Deck 15: Principles of Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 15: Principles of Chemical Equilibrium
1
One method to aid in working equilibrium problems is the ICE table.
True
2
The value of the equilibrium constant for a given reaction depends on the initial concentrations of reactants.
False
3
Given the following: I) N2O(g) + 1/2 O2(g) ⇌ 2NO(g) Kc = 1.7 × 10-13
II) N2(g) + O2(g) ⇌ 2NO(g) Kc = 4.1 × 10-31
Find the value of the equilibrium constant for the following equilibrium reaction:
N2(g) + 1/2 O2(g) ⇌ N2O(g)
A) 7.0 × 10-44
B) 4.2 × 1017
C) 2.4 × 10-18
D) 1.6 × 10-9
E) 2.6 × 10-22
II) N2(g) + O2(g) ⇌ 2NO(g) Kc = 4.1 × 10-31
Find the value of the equilibrium constant for the following equilibrium reaction:
N2(g) + 1/2 O2(g) ⇌ N2O(g)
A) 7.0 × 10-44
B) 4.2 × 1017
C) 2.4 × 10-18
D) 1.6 × 10-9
E) 2.6 × 10-22
2.4 × 10-18
4
Which of the following substances present in the chemical reaction would be excluded from the equilibrium constant expression?
A) Na+ (aq)
B) H2O(g)
C) Cl-
D) H2O(l) (reactant and solvent)
E) CO(g)
A) Na+ (aq)
B) H2O(g)
C) Cl-
D) H2O(l) (reactant and solvent)
E) CO(g)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
What is the value for Kc for the reaction below if the equilibrium concentrations are [N2] = 0.025, [H2] = 0.0013 and [NH3] = 0.028 for the following reaction? N2(g) + 3 H2(g) ⇌ 2 NH3(g)
A) 8.6 × 102
B) 1.4 × 107
C) 1.2 × 10-3
D) 7.1 × 10-8
E) 7.4 × 105
A) 8.6 × 102
B) 1.4 × 107
C) 1.2 × 10-3
D) 7.1 × 10-8
E) 7.4 × 105

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Choose the INCORRECT statement.
A) A certain amount of energy, called the activation energy, must be available if a reaction is to take place.
B) A reversible chemical reaction is one in which equilibrium is never established due to the constant decomposition of the products.
C) When the rate of the reverse reaction equals the rate of the forward reaction, equilibrium has been established.
D) Changes in temperature will change the value of an equilibrium constant.
E) Chemical equilibrium is a dynamic equilibrium.
A) A certain amount of energy, called the activation energy, must be available if a reaction is to take place.
B) A reversible chemical reaction is one in which equilibrium is never established due to the constant decomposition of the products.
C) When the rate of the reverse reaction equals the rate of the forward reaction, equilibrium has been established.
D) Changes in temperature will change the value of an equilibrium constant.
E) Chemical equilibrium is a dynamic equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
In a system in equilibrium, two opposing reactions occur at equal rates.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
For the reaction: H2(g) + I2(g) ⇌ 2 HI(g), Kc = 92.0 When equilibrium concentrations of HI and I2 are [HI] = 0.115 M and [I2] = 0.250 M, the equilibrium concentration of [H2] is:
A) 5.00 × 10-3 M
B) 5.75 × 10-4 M
C) 1.74 × 103 M
D) 0.135 M
E) 9.56 M
A) 5.00 × 10-3 M
B) 5.75 × 10-4 M
C) 1.74 × 103 M
D) 0.135 M
E) 9.56 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
For the following chemical equilibrium, Kp = 4.6 × 10-14 at 25°C, find the value of Kc for this reaction at 25°C. 2 Cl2(g) + 2 H2O(g) ⇌ 4 HCl(g) + O2(g)
A) Kc = 1.9 × 10-15
B) Kc = 2.2 × 10-14
C) Kc = 1.1 × 10-12
D) Kc = 9.4 × 10-14
E) Kc = 4.6 × 10-14
A) Kc = 1.9 × 10-15
B) Kc = 2.2 × 10-14
C) Kc = 1.1 × 10-12
D) Kc = 9.4 × 10-14
E) Kc = 4.6 × 10-14

