Deck 13: Solutions and Their Physical Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 13: Solutions and Their Physical Properties
1
Most volatile liquids can be separated from each other by fractional distillation.
True
2
Which compound is most likely to be soluble in water?
A) butyl alcohol (CH3CH2CH2CH2OH)
B) ethylene glycol (HOCH2CH2OH)
C) hexane (C6H14)
D) benzene (C6H12)
E) iodine (I2)
A) butyl alcohol (CH3CH2CH2CH2OH)
B) ethylene glycol (HOCH2CH2OH)
C) hexane (C6H14)
D) benzene (C6H12)
E) iodine (I2)
ethylene glycol (HOCH2CH2OH)
3
Liquid Q is a polar solvent and liquid R is a nonpolar solvent. On the basis of this information, you would expect:
A) both liquids to be miscible with a third liquid T
B) liquid Q to be miscible with liquid R
C) NaCl to be soluble to both Q and R
D) liquid Q and H2O to be miscible
E) liquid R and H2O to be miscible
A) both liquids to be miscible with a third liquid T
B) liquid Q to be miscible with liquid R
C) NaCl to be soluble to both Q and R
D) liquid Q and H2O to be miscible
E) liquid R and H2O to be miscible
liquid Q and H2O to be miscible
4
A magnesium sulfate heptahydrate solution, which is 18.00% by weight in the anhydrous compound, has a density at 20°C of 1.20 g/mL. What is the molality of the anhydrous compound in the solution? (Atomic weights: H = 1.0, O = 16.0, S = 32.1, Mg = 24.3)
A) 5.54 M
B) 1.79 M
C) 1.82 M
D) 1.25 M
E) 1.49 M
A) 5.54 M
B) 1.79 M
C) 1.82 M
D) 1.25 M
E) 1.49 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
The van't Hoff factor is due to ions being attracted to each other enough that they don't react as individual molecules.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
Osmotic pressure is a colligative property.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Molality is independent of temperature, molarity is dependent on temperature.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
In the solution, a solute is present in the greatest quantity.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
A solid solution of zinc in copper is an example of:
A) a solvent
B) a solute
C) the U.S. five-cent nickel
D) a non-uniform solute
E) an alloy
A) a solvent
B) a solute
C) the U.S. five-cent nickel
D) a non-uniform solute
E) an alloy

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
An unsaturated solution will have some solid undissolved in the bottom of the container.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
A solution prepared by dissolving 4.00 g KCl in 36.0 g H2O is said to be:
A) 11.1% KCl by mass
B) 0.100% KCl by mass
C) 0.111% KCl by mass
D) 10.0% KCl by mass
E) 9.00% KCl by mass
A) 11.1% KCl by mass
B) 0.100% KCl by mass
C) 0.111% KCl by mass
D) 10.0% KCl by mass
E) 9.00% KCl by mass

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
A sweetened cup of coffee is an example of a:
A) heterogeneous mixture
B) homogeneous mixture
C) solid solution
D) pure liquid
E) gaseous solute in a liquid solvent
A) heterogeneous mixture
B) homogeneous mixture
C) solid solution
D) pure liquid
E) gaseous solute in a liquid solvent

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
Which definition is INCORRECT?
A) mole fraction = moles of solute/moles of solution
B) molality = moles of solute/kilogram of solvent
C) weight percent = grams of solute/100 grams of solution
D) molarity = moles of solute/liter of solution
E) volume percent=volume of solute/volume of solution
A) mole fraction = moles of solute/moles of solution
B) molality = moles of solute/kilogram of solvent
C) weight percent = grams of solute/100 grams of solution
D) molarity = moles of solute/liter of solution
E) volume percent=volume of solute/volume of solution

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
Adding a solute to a solvent lowers the freezing point of the solution compared to the pure solvent.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
A 1.00 molal solution of NaCl in water contains:
A) 1.00 g NaCl per 1000.0 g H2O
B) 1.00 mol NaCl per 1000.0 g H2O
C) 1.00 mol NaCl per 1000.0 kg H2O
D) 1000.0 g NaCl per 1000.0 g H2O
E) 1.00 mol NaCl per 1000.0 mol H2O
A) 1.00 g NaCl per 1000.0 g H2O
B) 1.00 mol NaCl per 1000.0 g H2O
C) 1.00 mol NaCl per 1000.0 kg H2O
D) 1000.0 g NaCl per 1000.0 g H2O
E) 1.00 mol NaCl per 1000.0 mol H2O

