid
A) liquid; solid
B) gas; liquid
C) gas; solid
D) liquid; gas
E) solid; liquid

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
On a summer day, the temperature in Phoenix, Arizona, was recorded as 110°F. What is this temperature in °C?
A) 43°C
B) 78°C
C) 166°C
D) 93°C
E) 29oC
A) 43°C
B) 78°C
C) 166°C
D) 93°C
E) 29oC

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
The temperature of the surface of the sun is 5800 K. What is this in °F?
A) 5495°F
B) 5527°F
C) 9981°F
D) 3103°F
E) 10,899°F
A) 5495°F
B) 5527°F
C) 9981°F
D) 3103°F
E) 10,899°F

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
Liquid nitrogen boils at 77 K. What is this temperature in °F?
A) -196°F
B) -321°F
C) -256°F
D) -77°F
E) -352oF
A) -196°F
B) -321°F
C) -256°F
D) -77°F
E) -352oF

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
The density of quartz is 165 lb/ft3. A clear crystal with a mass of 26.5 g is found to displace 10.0 cm3 of water. The crystal has a density __________
A) of 165 lb/ft3 and therefore is most likely quartz.
B) of 2.65 g/cm3 and therefore is not quartz.
C) of 170 lb/ft3 and might be quartz. Better measurements are needed for a definitive test.
D) of 1.7 102 lb/ft3. Better measurements are needed for a definitive test.
E) very different from that of quartz.
A) of 165 lb/ft3 and therefore is most likely quartz.
B) of 2.65 g/cm3 and therefore is not quartz.
C) of 170 lb/ft3 and might be quartz. Better measurements are needed for a definitive test.
D) of 1.7 102 lb/ft3. Better measurements are needed for a definitive test.
E) very different from that of quartz.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
An object weighing 25.00 g has a volume of 9.3 cm3. What is the density of the object?
A) 2.688 g/cm3
B) 2.68 g/cm3
C) 2.7 g/cm3
D) 2.6882 g/cm3
E) 2.6881720 g/cm3
A) 2.688 g/cm3
B) 2.68 g/cm3
C) 2.7 g/cm3
D) 2.6882 g/cm3
E) 2.6881720 g/cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
If you had equal masses of each of the following substances, which would occupy the greatest volume?
A) ice (d = 0.917 g/mL)
B) water (d = 0.997 g/mL)
C) beeswax (d = 0.960 g/mL)
D) cocoa butter (d = 0.910 g/mL)
E) aluminum (d = 2.70 g/mL)
A) ice (d = 0.917 g/mL)
B) water (d = 0.997 g/mL)
C) beeswax (d = 0.960 g/mL)
D) cocoa butter (d = 0.910 g/mL)
E) aluminum (d = 2.70 g/mL)

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following statements is not correct?
A) Dry ice subliming is a physical change.
B) Methanol burning is a chemical change.
C) Sugar dissolving in water is a physical change.
D) Bleaching your hair is a chemical change, even though it changes your physical appearance.
E) Liquid water turning into steam is a chemical change.
A) Dry ice subliming is a physical change.
B) Methanol burning is a chemical change.
C) Sugar dissolving in water is a physical change.
D) Bleaching your hair is a chemical change, even though it changes your physical appearance.
E) Liquid water turning into steam is a chemical change.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
At what temperature do the Celsius and Fahrenheit scales read the same?
A) 40°
B) - 40°
C) 11.4°
D) - 11.4°
E) There is no temperature at which the two scales read the same.
A) 40°
B) - 40°
C) 11.4°
D) - 11.4°
E) There is no temperature at which the two scales read the same.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
Which one of the following is not classified correctly?
A) Distilled water is a compound.
B) Gold is an element.
C) Air is a solution.
D) Table salt (sodium chloride) is a mixture.
E) Tomato-basil pasta sauce is a food.
A) Distilled water is a compound.
B) Gold is an element.
C) Air is a solution.
D) Table salt (sodium chloride) is a mixture.
E) Tomato-basil pasta sauce is a food.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
Cheetahs can run at speeds of up to 60 mi per hour. How many seconds does it take a cheetah to run 10 m at this speed? (1 mi = 1.609 km)
A) 0.37 s
B) 0.10 s
C) 56 s
D) 18 s
E) 0.43 s
A) 0.37 s
B) 0.10 s
C) 56 s
D) 18 s
E) 0.43 s