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following equilibrium constants-reaction type is INCORRECT?
A) 1.4 × 1083 goes to completion.
B) 1.6 × 10-23 does not occur.
C) 1.8 × 10-5 equilibrium reaction more reactants than products at equilibrium.
D) 1.0 equilibrium reaction-equal amounts of products and reactants.
E) 3.2 × 103 equilibrium reaction-more reactants than products at equilibrium.
A) 1.4 × 1083 goes to completion.
B) 1.6 × 10-23 does not occur.
C) 1.8 × 10-5 equilibrium reaction more reactants than products at equilibrium.
D) 1.0 equilibrium reaction-equal amounts of products and reactants.
E) 3.2 × 103 equilibrium reaction-more reactants than products at equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
What is the value for Kc if [CO] = 0.025, [H2] = 0.013 and [CH3OH] = 0.0028 for the following reaction? CH3OH(g) ⇌ CO(g) + 2 H2(g)
A) 1.5 × 10-3
B) 0.12
C) 6.6 × 102
D) 8.6
E) 9.1 × 10-7
A) 1.5 × 10-3
B) 0.12
C) 6.6 × 102
D) 8.6
E) 9.1 × 10-7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Changes in temperature cause change in the equilibrium position.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Reaction quotient Q will always be equal to the reaction's equilibrium constant K.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
For which of the following reactions does Kp = Kc?
A) 3Fe(s) + 4H2O(g) ⇌ Fe3O4(s) + 4H2(g)
B) C(s) + H2O(g) ⇌ CO(g) + H2(g)
C) 2SO2(g) + O2(g) ⇌ 2SO3(g)
D) H2(g) + I2(s) ⇌ 2HI(g)
A) 3Fe(s) + 4H2O(g) ⇌ Fe3O4(s) + 4H2(g)
B) C(s) + H2O(g) ⇌ CO(g) + H2(g)
C) 2SO2(g) + O2(g) ⇌ 2SO3(g)
D) H2(g) + I2(s) ⇌ 2HI(g)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
The concentration of a pure solid is left out of a equilibrium constant expression but a pure liquid is included.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
Equilibrium reactions are noted by a single straight arrow for a yield sign.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
In an equilibrium process, the concentrations of products and of reactants are equal.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Both products and reactants will be present in an equilibrium reaction unless K is very small or very large.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Large value for equilibrium constant K means the reaction will be less complete at the equilibrium point.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Given that the equilibrium concentrations of [N2] = 0.035 M, [C2H2] = 0.057 M, and [HCN] = 6.8 × 10-4 M, find the value of the equilibrium constant expression for the reaction:
N2(g) + C2H2(g) ⇌ 2 HCN
A) 3.4 × 10-1
B) 2.9
C) 4300
D) 2.3 × 10-4
E) 6.8 × 10-1
N2(g) + C2H2(g) ⇌ 2 HCN
A) 3.4 × 10-1
B) 2.9
C) 4300
D) 2.3 × 10-4
E) 6.8 × 10-1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) what is the effect on equilibrium of increasing temperature of an exothermic reaction?
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the reaction: CH4(g) + 4 Cl2(g) ⇌ CCl4(l) + 4 HCl(g) ΔH° -398 kJ/mol
The equilibrium is displaced to the right if:
A) the temperature is raised
B) the pressure is lowered
C) some carbon tetrachloride is removed
D) some hydrogen chloride is added
E) some chlorine gas is removed
The equilibrium is displaced to the right if:
A) the temperature is raised
B) the pressure is lowered
C) some carbon tetrachloride is removed
D) some hydrogen chloride is added
E) some chlorine gas is removed

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
For the reaction CO(g) + 3 H2(g) ⇌ H2O(g) + CH4(g), Kc = 190 at 1000 K. If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.036 M, [H2] = 0.045, [H2O] = 0.020, and [CH4] = 0.031, in which direction will a reaction occur and why?
A) toward products because Q = 0.38
B) toward reactants because Q = 0.24
C) toward products because Q = 4.1
D) toward reactants because Q = 61
E) it is at equilibrium because Q = 1
A) toward products because Q = 0.38
B) toward reactants because Q = 0.24
C) toward products because Q = 4.1
D) toward reactants because Q = 61
E) it is at equilibrium because Q = 1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
For the reaction 2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g), Kc = 2.8 × 102 at 1000 K. If a vessel is filled with these gases such that the initial concentrations are [SO2] = 0.025, [O2] = 0.035, and [SO3] = 0.046, in which direction will a reaction occur and why?
A) toward products because Q = 53
B) toward reactants because Q = 0.019
C) toward products because Q = 96
D) toward reactants because Q = 2.8 × 103
E) it is at equilibrium because Q = 1
A) toward products because Q = 53
B) toward reactants because Q = 0.019
C) toward products because Q = 96
D) toward reactants because Q = 2.8 × 103
E) it is at equilibrium because Q = 1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
For the reaction: CH4(g) + 2 H2O(g) ⇌ CO2(g) + 4 H2(g) ΔH° = +190 kJ when catalyst is added:
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change, catalyst changes reaction rate only
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change, catalyst changes reaction rate only

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following keep the equilibrium position unchanged?
A) temperature decrease
B) concentration change
C) temperature
D) pressure change
E) homogeneous catalyst
A) temperature decrease
B) concentration change
C) temperature
D) pressure change
E) homogeneous catalyst