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
For the formation of an ideal solution from two liquid hydrocarbons, ΔHsoln < 0.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
Gas solubility always increases with temperature.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following pairs are more likely to form a solution?
A) chloroform (CHCl3) and acetone (CH3COCH3)
B) water and octane (C8H18)
C) methanol (CH3OH) and hexane (C6H14)
D) water and octyl alcohol (C8H17OH)
E) acetone (CH3COCH3) and octane (C8H13)
A) chloroform (CHCl3) and acetone (CH3COCH3)
B) water and octane (C8H18)
C) methanol (CH3OH) and hexane (C6H14)
D) water and octyl alcohol (C8H17OH)
E) acetone (CH3COCH3) and octane (C8H13)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
An aqueous solution containing 1.0 g ephedrine in 20.0 mL solution is said to be:
A) 5.0% ephedrine (mass/vol)
B) 1.0% ephedrine (mass/vol)
C) 0.50% ephedrine (mass/vol)
D) 0.050% ephedrine (mass/vol)
E) 20.0% ephedrine (mass/vol)
A) 5.0% ephedrine (mass/vol)
B) 1.0% ephedrine (mass/vol)
C) 0.50% ephedrine (mass/vol)
D) 0.050% ephedrine (mass/vol)
E) 20.0% ephedrine (mass/vol)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
The stronger the forces between solute and solvent molecules, the more exothermic the solution process.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
Henry's Law constants for aqueous solutions at 25°C are 8.20 × 10-7 molal/mmHg for N2 and 1.62 × 10-6 molal/mmHg for O2. Determine the solubility of nitrogen in water under an atmospheric pressure of 760 mmHg, assuming that air is 80% N2 and 20% O2.
A) 6.23 × 10-4 m
B) 7.79 × 10-4 m
C) 4.99 × 10-4 m
D) 1.25 × 10-4 m
E) 6.16 × 10-3 m
A) 6.23 × 10-4 m
B) 7.79 × 10-4 m
C) 4.99 × 10-4 m
D) 1.25 × 10-4 m
E) 6.16 × 10-3 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
The solubility of CO in water at 0°C and 1 atm CO pressure is 0.0354 mg of CO in 1 mL of water. Calculate the molarity of aqueous CO solution at the normal partial pressure of CO of 0.00036 atm?
A) 4.5 × 10-7 M
B) 1.6 × 10-3 M
C) 1.3 × 10-5 M
D) 2.9 × 10-4 M
E) 3.2 × 10-3 M
A) 4.5 × 10-7 M
B) 1.6 × 10-3 M
C) 1.3 × 10-5 M
D) 2.9 × 10-4 M
E) 3.2 × 10-3 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following pairs of liquids would form a nonideal solution?
A) CHCl3 and (CH3)2CO
B) C6H5-CH3 (toluene) and C6H6 (benzene)
C) CH3CH2CH2OH and CH3CH(OH)CH3
D) H5C6-C6H5 (diphenyl) and C6H6 (benzene)
A) CHCl3 and (CH3)2CO
B) C6H5-CH3 (toluene) and C6H6 (benzene)
C) CH3CH2CH2OH and CH3CH(OH)CH3
D) H5C6-C6H5 (diphenyl) and C6H6 (benzene)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
A solution is called "ideal" when:
A) all intermolecular forces of attraction are the same, resulting in no net enthalpy change when the solution is formed from its components.
B) the forces of attraction between solute molecules exceed those between solute and solvent molecules.
C) the forces of attraction between solute and solvent molecules are somewhat smaller than between solute-solute molecules or solvent-solvent molecules.
D) the forces of attraction between solvent molecules exceed those between solute and solvent molecules.
E) the forces of attraction between solute molecules and solvent molecules are much smaller than between solute-solute molecules or solvent-solvent molecules.
A) all intermolecular forces of attraction are the same, resulting in no net enthalpy change when the solution is formed from its components.
B) the forces of attraction between solute molecules exceed those between solute and solvent molecules.
C) the forces of attraction between solute and solvent molecules are somewhat smaller than between solute-solute molecules or solvent-solvent molecules.
D) the forces of attraction between solvent molecules exceed those between solute and solvent molecules.
E) the forces of attraction between solute molecules and solvent molecules are much smaller than between solute-solute molecules or solvent-solvent molecules.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
What is the osmotic pressure in mmHg of 6.00 L of a 0.108 M solution at 30°C if three moles of ions are produced in aqueous solution for every mole of solute dissolved?
A) 8.05 mmHg
B) 3.68 × 104 mmHg
C) 2.04 × 103 mmHg
D) 0.0613 mmHg
E) 6.13 × 103 mmHg
A) 8.05 mmHg
B) 3.68 × 104 mmHg
C) 2.04 × 103 mmHg
D) 0.0613 mmHg
E) 6.13 × 103 mmHg