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
What might be the air temperature on a really hot day?
A) 25°C
B) 273 K
C) 298 K
D) 40°C
E) 373 K
A) 25°C
B) 273 K
C) 298 K
D) 40°C
E) 373 K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
Using __________, scientists can image individual atoms and molecules on a surface.
A) transmission microscopy
B) electron microscopy
C) scanning tunneling microscopy
D) magnetic resonance
E) X-ray spectroscopy
A) transmission microscopy
B) electron microscopy
C) scanning tunneling microscopy
D) magnetic resonance
E) X-ray spectroscopy

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
Spanish mahogany has a density of 53 lb/ft3. Would you be able to lift a piece of mahogany that measured 10 in 12 in 14 in?
A) No, it would weigh approximately 200 lb.
B) No, it would be too awkward.
C) Yes, it would weigh approximately 25 lb.
D) Yes, it would weigh approximately 50 lb.
E) Yes, it would weigh approximately 5 lb.
A) No, it would weigh approximately 200 lb.
B) No, it would be too awkward.
C) Yes, it would weigh approximately 25 lb.
D) Yes, it would weigh approximately 50 lb.
E) Yes, it would weigh approximately 5 lb.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
Which one of the following is not a chemical change?
A) dynamite exploding
B) iron rusting
C) wood burning
D) water turning to steam
E) eggs cooking
A) dynamite exploding
B) iron rusting
C) wood burning
D) water turning to steam
E) eggs cooking

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
Room temperature is often taken to be 25°C. What is this temperature in °F?
A) 46°F
B) 45°F
C) 14°F
D) 77°F
E) 72oF
A) 46°F
B) 45°F
C) 14°F
D) 77°F
E) 72oF

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
Air is an example of __________
A) an element.
B) a compound.
C) a pure substance.
D) a heterogeneous mixture.
E) a homogeneous mixture.
A) an element.
B) a compound.
C) a pure substance.
D) a heterogeneous mixture.
E) a homogeneous mixture.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
The summit of Mt. Humphreys, the highest point in Arizona, is 12,600 ft. How many meters is this? (1 m = 1.0936 yd)
A) 4593 m
B) 3841 m
C) 34,565 m
D) 41,338 m
E) 37,800 m
A) 4593 m
B) 3841 m
C) 34,565 m
D) 41,338 m
E) 37,800 m

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
Which one of the following is not a physical property?
A) flammability
B) electrical conductivity
C) color
D) density
E) boiling point
A) flammability
B) electrical conductivity
C) color
D) density
E) boiling point

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the number of atoms across the diameter of a human hair given that the diameter of an atom is 0.1 nm and the diameter of a human hair is 0.1 mm.
A) 10-12
B) 1012
C) 103
D) 106
E) 109
A) 10-12
B) 1012
C) 103
D) 106
E) 109

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
The symbol and name corresponding to the factor 10-9 is
A) f, femto
B) p, pico
C) n, nano
D) "", micro
E) m, milli
A) f, femto
B) p, pico
C) n, nano
D) "", micro
E) m, milli

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
In the movie The Italian Job, thieves steal gold bullion. One plan was to carry the ingots of gold off in suitcases. If each suitcase were 20 inches 14 inches 10 inches, approximately how much would each suitcase weigh when filled with gold? The volume of the suitcase is 4.4 104 mL, the molar mass of gold is 197 g/mol, and the density of gold is 19.3 g/mL.
A) 2,300 g
B) 850 kg
C) 4,300 g
D) 167 mg
E) 550 kg
A) 2,300 g
B) 850 kg
C) 4,300 g
D) 167 mg
E) 550 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
What would you report for the total mass of three samples weighing 106.2 g, 33.15 g, and 0.028 g?
A) 139 g
B) 139.3 g
C) 139.4 g
D) 139.38 g
E) 139.378 g
A) 139 g
B) 139.3 g
C) 139.4 g
D) 139.38 g
E) 139.378 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
If the following arithmetic operations were carried out, how many significant figures should the answer be reported to? 5.70 16.90 / 7.2356
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
How many carbon atoms does it take to produce a layer one atom deep that is the size of the period at the end of the next sentence? Assume the area covered by the period is 0.2 mm2 and that one carbon atom has a diameter of 160 pm and covers an area of 0.02 nm2.
A) 1.0 1012
B) 1.0 1011
C) 1.0 107
D) 1.0 1013
E) 2.0 106
A) 1.0 1012
B) 1.0 1011
C) 1.0 107
D) 1.0 1013
E) 2.0 106