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Choose the correct statement about a container in which the chemical equilibrium is established: 2 SO2(g) + O2(g) ⇌ 2 SO3(g) + heat
A) A decrease in amount of O2 will decrease the amount of SO2 present.
B) A decrease in the volume will decrease the amount of SO2 present.
C) A decrease in temperature will increase the amount of SO2 present.
D) A decrease in the amount of SO3 present will increase the amount of SO2 present.
E) An increase in amount of O2 will increase the amount of SO2 present.
A) A decrease in amount of O2 will decrease the amount of SO2 present.
B) A decrease in the volume will decrease the amount of SO2 present.
C) A decrease in temperature will increase the amount of SO2 present.
D) A decrease in the amount of SO3 present will increase the amount of SO2 present.
E) An increase in amount of O2 will increase the amount of SO2 present.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
For the reaction: CH4(g) + 2 H2O(g) ⇌ CO2(g) + 4 H2(g) ΔH° = +190 kJ when CH4 is added:
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change in equilibrium position
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change in equilibrium position

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
For the reaction: CH4(g) + 2 H2O(g) ⇌ CO2(g) + 4 H2(g) ΔH° = +190 kJ raise the temperature to 1200 K:
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change in equilibrium position
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change in equilibrium position

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following reaction. C(s) + H2O(g) ⇌ CO(g) + H2(g)
At equilibrium at a certain temperature, [H2O(g)] = 0.12 M, and [CO(g)] = [H2(g)] = 1.2 M. If suddenly these concentrations are increased by 0.50 M, which of the following is true?
A) more products are formed
B) Kc = 4.66
C) more H2O(g) will be formed
D) Since Kc does not change, nothing happens.
At equilibrium at a certain temperature, [H2O(g)] = 0.12 M, and [CO(g)] = [H2(g)] = 1.2 M. If suddenly these concentrations are increased by 0.50 M, which of the following is true?
A) more products are formed
B) Kc = 4.66
C) more H2O(g) will be formed
D) Since Kc does not change, nothing happens.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Which factor influences the value of the equilibrium constant for a reversible reaction?
A) addition of a catalyst
B) raising the temperature
C) removing product
D) removing reactant
E) increase in mixing rate
A) addition of a catalyst
B) raising the temperature
C) removing product
D) removing reactant
E) increase in mixing rate

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Choose the correct statement about the equilibrium: N2(g) + 3 H2(g) ⇌ 2 NH3(g), Kp = 1 × 10-4 atm-2
A) The rate constant for the forward reaction is greater than that of the reverse reaction.
B) Since the reaction has a high activation energy, a catalyst is not needed.
C) The equilibrium constant is given by Kp = [N2][H2]3/[NH3]2.
D) Conducting the reaction under high pressures will increase the yield of ammonia.
E) The Kp is independent of temperature.
A) The rate constant for the forward reaction is greater than that of the reverse reaction.
B) Since the reaction has a high activation energy, a catalyst is not needed.
C) The equilibrium constant is given by Kp = [N2][H2]3/[NH3]2.
D) Conducting the reaction under high pressures will increase the yield of ammonia.
E) The Kp is independent of temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
For the reaction CO(g) + 3 H2(g) ⇌ H2O(g) + CH4(g) , Kc = 190 at 1000 K. If a vessel is filled with these gases such that the initial concentrations are [CO] = 0.025, [H2] = 0.045, [H2O] = 0.025, and [CH4] = 0.046M, in which direction will a reaction occur and why?
A) toward products because Q = 0.17
B) toward reactants because Q = 0.0029
C) toward products because Q = 0.35
D) toward reactants because Q = 504
E) it is at equilibrium because Q = 1
A) toward products because Q = 0.17
B) toward reactants because Q = 0.0029
C) toward products because Q = 0.35
D) toward reactants because Q = 504
E) it is at equilibrium because Q = 1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) what is the effect of removing H2?
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
For the reaction PCl5 (g) ⇌ PCl3 (g) + Cl2 (g) Kc = 0.0454 at 261°C. If a vessel is filled with these gases such that the initial concentrations are [PCl5] = 0.2 M, [PCl3] = 0.20 M, and [Cl2] = 2.25 M, in which direction will a reaction occur and why?
A) toward products because Q = 0.56
B) toward reactants because Q = 1.8
C) toward products because Q = 2.8
D) toward reactants because Q = 0.0454
E) it is at equilibrium because Q = 1
A) toward products because Q = 0.56
B) toward reactants because Q = 1.8
C) toward products because Q = 2.8
D) toward reactants because Q = 0.0454
E) it is at equilibrium because Q = 1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
For the reaction: CH4(g) + 2H2O(g) ⇌ CO2(g) + 4H2(g) ΔH° = +190 kJ add H2(g):
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change in equilibrium position
A) the reaction reacts to the right
B) the reaction reacts to the left
C) the ΔH° increases
D) the temperature increases
E) there is no change in equilibrium position