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
Henry's Law states that:
A) a supersaturated solution is unstable
B) the solubility of a gas increases as the gas pressure is increased
C) the solubility of a gas decreases as the gas pressure is increased
D) a concentrated solution lowers the freezing point of a solution
E) a concentrated solution increases the boiling point of a solution
A) a supersaturated solution is unstable
B) the solubility of a gas increases as the gas pressure is increased
C) the solubility of a gas decreases as the gas pressure is increased
D) a concentrated solution lowers the freezing point of a solution
E) a concentrated solution increases the boiling point of a solution

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
Nitrogen gas has a Henry's law constant k = 6.3 × 10-4 M/atm at 25°C. The "bends" in divers results from bubbles of N2(g) being rapidly released from body fluids when a diver ascends to the surface too quickly. Which of the following would be a good substitute for N2(g) in order to make the "bends" less severe?
A) He(g), k = 3.7 × 10-4 M/atm
B) Ar(g), k = 1.5 × 10-3 M/atm
C) H2(g), k = 8.1 × 10-4 M/atm
D) CO2(g), k = 3.4 × 10-2 M/atm
A) He(g), k = 3.7 × 10-4 M/atm
B) Ar(g), k = 1.5 × 10-3 M/atm
C) H2(g), k = 8.1 × 10-4 M/atm
D) CO2(g), k = 3.4 × 10-2 M/atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following organic substances is most readily soluble in water? 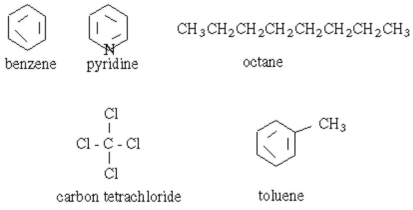
A) benzene
B) pyridine
C) octane
D) carbon tetrachloride
E) toluene