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
If the following arithmetic operations are carried out, how many significant figures should be reported in the answer? 132.0 + 0.56 + 0.01 + 3.33
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
Which one of the following is not equal to exactly one cubic meter (1 m3)?
A) 106 cm3
B) 103 L
C) 109 mm3
D) 106 mL
E) 100 cm3
A) 106 cm3
B) 103 L
C) 109 mm3
D) 106 mL
E) 100 cm3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
The bubbles that form in water after it has been boiling for some time are __________
A) empty space.
B) H2(g) and O2(g) gases.
C) the vapor phase of water, H2O(g).
D) filled with air.
E) superhot water, H2O(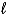 ).
).
A) empty space.
B) H2(g) and O2(g) gases.
C) the vapor phase of water, H2O(g).
D) filled with air.
E) superhot water, H2O(
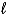 ).
).
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
This problem is from a New York Times article. Researchers tested a group of 28 doctors. The doctors were told that a five-year-old child suffering a potentially fatal allergic reaction to peanuts needed an emergency injection of 0.12 mg of epinephrine. The bottle of epinephrine is labeled 1 mg in 1 mL of solution. What volume of this solution would you inject if you were the doctor?
A) 0.12 mL
B) 120 mL
C) 1.2 10-4 mL
D) 12 mL
E) 1.2 mL
A) 0.12 mL
B) 120 mL
C) 1.2 10-4 mL
D) 12 mL
E) 1.2 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
Which one of the following is not a correct statement?
A) Vodka is a solution.
B) Water (H2O) is a compound.
C) Sodium chloride (table salt) is a compound.
D) Silver is an element.
E) Sugar dissolved in water is a heterogeneous mixture.
A) Vodka is a solution.
B) Water (H2O) is a compound.
C) Sodium chloride (table salt) is a compound.
D) Silver is an element.
E) Sugar dissolved in water is a heterogeneous mixture.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
Determine the maximum number of atoms that could comprise a baseball given that the volume of a baseball is 200 cm3 and the volume of an atom is 0.004 nm3.
A) 5 105
B) 5 1025
C) 5 1017
D) 6.0 1023
E) 6.0 1037
A) 5 105
B) 5 1025
C) 5 1017
D) 6.0 1023
E) 6.0 1037

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
The symbol and name corresponding to the factor 10-6 is
A) f, femto
B) p, pico
C) n, nano
D) "", micro
E) m, milli
A) f, femto
B) p, pico
C) n, nano
D) "", micro
E) m, milli

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following statements is not correct?
A) Sodium and chlorine are elements.
B) Sodium chloride (table salt) is a compound.
C) Sodium chloride is a pure substance.
D) Sodium chloride is a heterogeneous mixture.
E) Sodium chloride added to water forms a solution.
A) Sodium and chlorine are elements.
B) Sodium chloride (table salt) is a compound.
C) Sodium chloride is a pure substance.
D) Sodium chloride is a heterogeneous mixture.
E) Sodium chloride added to water forms a solution.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
If an atom is 0.1 nm in diameter, how many atoms must be lined up to make a row 1 cm long?
A) 104
B) 106
C) 108
D) 1010
E) 1012
A) 104
B) 106
C) 108
D) 1010
E) 1012

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
How thick is a piece of aluminum foil that measures 5 cm on each side and has a mass of 675 mg? The density of aluminum is 2.70 g/cm3.
A) 1.0 mm
B) 0.1 mm
C) 0.01 mm
D) 10 m
E) 1.0 m
A) 1.0 mm
B) 0.1 mm
C) 0.01 mm
D) 10 m
E) 1.0 m

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