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
In a reaction at equilibrium involving only gases, a change in pressure of the reaction mixture shifts the position of equilibrium only when:
A) heat is absorbed by the reaction proceeding to the right
B) the gases are impure
C) the collision rate increases
D) the reaction is exothermic as written
E) the moles of gas are not equal on the two sides of the equation.
A) heat is absorbed by the reaction proceeding to the right
B) the gases are impure
C) the collision rate increases
D) the reaction is exothermic as written
E) the moles of gas are not equal on the two sides of the equation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the exothermic reaction: 4 HCl(aq) + MnO2(s) ⇌ Cl2(g) + 2 H2O(l) + MnCl2(aq)
The equilibrium is displaced to the left if:
A) catalyst is added
B) pressure is lowered
C) temperature is lowered
D) H2O(l) is added
E) MnO2 (s) is added
The equilibrium is displaced to the left if:
A) catalyst is added
B) pressure is lowered
C) temperature is lowered
D) H2O(l) is added
E) MnO2 (s) is added

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
According to Le Chatelier's Principle:
A) an increase in pressure always causes a change in the position of equilibrium for any reaction
B) the greatest yield of ammonia in the exothermic reaction N2 + 3H2 ⇌ 2NH3 is attained at a high temperature
C) the equilibrium constant is increased for the reaction A + B ⇌ C if the concentration of A is increased
D) an increase of temperature causes a decrease in the value of the equilibrium constant for an exothermic reaction
E) when an equilibrium system is stressed, the system reacts to offset the stress
A) an increase in pressure always causes a change in the position of equilibrium for any reaction
B) the greatest yield of ammonia in the exothermic reaction N2 + 3H2 ⇌ 2NH3 is attained at a high temperature
C) the equilibrium constant is increased for the reaction A + B ⇌ C if the concentration of A is increased
D) an increase of temperature causes a decrease in the value of the equilibrium constant for an exothermic reaction
E) when an equilibrium system is stressed, the system reacts to offset the stress

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the reaction: 2SO2(g) + O2(g) ⇌ 2SO3(g) ΔH° = -196.6 kJ/mol
The equilibrium is displaced to the left if:
A) some sulfur trioxide is removed
B) the temperature is raised
C) some sulfur dioxide is added
D) the pressure is raised
E) the temperature is lowered
The equilibrium is displaced to the left if:
A) some sulfur trioxide is removed
B) the temperature is raised
C) some sulfur dioxide is added
D) the pressure is raised
E) the temperature is lowered

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
2.5 moles H2O and 100 g of C are placed in a 50-L container. At equilibrium for the reaction C(s) + H2O(g) ⇌ CO(g) + H2(g), [H2] = 0.040 M. Which of the following is true?
A) [CO] = 0.020 M
B) [H2O] = 0.010 M
C) no carbon is left
D) [H2O] = 0.020 M
E) [C(s)] = 0.04 M
A) [CO] = 0.020 M
B) [H2O] = 0.010 M
C) no carbon is left
D) [H2O] = 0.020 M
E) [C(s)] = 0.04 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
What is the equilibrium constant expression for: 4 NH3(g) + 5 O2(g) ⇌ 4 NO(g) + 6 H2O(g)
A)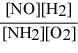
B)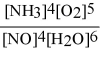
C)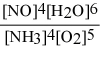
D)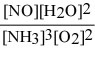
E)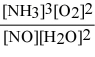
A)
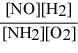
B)
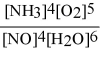
C)
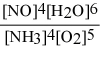
D)
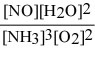
E)
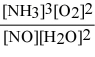

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
For the decomposition of SO3(g), Kc = [SO2]2[O2]/[SO3]2, at equilibrium, there are 0.090 mol SO2, 0.110 mol O2, 0.100 mol SO3 in a 25.0-L container. What is the value of Kc?
A) 3.6 × 10-3
B) 0.040
C) 2.23
D) 0.089
E) 7.89
A) 3.6 × 10-3
B) 0.040
C) 2.23
D) 0.089
E) 7.89

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
For the following reaction O2(g) ⇌ 2O(g)
What conditions favor production of oxygen atoms?
A) high temperature and low pressure
B) high temperature and high pressure
C) low temperature and low pressure
D) low temperature and high pressure
What conditions favor production of oxygen atoms?
A) high temperature and low pressure
B) high temperature and high pressure
C) low temperature and low pressure
D) low temperature and high pressure

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following equilibrium reaction: PCl3(g) + Cl2(g) ⇌ PCl5(g) Kc = 96.2 The equilibrium constant for the decomposition of PCl5(g) to PCl3(g) and Cl2(g) is ________.
A) -Kc
B)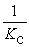
C)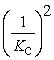
D)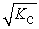
E)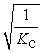
A) -Kc
B)
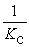
C)
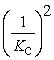
D)
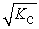
E)
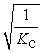

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
In a reaction at equilibrium involving only gases, a pressure change will shift the reaction only when:
A) heat is absorbed by the reaction proceeding to the right
B) the number of molecules on one side is greater than the number on the other side of the balanced equation
C) the number of molecules increases during the chemical reaction
D) the gases are impure
E) the collision rate increases
A) heat is absorbed by the reaction proceeding to the right
B) the number of molecules on one side is greater than the number on the other side of the balanced equation
C) the number of molecules increases during the chemical reaction
D) the gases are impure
E) the collision rate increases