A) benzene
B) pyridine
C) octane
D) carbon tetrachloride
E) toluene

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Henry's Law constants for aqueous solutions at 25°C are 8.20 × 10-7 molal/mmHg for N2 and 1.62 × 10-6 molal/mmHg for O2. Determine the solubility of oxygen in water under an atmospheric pressure of 760 mmHg, assuming that air is 80% N2 and 20% O2.
A) 6.2 × 10-3 m
B) 2.5 × 10-4 m
C) 1.3 × 10-4 m
D) 5.0 × 10-4 m
E) 1.2 × 10-3 m
A) 6.2 × 10-3 m
B) 2.5 × 10-4 m
C) 1.3 × 10-4 m
D) 5.0 × 10-4 m
E) 1.2 × 10-3 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
A solution composed of 5 moles of acetone (CH3COCH3, P°A = 324 mmHg) and 5 moles of chloroform (CHCl3, P°C = 274 mmHg) has a vapor pressure of 236 mmHg. Which one of the following statements is completely true about this solution?
A) The solution shows a negative deviation from Raoult's law and thus possesses a maximum boiling azeotrope.
B) The solution process is exothermic because the forces between unlike molecules are weaker than those between like molecules.
C) The solution shows a positive deviation from Raoult's law.
D) The solution possesses a minimum boiling azeotrope because it shows a negative deviation from Raoult's law.
E) The solution obeys Raoult's Law.
A) The solution shows a negative deviation from Raoult's law and thus possesses a maximum boiling azeotrope.
B) The solution process is exothermic because the forces between unlike molecules are weaker than those between like molecules.
C) The solution shows a positive deviation from Raoult's law.
D) The solution possesses a minimum boiling azeotrope because it shows a negative deviation from Raoult's law.
E) The solution obeys Raoult's Law.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
An azeotropic mixture is a:
A) mixture of two or more substances where boiling point cannot be determined.
B) solution of two or more substances present in the same amounts in the liquid phase.
C) liquid mixture of two or more substances in which the vapor has the same composition as the liquid.
D) liquid mixture of two or more substances linked by azide functional groups.
E) solution of two or more substances that cannot be made to freeze.
A) mixture of two or more substances where boiling point cannot be determined.
B) solution of two or more substances present in the same amounts in the liquid phase.
C) liquid mixture of two or more substances in which the vapor has the same composition as the liquid.
D) liquid mixture of two or more substances linked by azide functional groups.
E) solution of two or more substances that cannot be made to freeze.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following compounds has the highest solubility?
A) CaCl2
B) SrI2
C) CaI2
D) MgI2
E) CaBr2
A) CaCl2
B) SrI2
C) CaI2
D) MgI2
E) CaBr2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is a correct statement about azeotropes?
A) The components boil at distinct temperatures.
B) The mixture cannot be separated by fractional distillation.
C) The solution behaves as an ideal solution.
D) The vapor has a different composition than the liquid.
E) The solution has the same pressure as the pure solvent.
A) The components boil at distinct temperatures.
B) The mixture cannot be separated by fractional distillation.
C) The solution behaves as an ideal solution.
D) The vapor has a different composition than the liquid.
E) The solution has the same pressure as the pure solvent.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
The solubility of CO in water at 0°C and 1 atm CO pressure is 0.0354 mg of CO in 1 mL of water. Calculate the molarity of aqueous CO solution at 2 atm CO pressure.
A) 1.58 × 10-3 M
B) 6.32 × 10-2 M
C) 1.59 M
D) 2.53 × 10-3 M
E) 3.16 × 10-3 M
A) 1.58 × 10-3 M
B) 6.32 × 10-2 M
C) 1.59 M
D) 2.53 × 10-3 M
E) 3.16 × 10-3 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
The solubilities of ammonium bromide, NH4Br, in water at 0°C, 20°C, and 80°C are as follows: T (°C) Sol. g NH4Br/100 g H2O
0 60.5
20 76.4
80 125
Which of the following fractional crystallization schemes would produce the highest percent yield for the recrystallization of ammonium bromide?
A) A solution containing 50.5 g NH4Br in 100.0 g H2O at 20°C is cooled to 0°C.
B) A solution containing 115 g NH4Br in 200 g H2O at 80°C is cooled to 0°C.
C) A solution containing 120 g NH4Br in 100 g H2O at 80°C is cooled to 20°C.
D) A solution containing 100 g NH4Br in 100.0 g H2O at 80°C is cooled to 20°C.
E) A solution containing 95 g NH4Br in 175 g H2O at 80°C is cooled to 0°C.
0 60.5
20 76.4
80 125
Which of the following fractional crystallization schemes would produce the highest percent yield for the recrystallization of ammonium bromide?
A) A solution containing 50.5 g NH4Br in 100.0 g H2O at 20°C is cooled to 0°C.
B) A solution containing 115 g NH4Br in 200 g H2O at 80°C is cooled to 0°C.
C) A solution containing 120 g NH4Br in 100 g H2O at 80°C is cooled to 20°C.
D) A solution containing 100 g NH4Br in 100.0 g H2O at 80°C is cooled to 20°C.
E) A solution containing 95 g NH4Br in 175 g H2O at 80°C is cooled to 0°C.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements about ideal solutions is(are) true? I) Entropy effects are important for the formation of ideal solutions.
II) The heat of solution is zero.
III) The vapor pressure varies linearly with the composition.
IV) The forces between like kinds of molecules are (almost) equal to the forces between unlike kinds of molecules.
A) I), II), IV)
B) I), III), IV)
C) II), III), IV)
D) I), II), III)
E) I), II), III), IV)
II) The heat of solution is zero.
III) The vapor pressure varies linearly with the composition.
IV) The forces between like kinds of molecules are (almost) equal to the forces between unlike kinds of molecules.
A) I), II), IV)
B) I), III), IV)
C) II), III), IV)
D) I), II), III)
E) I), II), III), IV)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
Which compound is most likely to be soluble in hexane?
A) water
B) acetone (CH3COCH3)
C) benzene (C6H12)
D) ethyl alcohol (CH3CH2OH)
E) chloroform (CHCl3)
A) water
B) acetone (CH3COCH3)
C) benzene (C6H12)
D) ethyl alcohol (CH3CH2OH)
E) chloroform (CHCl3)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
The vapor pressures of pure propyl alcohol and isopropyl alcohol are 21.0 mmHg and 45.2 mmHg, respectively, at 25°C. What is the composition of the vapor in equilibrium with a propyl alcohol - isopropyl alcohol solution in which the mole fraction of propyl alcohol is 0.250?
A) xiso = 0.866, xprop = 0.134
B) xiso = 0.750, xprop = 0.250
C) xiso = 0.317, xprop = 0.683
D) xiso = 0.512, xprop = 0.488
A) xiso = 0.866, xprop = 0.134
B) xiso = 0.750, xprop = 0.250
C) xiso = 0.317, xprop = 0.683
D) xiso = 0.512, xprop = 0.488

