Deck 14: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 14: Chemical Kinetics
1
If the half-life of a reactant is independent of its initial concentration, the reaction order is 0.
False
2
For the reaction: C2H4Br2 + 3 KI → C2H4 + 2 KBr + KI3, when the rate of reaction is 2.0 × 10-5, what is the rate of appearance of C2H4?
A) 0.67 × 10-5
B) 2.0 × 10-5
C) 4.0 × 10-5
D) 6.0 × 10-5
E) 1.0 × 10-5
A) 0.67 × 10-5
B) 2.0 × 10-5
C) 4.0 × 10-5
D) 6.0 × 10-5
E) 1.0 × 10-5
2.0 × 10-5
3
In the reaction C4H9Cl(aq) + H2O(l) → C4H9OH(aq) + HCl(aq) the concentration of the reactant changes from 0.0562 M to 0.0431 M in 85 sec. What is the average rate of decomposition over this interval?
A) 1.54 × 10-4 M/s
B) 1.54 × 10-4 moles
C) 1.54 × 10-4 moles/s
D) 0.0154 M
E) 0.0154 M/s
A) 1.54 × 10-4 M/s
B) 1.54 × 10-4 moles
C) 1.54 × 10-4 moles/s
D) 0.0154 M
E) 0.0154 M/s
1.54 × 10-4 M/s
4
Temperature has no effect on reaction rate.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
If increasing the concentration of A in a chemical reaction causes no increase in the rate of the reaction, then we may say the reaction rate is first order in [A].

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
Energy of activation has no effect on reaction rate.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
Define rate law.
A) A theoretical equation that describes how the rate of reaction depends on the concentration of reactants.
B) An experimentally determined equation that describes how the rate of reaction depends on the concentration of reactants.
C) A theoretical equation that describes how the rate of reaction depends on temperature, orientation and number of collisions.
D) An experimentally determined equation that describes how the rate of reaction depends on temperature, orientation and number of collisions.
E) A statement that describes how the ratio of reaction depends on concentration of reactants developed from the balanced equation.
A) A theoretical equation that describes how the rate of reaction depends on the concentration of reactants.
B) An experimentally determined equation that describes how the rate of reaction depends on the concentration of reactants.
C) A theoretical equation that describes how the rate of reaction depends on temperature, orientation and number of collisions.
D) An experimentally determined equation that describes how the rate of reaction depends on temperature, orientation and number of collisions.
E) A statement that describes how the ratio of reaction depends on concentration of reactants developed from the balanced equation.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
For the reaction: C2H4Br2 + 3 KI → C2H4 + 2 KBr + KI3, when the rate of reaction is 2.0 × 10-5, what is the rate of disappearance of C2H4Br2?
A) -0.67 × 10-5
B) -2.0 × 10-5
C) -4.0 × 10-5
D) -6.0 × 10-5
E) -1.0 × 10-5
A) -0.67 × 10-5
B) -2.0 × 10-5
C) -4.0 × 10-5
D) -6.0 × 10-5
E) -1.0 × 10-5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
For a reaction with a second order rate constant, the correct unit is M ∙ time-1.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
In the Arrhenius equation, ln k = -Ea/RT + ln A, the symbol A is a constant that represents the frequency of collisions with the proper orientation and other steric conditions favorable for a reaction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
The reaction has the rate law Rate = k[A][B]2. Which will cause the rate to increase the most?
A) doubling [A]
B) lowering temperature
C) tripling [B]
D) quadrupling [A]
E) doubling [B]
A) doubling [A]
B) lowering temperature
C) tripling [B]
D) quadrupling [A]
E) doubling [B]

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: ![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol O2. At the time when N2O5 is being consumed at a rate of -1.2 × 10-4 M/s, what is the rate at which NO2 is being formed?</strong> A) 1.2 × 10-4 M/s B) 2.4 × 10-4 M/s C) 6.0 × 10-5 M/s D) 3.0 × 10-5 M/s E) 4.8 × 10-4 M/s](https://storage.examlex.com/TB2799/11eaaed0_3e85_fb78_abb0_0b444285465d_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol O2. At the time when N2O5 is being consumed at a rate of -1.2 × 10-4 M/s, what is the rate at which NO2 is being formed?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol O2. At the time when N2O5 is being consumed at a rate of -1.2 × 10-4 M/s, what is the rate at which NO2 is being formed?
A) 1.2 × 10-4 M/s
B) 2.4 × 10-4 M/s
C) 6.0 × 10-5 M/s
D) 3.0 × 10-5 M/s
E) 4.8 × 10-4 M/s
![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol O2. At the time when N2O5 is being consumed at a rate of -1.2 × 10-4 M/s, what is the rate at which NO2 is being formed?</strong> A) 1.2 × 10-4 M/s B) 2.4 × 10-4 M/s C) 6.0 × 10-5 M/s D) 3.0 × 10-5 M/s E) 4.8 × 10-4 M/s](https://storage.examlex.com/TB2799/11eaaed0_3e85_fb78_abb0_0b444285465d_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol O2. At the time when N2O5 is being consumed at a rate of -1.2 × 10-4 M/s, what is the rate at which NO2 is being formed?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol O2. At the time when N2O5 is being consumed at a rate of -1.2 × 10-4 M/s, what is the rate at which NO2 is being formed?A) 1.2 × 10-4 M/s
B) 2.4 × 10-4 M/s
C) 6.0 × 10-5 M/s
D) 3.0 × 10-5 M/s
E) 4.8 × 10-4 M/s