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
0.75 mol of N2 and 1.20 mol of H2 are placed in a 3.0 liter container. When the reaction N2(g) + 3H2(g) ⇌ 2 NH3(g) reaches equilibrium, [H2] = 0.100 M. Which of the following is true?
A) [NH3] = 0.150 M
B) [NH3] = 0.200
C) [N2] = 0.650 M
D) [N2] = 0.250
E) [NH3] = [H2] = 0.05 M
A) [NH3] = 0.150 M
B) [NH3] = 0.200
C) [N2] = 0.650 M
D) [N2] = 0.250
E) [NH3] = [H2] = 0.05 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following chemical reaction at equilibrium: 2 Cl2(g) + 2 H2O(g) ⇌ 4 HCl(g) + O2(g)
This equilibrium can be shifted to the right by:
A) removing H2O(g) from the mixture.
B) adding more O2(g) to the mixture.
C) adding Ne(g) to the mixture.
D) decreasing the volume of the mixture.
E) increasing the volume of the mixture.
This equilibrium can be shifted to the right by:
A) removing H2O(g) from the mixture.
B) adding more O2(g) to the mixture.
C) adding Ne(g) to the mixture.
D) decreasing the volume of the mixture.
E) increasing the volume of the mixture.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
For 2 NO2(g) ⇌ N2O4(g), Kc = [N2O4]/[NO2]2. At equilibrium there are 0.0270 mol N2O4 and 0.450 mol NO2 in a 50.0-L container. What is Kc?
A) 0.00267
B) 6.81
C) 6.67
D) 0.133
E) 2.45
A) 0.00267
B) 6.81
C) 6.67
D) 0.133
E) 2.45

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction: CH4(g) + 2H2O(g) ⇌ CO2(g) + 4H2(g) ΔH° = +190 kJ add N2(g) at constant volume and:
A) the reaction reacts to the right.
B) the reaction reacts to the left.
C) the ΔH° increases.
D) the temperature increases.
E) there is no change.
A) the reaction reacts to the right.
B) the reaction reacts to the left.
C) the ΔH° increases.
D) the temperature increases.
E) there is no change.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following gas phase reaction at 25°C. N2(g) + C2H2(g) ⇌ 2 HCN(g)
1)600 mol N2(g) and 1.750 mol C2H2(g) are placed in a 1.000 L vessel and the mixture is allowed to react. At equilibrium, there are 1.587 mol N2(g) in the mixture. What is Kc for this reaction at 25 °C?
A) 9.8 × 10-4
B) 9.4 × 10-3
C) 2.5 × 10-4
D) 4.7 × 10-3
E) 6.7 × 10-5
1)600 mol N2(g) and 1.750 mol C2H2(g) are placed in a 1.000 L vessel and the mixture is allowed to react. At equilibrium, there are 1.587 mol N2(g) in the mixture. What is Kc for this reaction at 25 °C?
A) 9.8 × 10-4
B) 9.4 × 10-3
C) 2.5 × 10-4
D) 4.7 × 10-3
E) 6.7 × 10-5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
For CO2(g) + H2(g) ⇌ CO(g) + H2O(g), Kc = [CO][H2]/[CO2][H2], if there are 1.43 mols each of CO and H2, 0.572 mol H2 and 4.572 mols CO2, in a 4.0 L container at equilibrium, what is Kc?
A) 0.547
B) 0.782
C) 1.28
D) 0.137
E) 2.34
A) 0.547
B) 0.782
C) 1.28
D) 0.137
E) 2.34

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the following equation: N2O4(g) ⇌ 2 NO2(g) Kc = 5.8 × 10-3
If the initial concentration of N2O4(g) = 0.040 M and the initial concentration of NO2(g) is 0 M, what is the equilibrium concentration of N2O4(g)?
A) 1.7 × 10-2 M
B) 1.9 × 10-2 M
C) 3.3 × 10-2 M
D) 2.6 × 10-2 M
E) 2.3 × 10-6 M
If the initial concentration of N2O4(g) = 0.040 M and the initial concentration of NO2(g) is 0 M, what is the equilibrium concentration of N2O4(g)?
A) 1.7 × 10-2 M
B) 1.9 × 10-2 M
C) 3.3 × 10-2 M
D) 2.6 × 10-2 M
E) 2.3 × 10-6 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
What will happen to the equilibrium in the reaction 2 A(g) ⇌ B(g) + C(g), Kc = 1.25 at 300 K if a catalyst is added?
A) The reaction is forced to the right.
B) The reaction is forced to the left.
C) No change, catalyst only changes the rate.
D) Kc is increased.
E) Kc is decreased.
A) The reaction is forced to the right.
B) The reaction is forced to the left.
C) No change, catalyst only changes the rate.
D) Kc is increased.
E) Kc is decreased.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
For the production of NO2, Kc = [NO2]2/[NO]2[O2]. At equilibrium in a 2.50 L container, there are 3.00 mol NO, 4.00 mol O2 and 22.0 mol NO2. The value of Kc is ________.
A) 13.4
B) 33.6
C) 5.38
D) 0.0116
E) 3.75
A) 13.4
B) 33.6
C) 5.38
D) 0.0116
E) 3.75