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
A solution that can dissolve no more solute is called:
A) unsaturated
B) saturated
C) concentrated
D) supersaturated
E) oversaturated
A) unsaturated
B) saturated
C) concentrated
D) supersaturated
E) oversaturated

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
A crystal is placed into a solution and the solution seems to solidify while getting much warmer at the same time. The solution must have been:
A) supersaturated
B) dilute
C) saturated
D) unsaturated
E) concentrated
A) supersaturated
B) dilute
C) saturated
D) unsaturated
E) concentrated

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following aqueous solutions has the lowest freezing point?
A) 1.5 m aluminum perchlorate.
B) 1.0 m magnesium phosphate
C) 1.5 m calcium nitrate
D) 2.0 m potassium chloride
A) 1.5 m aluminum perchlorate.
B) 1.0 m magnesium phosphate
C) 1.5 m calcium nitrate
D) 2.0 m potassium chloride

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is an example of a colloid?
A) sucrose in water
B) whipped cream
C) steel
D) salt in water
A) sucrose in water
B) whipped cream
C) steel
D) salt in water

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following aqueous solutions has the highest boiling point?
A) 1.0 m magnesium phosphate
B) 1.5 m sodium sulfate
C) 2.0 m lithium perchlorate
D) 1.5 m magnesium sulfate
A) 1.0 m magnesium phosphate
B) 1.5 m sodium sulfate
C) 2.0 m lithium perchlorate
D) 1.5 m magnesium sulfate

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
Solutions are made that contain 0.10 moles of each of the following compounds in 100 g of H2O. Choose the compound whose solution will have the highest freezing point.
A) K2SO4
B) NaI
C) Mg(CH3CO2)2
D) Ca3(PO4)2
E) Sr(NO3)2
A) K2SO4
B) NaI
C) Mg(CH3CO2)2
D) Ca3(PO4)2
E) Sr(NO3)2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is a true solution with particles less than 1 nm in size?
A) calcium chloride in water.
B) oil in water.
C) whipped cream.
D) milk.
A) calcium chloride in water.
B) oil in water.
C) whipped cream.
D) milk.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
Colligative properties are similar in that they all:
A) are due to solvent chemical interactions
B) depend on the number of solute particles in solution
C) depend on the solvent
D) have no effect on the properties of solution
E) describe colloids
A) are due to solvent chemical interactions
B) depend on the number of solute particles in solution
C) depend on the solvent
D) have no effect on the properties of solution
E) describe colloids

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
85.0 grams of potassium bromide are to be used to produce a 0.300 M solution. What will be the total volume of this solution?
A) 2.38 L
B) 2.83 L
C) 283 L
D) 0.214 L
E) 0.420 L
A) 2.38 L
B) 2.83 L
C) 283 L
D) 0.214 L
E) 0.420 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
Moles of solute per mole of solution is a definition of ________.
A) molarity
B) mole fraction
C) percent by weight
D) molality
E) normality
A) molarity
B) mole fraction
C) percent by weight
D) molality
E) normality

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Solutions are made that contain 0.1 moles of each of the following compounds below in 100 g of H2O. Choose the compound whose solution will have the lowest freezing point.
A) MgCl2
B) KClO3
C) CO2
D) MgSO4
E) NaBr
A) MgCl2
B) KClO3
C) CO2
D) MgSO4
E) NaBr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
Moles of solute per liter of solution is the definition of ________.
A) molality
B) solubility
C) molarity
D) normality
E) mole fraction
A) molality
B) solubility
C) molarity
D) normality
E) mole fraction

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
A solution containing 3.24 g of sulfur in 40 g of benzene boils 0.81°C above the boiling point of pure benzene. Determine the number of S atoms in one molecule of dissolved sulfur. Kb for benzene is 2.53°C m-1.
A) 10
B) 12
C) 6
D) 8
E) 4
A) 10
B) 12
C) 6
D) 8
E) 4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is a non-colligative property?
A) freezing point depression
B) boiling point elevation
C) osmotic pressure
D) solubility
E) vapour pressure lowering
A) freezing point depression
B) boiling point elevation
C) osmotic pressure
D) solubility
E) vapour pressure lowering