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Equilibrium constant K is constant except when one varies the:
A) concentrations of the reactants
B) temperature of the reaction
C) concentration of the products
D) partial pressures of the reactants
E) K always remains constant
A) concentrations of the reactants
B) temperature of the reaction
C) concentration of the products
D) partial pressures of the reactants
E) K always remains constant

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
For the reaction; N2(g) + 3 H2(g) ⇌ 2 NH3(g), the equilibrium amount of NH3 will be increased by: I) increasing the pressure
II) adding H2
III) removing N2
IV) decreasing the pressure
A) I, III
B) III only
C) II, III
D) I, II
E) II, IV
II) adding H2
III) removing N2
IV) decreasing the pressure
A) I, III
B) III only
C) II, III
D) I, II
E) II, IV

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
Write the equilibrium constant expression for the following reaction: N2(g) + 3 H2(g) ⇌ 2 NH3(g)
A)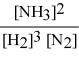
B)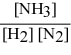
C)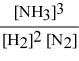
D)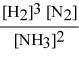
E)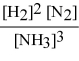
A)
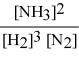
B)
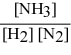
C)
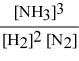
D)
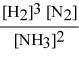
E)
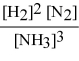

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the following reaction at a certain temperature. 2SO3(g) ⇌ 2SO2(g) + O2(g)
When the initial concentration of SO3(g) is 0.128 M, the concentration of oxygen gas at equilibrium is found to be 0.0130 M. Calculate Kc for this reaction.
A) 8.45 x 10-4
B) 1.62 x 10-2
C) 7.64 x 10-5
D) 1.47 x 10-3
When the initial concentration of SO3(g) is 0.128 M, the concentration of oxygen gas at equilibrium is found to be 0.0130 M. Calculate Kc for this reaction.
A) 8.45 x 10-4
B) 1.62 x 10-2
C) 7.64 x 10-5
D) 1.47 x 10-3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
For the reaction below CO(g) + 2H2(g) ⇌ CH3OH(g)
The equilibrium concentrations at 483 K are [CO(g)] = 0.0753 M, [H2(g)] = 0.151 M, and [CH3OH(g)] = 0.0247 M. Calculate the value of Kc.
A) 14.4
B) 1.09
C) 2.17
D) 0.0694
The equilibrium concentrations at 483 K are [CO(g)] = 0.0753 M, [H2(g)] = 0.151 M, and [CH3OH(g)] = 0.0247 M. Calculate the value of Kc.
A) 14.4
B) 1.09
C) 2.17
D) 0.0694

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
For the reaction: PCl3(g) + Cl2(g) ⇌ PCl5(g) at 70.5°C, Kp = 1.05.
If one starts with 1.80 atm pressure of PCl3(g), 1.72 atm pressure of Cl2(g), and no PCl5(g), what is the partial pressure of PCl5(g) at equilibrium?
A) 0.827 atm
B) 0.856 atm
C) 0.818 atm
D) 0.599 atm
E) 0.080 atm
If one starts with 1.80 atm pressure of PCl3(g), 1.72 atm pressure of Cl2(g), and no PCl5(g), what is the partial pressure of PCl5(g) at equilibrium?
A) 0.827 atm
B) 0.856 atm
C) 0.818 atm
D) 0.599 atm
E) 0.080 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)
A)![<strong>Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)</strong> A) B) Kc = [I2] C) Kc = [I2]2 D) E)](https://storage.examlex.com/TB2799/11eaaed0_3e84_269e_abb0_498b12736439_TB2799_11.jpg)
B) Kc = [I2]
C) Kc = [I2]2
D)![<strong>Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)</strong> A) B) Kc = [I2] C) Kc = [I2]2 D) E)](https://storage.examlex.com/TB2799/11eaaed0_3e84_4daf_abb0_d385186180a2_TB2799_11.jpg)
E)![<strong>Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)</strong> A) B) Kc = [I2] C) Kc = [I2]2 D) E)](https://storage.examlex.com/TB2799/11eaaed0_3e84_4db0_abb0_3b71e1941880_TB2799_11.jpg)
A)
![<strong>Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)</strong> A) B) Kc = [I2] C) Kc = [I2]2 D) E)](https://storage.examlex.com/TB2799/11eaaed0_3e84_269e_abb0_498b12736439_TB2799_11.jpg)
B) Kc = [I2]
C) Kc = [I2]2
D)
![<strong>Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)</strong> A) B) Kc = [I2] C) Kc = [I2]2 D) E)](https://storage.examlex.com/TB2799/11eaaed0_3e84_4daf_abb0_d385186180a2_TB2799_11.jpg)
E)
![<strong>Write the equilibrium constant expression for the following reaction: 2 KI(aq) + H2O2(aq) ⇌ 2 KOH(aq) + I2(aq)</strong> A) B) Kc = [I2] C) Kc = [I2]2 D) E)](https://storage.examlex.com/TB2799/11eaaed0_3e84_4db0_abb0_3b71e1941880_TB2799_11.jpg)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
For the reaction: 2 Cl2(g) + 2 H2O(g) ⇌ 4 HCl(g) + O2(g), Kp = 6.4 × 10-6 at 500 K. If a fixed volume is filled with initial concentrations of these gases at 227°C such that [Cl2] = 0.5 M, [H2O] = 0.40 M, [HCl] = 0.5 M, and [O2] = 0.015 M, in which direction will the reaction proceed?
A) The reaction proceeds to the right.
B) The reaction proceeds to the left.
C) The reaction is already at equilibrium.
D) The reaction volume must be specified to answer this question.
E) The value of Kp at 25 °C must be specified to answer this question.
A) The reaction proceeds to the right.
B) The reaction proceeds to the left.
C) The reaction is already at equilibrium.
D) The reaction volume must be specified to answer this question.
E) The value of Kp at 25 °C must be specified to answer this question.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Find Kp for the following reaction at 25.0°C. SbCl5(g) ⇌ SbCl3(g) + Cl2(g) Kc = 2.51 × 10-2
A) 0.614
B) 1.03 × 10-3
C) 5.15 × 10-2
D) 9.74 × 102
E) 39.8
A) 0.614
B) 1.03 × 10-3
C) 5.15 × 10-2
D) 9.74 × 102
E) 39.8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
For a reaction, the reaction quotient, Qc > Kc, the reaction ________.
A) is at equilibrium
B) is exothermic
C) shifts to the right
D) shifts to the left
E) is endothermic
A) is at equilibrium
B) is exothermic
C) shifts to the right
D) shifts to the left
E) is endothermic