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
We wish to lower the freezing point of the water in an automobile radiator to -40°C. The total mass of water present is 12.0 kg. For which one of the following compounds would we have to use the greatest mass of solute to achieve this? You may assume that Kf for water = -1.86°C/m and that all ionizable solutes are completely ionized with unit activity even at this high concentration.
A) AlF3 (M.W. = 84 g/mol)
B) C2H5OH (M.W. = 46 g/mol)
C) CaCl2 (M.W. = 111 g/mol)
D) C3H6(OH)2 (M.W. = 76 g/mol)
E) NaCl (M.W. = 58.5 g/mol)
A) AlF3 (M.W. = 84 g/mol)
B) C2H5OH (M.W. = 46 g/mol)
C) CaCl2 (M.W. = 111 g/mol)
D) C3H6(OH)2 (M.W. = 76 g/mol)
E) NaCl (M.W. = 58.5 g/mol)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
An aqueous solution contains 64.0 g of ethanol (C2H5OH) in 122.0 g of solution. What is the mole fraction of ethanol in the solution?
A) 0.301
B) 0.432
C) 0.205
D) 0.698
E) 0.317
A) 0.301
B) 0.432
C) 0.205
D) 0.698
E) 0.317

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
Choose the correct statement.
A) Colloids are homogeneous solutions.
B) A true colloidal particle will not settle out of the dispersing membrane.
C) Colloidal particles can usually pass through a semipermeable membrane.
D) Electrolytes are protective colloids.
E) Dispersion is the method of preparing colloids from ions or molecules.
A) Colloids are homogeneous solutions.
B) A true colloidal particle will not settle out of the dispersing membrane.
C) Colloidal particles can usually pass through a semipermeable membrane.
D) Electrolytes are protective colloids.
E) Dispersion is the method of preparing colloids from ions or molecules.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
A solution component present in lesser quantity than the solvent is called ________.
A) a diluent
B) an alloy
C) a heterogeneous solid
D) a solute
E) a mixture
A) a diluent
B) an alloy
C) a heterogeneous solid
D) a solute
E) a mixture

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following combination of dispersed phase in a dispersion medium with the colloid type is INCORRECT?
A) Solid in liquid is a sol.
B) Solid in solid is a solid sol.
C) Solid in gas is an aerosol.
D) Liquid in solid is a solid foam.
E) Liquid in gas is an aerosol.
A) Solid in liquid is a sol.
B) Solid in solid is a solid sol.
C) Solid in gas is an aerosol.
D) Liquid in solid is a solid foam.
E) Liquid in gas is an aerosol.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
Arrange the following three water solutions (all 250 cm3 in volume) according to decreasing osmotic pressure at 273 K: 5 g of C2H5OH, 5 g C6H12O6 ,and 5 g C12H22O11.
A) C2H5OH > C6H12O6 > C12H22O11
B) C2H5OH > C6H12O6 = C12H22O11
C) C6H12O6 > C2H5OH > C12H22O11
D) C12H22O11 > C6H12O6 > C2H5OH
E) C12H22O11 > C2H5OH> C6H12O6
A) C2H5OH > C6H12O6 > C12H22O11
B) C2H5OH > C6H12O6 = C12H22O11
C) C6H12O6 > C2H5OH > C12H22O11
D) C12H22O11 > C6H12O6 > C2H5OH
E) C12H22O11 > C2H5OH> C6H12O6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following material-colloid type combinations is INCORRECT?
A) mayonnaise - emulsion
B) whipped cream - foam
C) smoke - aerosol
D) lava - solid foam
E) opal - solid sol
A) mayonnaise - emulsion
B) whipped cream - foam
C) smoke - aerosol
D) lava - solid foam
E) opal - solid sol

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
Choose the INCORRECT statement.
A) Since colloidal particles are large aggregates of atoms they are electrically neutral.
B) Colloidal suspensions can be prepared in which the particles do not settle.
C) The addition of electrolytes to a colloidal suspension will sometimes cause the particles to coalesce and precipitate.
D) Colloidal particles reflect light from a beam.
E) Colloidal suspensions exhibit the Tyndall Effect.
A) Since colloidal particles are large aggregates of atoms they are electrically neutral.
B) Colloidal suspensions can be prepared in which the particles do not settle.
C) The addition of electrolytes to a colloidal suspension will sometimes cause the particles to coalesce and precipitate.
D) Colloidal particles reflect light from a beam.
E) Colloidal suspensions exhibit the Tyndall Effect.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
Commercial perchloric acid is 70.0% by mass, HClO4(aq), and has a density of 1.67 g/mL. Calculate the mole fraction of perchloric acid in the solution.
A) 0.295
B) 0.420
C) 0.705
D) 0.697
A) 0.295
B) 0.420
C) 0.705
D) 0.697

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
The solubility of ammonium permanganate is 7.90 g/100 mL of water. 29.0 grams of ammonium permanganate are placed in a container and 220 mL of water is added. The liquid is allowed to stand in contact with the solid until equilibrium is achieved and then the liquid is poured off. What is the weight of solid left?
A) 17.4 g
B) 79.0 g
C) 7.90 g
D) 11.6 g
E) 29.0 g
A) 17.4 g
B) 79.0 g
C) 7.90 g
D) 11.6 g
E) 29.0 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
The vapor pressures of pure hexane and pure heptane at 25°C are 151.4 mmHg and 45.62 mmHg respectively. A solution contains 0.800 mol fraction n-hexane and 0.200 mol fraction n-heptane. What is the composition of the vapor in equilibrium with this solution at 25°C?
A) 80.0% hexane, 20.0% heptane
B) 50.0% hexane, 50.0% heptane
C) 77.0% hexane, 23.0% heptane
D) 45.0% hexane, 55.0% heptane
E) 93.0% hexane, 7.0% heptane
A) 80.0% hexane, 20.0% heptane
B) 50.0% hexane, 50.0% heptane
C) 77.0% hexane, 23.0% heptane
D) 45.0% hexane, 55.0% heptane
E) 93.0% hexane, 7.0% heptane

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
252 mL of 3.00 M H2SO4 are added to 1.50 L of 0.500 M H2SO4. What is the concentration of the resulting solution?
A) 1.50 M
B) 0.860 M
C) 2.64 M
D) 1.75 M
E) 1.25 M
A) 1.50 M
B) 0.860 M
C) 2.64 M
D) 1.75 M
E) 1.25 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
Commercial perchloric acid is 70.0% by mass, HClO4(aq), and has a density of 1.67 g/mL. Calculate the molarity of perchloric acid in the solution.
A) 11.5 M
B) 1.17 M
C) 23.7 M
D) 16.5 M
A) 11.5 M
B) 1.17 M
C) 23.7 M
D) 16.5 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
What is the molarity of a saturated solution of potassium sulfate if the solubility is 13 g per 100 g H2O at 25°C? The density of the solution is 1.1 g/mL
A) 0.14 M
B) 1.1 M
C) 0.96 M
D) 0.73 M
E) 0.79 M
A) 0.14 M
B) 1.1 M
C) 0.96 M
D) 0.73 M
E) 0.79 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
A saturated solution of ammonium sulfate, (NH4)2SO4, in water at 30°C contains 78.0 g (NH4)2SO4 per 100.0 g H2O. What is the molality of this solution?
A) 0.780 m
B) 1.69 m
C) 0.590 m
D) 0.0590 m
E) 5.90 m
A) 0.780 m
B) 1.69 m
C) 0.590 m
D) 0.0590 m
E) 5.90 m

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
If a solution containing 4 mols of A and 6 mols of B boils at 85°C and 1 atm of pressure, and the vapor pressure of pure A at 85°C is 500 mmHg, what is the vapor pressure of pure B at this temperature?
A) 933 mmHg
B) 336 mmHg
C) 260 mmHg
D) 576 mmHg
E) 960 mmHg
A) 933 mmHg
B) 336 mmHg
C) 260 mmHg
D) 576 mmHg
E) 960 mmHg

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
138.0 grams of ethanol (46.0 g/mol), 99.0 grams of water (18.0 g/mol), and 80.0 grams of methanol (32.0 g/mol) comprise a solution. What is the mole fraction of methanol present in the solution?
A) 0.273
B) 0.338
C) 0.294
D) 0.252
E) 0.227
A) 0.273
B) 0.338
C) 0.294
D) 0.252
E) 0.227