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
For the reaction 2 NO(g)⇌ N2O4(g) Kp equals ________.
A) Kc
B) RT/Kc
C) Kc(RT)
D) Kc/RT
E) Kc(RT)2
A) Kc
B) RT/Kc
C) Kc(RT)
D) Kc/RT
E) Kc(RT)2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
For the reaction 2 A(g) ⇌ B(g) + C(g), Kc = 1.25 at 300 K. If a 1.00 L mixture contains 0.619 mol A, 0.693 mol B, and 0.689 mol C at 300 K, will the mixture be in equilibrium? If not, in what direction will a net reaction occur?
A) Yes, at equilibrium.
B) No, net reaction to the left.
C) No, net reaction to the right.
D) No, but there is no net reaction.
E) Yes, net reaction to the right
A) Yes, at equilibrium.
B) No, net reaction to the left.
C) No, net reaction to the right.
D) No, but there is no net reaction.
E) Yes, net reaction to the right

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
At a certain temperature, Kc = 0.0500 and △H = +39.6 kJ for the reaction below. 2MgCl2(s) + O2(g) ⇌ 2MgO(s) + 2Cl2(g)
Calculate Kc for the reaction
MgO(s) + Cl2(g) ⇌ MgCl2(s) +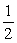 O2(g)
O2(g)
And indicate whether the value will be larger or smaller at a lower temperature.
A) 4.47, larger
B) 400, smaller
C) 0.224, larger
D) 0.224, smaller
Calculate Kc for the reaction
MgO(s) + Cl2(g) ⇌ MgCl2(s) +
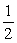 O2(g)
O2(g)And indicate whether the value will be larger or smaller at a lower temperature.
A) 4.47, larger
B) 400, smaller
C) 0.224, larger
D) 0.224, smaller

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
What is the relationship between Kp and Kc for the reaction below? 4NH3(g) + 7O2(g) ⇌ 2N2O4(g) + 6H2O(g)
A) Kp = Kc(RT)-3
B) Kc = Kp(RT)-3
C) Kp = Kc(RT)3
D) Kp = Kc(RT)
A) Kp = Kc(RT)-3
B) Kc = Kp(RT)-3
C) Kp = Kc(RT)3
D) Kp = Kc(RT)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Write the equilibrium constant expression for the following reaction: 6 CO2(g) + 6 H2O(l) ⇌ C6H12O6(s) + 6 O2(g)
A)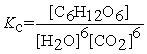
B)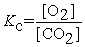
C)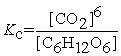
D)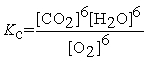
E)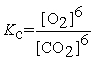
A)
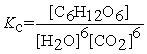
B)
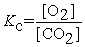
C)
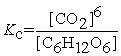
D)
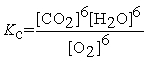
E)
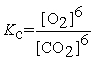

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Write the equilibrium expression Kc for the reaction: sodium sulfite(aq) + chloric acid (aq) ⇌ sodium chlorite(aq) + sulfur dioxide(g) + water(l).
A)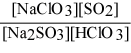
B)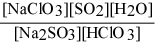
C)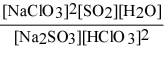
D)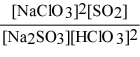
E)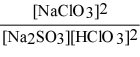
A)
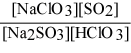
B)
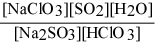
C)
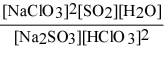
D)
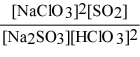
E)
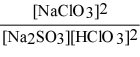