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
A magnesium sulfate heptahydrate solution, which is 18.00% by weight in the anhydrous compound, has a density at 20°C of 1.20 g/mL. What is the molarity of the anhydrous compound in the solution? (Atomic weights: H = 1.0, O = 16.0, S = 32.1, Mg = 24.3)
A) 5.54 M
B) 1.79 M
C) 1.82 M
D) 1.25 M
E) 1.49 M
A) 5.54 M
B) 1.79 M
C) 1.82 M
D) 1.25 M
E) 1.49 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
The vapor pressures of pure propyl alcohol and isopropyl alcohol are 21.0 mmHg and 45.2 mmHg, respectively, at 25°C. Calculate the partial pressure of isopropyl alcohol above a solution in which the mole fraction of propyl alcohol is 0.250.
A) 33.9 mmHg
B) 11.3 mmHg
C) 5.25 mmHg
D) 15.8 mmHg
A) 33.9 mmHg
B) 11.3 mmHg
C) 5.25 mmHg
D) 15.8 mmHg

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
An aqueous solution containing 12.0% MgCl2 by mass has a density of 1.105 g/mL. What is the mol fraction of water in this solution?
A) 0.975
B) 0.880
C) 0.868
D) 0.133
E) 0.952
A) 0.975
B) 0.880
C) 0.868
D) 0.133
E) 0.952

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
A 1.38 M solution of nitric acid (63. g/mol) in water (18.0 g/mol) has a density of 1.04 g/mL. What is the mole fraction of nitric acid in the solution?
A) 0.0254
B) 0.0239
C) 0.0261
D) 0.0228
E) 0.0233
A) 0.0254
B) 0.0239
C) 0.0261
D) 0.0228
E) 0.0233

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
Commercial nitric acid is 16.0 M HNO3(aq) and has a density of 1.42 g/mL. What is the mole fraction of HNO3 in this solution?
A) 0.412
B) 0.699
C) 0.589
D) 0.704
A) 0.412
B) 0.699
C) 0.589
D) 0.704

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Given that the vapor pressure of pure n-hexane and pure n-heptane at 25°C are 151.4 mmHg and 45.62 mmHg respectively, calculate the total vapor pressure above a solution containing only n-hexane and n-heptane in which the mole fraction of n-hexane is 0.600.
A) 87.9 mmHg
B) 197 mmHg
C) 106 mmHg
D) 109 mmHg
E) 170 mmHg
A) 87.9 mmHg
B) 197 mmHg
C) 106 mmHg
D) 109 mmHg
E) 170 mmHg

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
What is the correct value for the weight of Na3PO4 needed to make 100 mL of a 0.50 molar solution?
A) 16.4 g
B) 8.2 g
C) 0.05 g
D) 82 g
E) 4.1 g
A) 16.4 g
B) 8.2 g
C) 0.05 g
D) 82 g
E) 4.1 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
A mixture of benzene and toluene has a total vapor pressure at 25°C of 45.06 mmHg. What is the partial pressure of benzene in this solution? The vapor pressure of pure benzene and pure toluene at 25°C are 95.03 mmHg and 28.40 mmHg respectively.
A) 0.7500 mmHg
B) 23.76 mmHg
C) 0.3217 mmHg
D) 21.30 mmHg
E) 16.66 mmHg
A) 0.7500 mmHg
B) 23.76 mmHg
C) 0.3217 mmHg
D) 21.30 mmHg
E) 16.66 mmHg

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
The concentration unit used in Raoult's Law calculations is ________.
A) mole fraction
B) percent by weight
C) molarity
D) molality
E) grams per L
A) mole fraction
B) percent by weight
C) molarity
D) molality
E) grams per L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
100 mL of LiNO3, 0.241 M is mixed with 240 mL of 0.618 M Ca(NO3)2. What is the final concentration of NO3- in the solution?
A) 0.508 M
B) 1.01 M
C) 0.943 M
D) 0.756 M
E) 1.38 M
A) 0.508 M
B) 1.01 M
C) 0.943 M
D) 0.756 M
E) 1.38 M

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
What is the mass/vol % ethanol in an ethanol-water solution with a density of 0.875 g/mL and containing 65.0% ethanol by volume? The density of pure ethanol is 0.789 g/mL.
A) 90.2% ethanol (mass/vol)
B) 56.9% ethanol (mass/vol)
C) 51.3% ethanol (mass/vol)
D) 58.6% ethanol (mass/vol)
E) 74.5% ethanol (mass/vol)
A) 90.2% ethanol (mass/vol)
B) 56.9% ethanol (mass/vol)
C) 51.3% ethanol (mass/vol)
D) 58.6% ethanol (mass/vol)
E) 74.5% ethanol (mass/vol)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