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g)
Write the expression for Kp.
A)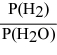
B)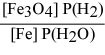
C)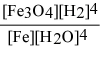
D)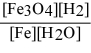
E)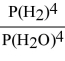
Write the expression for Kp.
A)
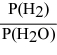
B)
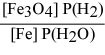
C)
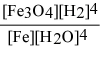
D)
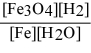
E)
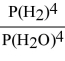

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
For the reaction: 2 SO2(g) + O2(g) ⇌ 2 SO3(g), Kc = 6.9 × 1024 at 25°C. If a reaction vessel is filled with these gases, such that [SO2] = 6.0 M, [O2] = 6.0 M and [SO3] = 6.0 M, in which direction will the reaction proceed?
A) The reaction proceeds to the right.
B) The reaction proceeds to the left.
C) The reaction is already at equilibrium.
D) The reaction volume must be specified to answer this question.
E) The value of Kp must be specified to answer this question.
A) The reaction proceeds to the right.
B) The reaction proceeds to the left.
C) The reaction is already at equilibrium.
D) The reaction volume must be specified to answer this question.
E) The value of Kp must be specified to answer this question.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Two moles of NH3 are initially present for the reaction: 2 NH3(g) ⇌ N2(g) + 3 H2(g)
At equilibrium there is 1.00 mol NH3. How many moles of H2 are present at equilibrium?
A) 3.00
B) 1.00
C) 1.50
D) 0.67
E) 0.75
At equilibrium there is 1.00 mol NH3. How many moles of H2 are present at equilibrium?
A) 3.00
B) 1.00
C) 1.50
D) 0.67
E) 0.75

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Given the following reactions, 2PCl3(g) ⇌ 2P(g) + 3Cl2(g) Kc = 0.0667
PCl3(g) + Cl2(g) ⇌ PCl5(g) Kc = 4.0
Calculate Kc for the reaction below.
2P(g) + 5Cl2(g) ⇌ 2PCl5(g)
A) 240
B) 1.1
C) 60
D) 23
PCl3(g) + Cl2(g) ⇌ PCl5(g) Kc = 4.0
Calculate Kc for the reaction below.
2P(g) + 5Cl2(g) ⇌ 2PCl5(g)
A) 240
B) 1.1
C) 60
D) 23

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) write the expression for Kc in terms of Kp.
A) Kc = Kp
B) Kc =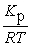
C) Kc = KpRT
D) Kc = Kp2
E) Kc =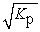
A) Kc = Kp
B) Kc =
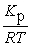
C) Kc = KpRT
D) Kc = Kp2
E) Kc =
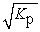

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Write the equilibrium constant expression for the reaction: 3 Sn(s) + 4 HNO3(aq) + H2O(l) ⇌ 3 H2SnO3(s) + 4 NO(g)
A) Kc =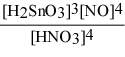
B) Kc =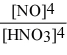
C) Kc =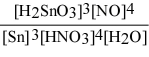
D) Kc =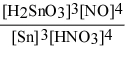
E) Kc =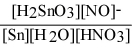
A) Kc =
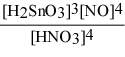
B) Kc =
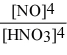
C) Kc =
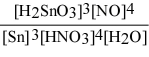
D) Kc =
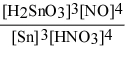
E) Kc =
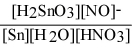

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the following hypothetical equilibrium reaction: A2(g) + B2(g) ⇌ 2AB(g) where Kc = 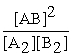 The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇔ 4 AB(g) is ________.
The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇔ 4 AB(g) is ________.
A)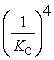
B) Kc4
C)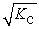
D)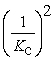
E) Kc2
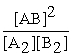 The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇔ 4 AB(g) is ________.
The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇔ 4 AB(g) is ________.A)
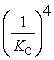
B) Kc4
C)
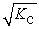
D)
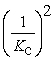
E) Kc2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) what is the effect of adding Fe(s)?
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) The Kp is decreased.
E) The Kp is doubled.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
In the reaction: 2 N2O(g) + N2H4(g) ⇌ 3 N2(g) + 2 H2O(g),
One starts with 0.10 mol N2O and 0.25 mol N2H4 in a 10.0 L container. If there are 0.06 mol N2O at equilibrium, how many moles of N2 are present?
A) 0.02
B) 0.04
C) 0.06
D) 0.09
E) 0.07
One starts with 0.10 mol N2O and 0.25 mol N2H4 in a 10.0 L container. If there are 0.06 mol N2O at equilibrium, how many moles of N2 are present?
A) 0.02
B) 0.04
C) 0.06
D) 0.09
E) 0.07

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