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
If the rate of a specific chemical reaction is independent of the concentrations of the reactants, the reaction is zero order.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
A heterogeneous catalyst is a catalyst that is in two phases of matter.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
Adding a catalyst lowers the activation energy of a reaction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) at the time when N2O5 is being consumed at a rate of -1.2 × 10-4 M/s, what is the rate at which O2 is being formed?
A) 1.2 × 10-4 M/s
B) 2.4 × 10-4 M/s
C) 6.0 × 10-5 M/s
D) 3.0 × 10-5 M/s
E) 4.8 × 10-4 M/s
A) 1.2 × 10-4 M/s
B) 2.4 × 10-4 M/s
C) 6.0 × 10-5 M/s
D) 3.0 × 10-5 M/s
E) 4.8 × 10-4 M/s

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
For the reaction: C2H4Br2 + 3 KI → C2H4 + 2 KBr + KI3, when the rate of reaction is 2.0 × 10-5, what is the rate of disappearance of KI?
A) -0.67 × 10-5
B) -2.0 × 10-5
C) -4.0 × 10-5
D) -6.0 × 10-5
E) -1.0 × 10-5
A) -0.67 × 10-5
B) -2.0 × 10-5
C) -4.0 × 10-5
D) -6.0 × 10-5
E) -1.0 × 10-5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
If a reaction has a rate equation of rate = k[A][B][C] then it is:
A) overall second order
B) overall first order
C) overall third order
D) zero order in A
E) second order in B
A) overall second order
B) overall first order
C) overall third order
D) zero order in A
E) second order in B

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
In the Arrhenius equation, ln k = -Ea/RT + ln A, the symbol A denotes the initial concentration of A.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
For the reaction: C2H4Br2 + 3 KI → C2H4 + 2 KBr + KI3, when the rate of reaction is 2.0 × 10-5, what is the rate of appearance of KBr?
A) 0.67 × 10-5
B) 2.0 × 10-5
C) 4.0 × 10-5
D) 6.0 × 10-5
E) 1.0 × 10-5
A) 0.67 × 10-5
B) 2.0 × 10-5
C) 4.0 × 10-5
D) 6.0 × 10-5
E) 1.0 × 10-5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
For a reaction Rate = k[A][B]2, what factor will keep k unchanged?
A) raising temperature
B) adding inhibitor
C) increasing [A]
D) adding catalyst
A) raising temperature
B) adding inhibitor
C) increasing [A]
D) adding catalyst

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
For the second order reaction A → products, the following data are obtained: [A] = 3.024 M, t = 0 min
[A] = 2.935 M, t = 1.0 min
[A] = 2.852 M, t = 2.0 min
What is the rate constant, k?
A) 3.6 × 10-3 M-1 min-1
B) 1.4 × 10-2 M-1 min-1
C) 2.2 × 10-2 M-1 min-1
D) 9.7 × 10-3 M-1 min-1
E) 1.0 × 10-2 M-1 min-1
[A] = 2.935 M, t = 1.0 min
[A] = 2.852 M, t = 2.0 min
What is the rate constant, k?
A) 3.6 × 10-3 M-1 min-1
B) 1.4 × 10-2 M-1 min-1
C) 2.2 × 10-2 M-1 min-1
D) 9.7 × 10-3 M-1 min-1
E) 1.0 × 10-2 M-1 min-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
For 2NO + O2 → 2NO2, initial rate data are: [NO] 0.010 0.010 0.030 M
[O2] 0.010 0.020 0.020 M
Rate 2.5 5.0 45.0 mM/sec
The rate law is Rate = k[NO]x[O2]y:
A) x = 1, y = 2
B) x = 2, y = 1
C) x = 1, y = 1
D) x = 2, y = 2
E) x = 0, y = 2
[O2] 0.010 0.020 0.020 M
Rate 2.5 5.0 45.0 mM/sec
The rate law is Rate = k[NO]x[O2]y:
A) x = 1, y = 2
B) x = 2, y = 1
C) x = 1, y = 1
D) x = 2, y = 2
E) x = 0, y = 2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements is true about the reaction 2A → B + C which is first order in A and first order overall?
A) The rate of the reaction will decrease at higher concentrations of B and C.
B) The time required for one half of A to react is directly proportional to the quantity of A.
C) The rate of formation of C is twice the rate of reaction of A.
D) The rate of formation of B is the same as the rate of reaction of A.
E) The initial rate doubles with doubling of initial concentration of A.
A) The rate of the reaction will decrease at higher concentrations of B and C.
B) The time required for one half of A to react is directly proportional to the quantity of A.
C) The rate of formation of C is twice the rate of reaction of A.
D) The rate of formation of B is the same as the rate of reaction of A.
E) The initial rate doubles with doubling of initial concentration of A.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements is INCORRECT?
A) Radioactive decay is a first order reaction.
B) Half-life in a first order reaction is constant.
C) For a first order reaction ln [A]t/[A]o = kt.
D) In gaseous reactions [A] can be expressed as concentration or as pressure.
E) In a zero order reaction the rate remains constant throughout the reaction.
A) Radioactive decay is a first order reaction.
B) Half-life in a first order reaction is constant.
C) For a first order reaction ln [A]t/[A]o = kt.
D) In gaseous reactions [A] can be expressed as concentration or as pressure.
E) In a zero order reaction the rate remains constant throughout the reaction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
The reaction A + B → C + D is second order in A and zero order in B. The value of k is 0.012 M-1 min-1. What is the rate of this reaction when [A] = 0.125 M and [B] = 0.435 M?
A) 5 × 10-4 M min-1
B) 3.4 × 10-3 M min-1
C) 1.3 M min-1
D) 1.9 × 10-4 M min-1
E) 1.5 × 10-3 M min-1
A) 5 × 10-4 M min-1
B) 3.4 × 10-3 M min-1
C) 1.3 M min-1
D) 1.9 × 10-4 M min-1
E) 1.5 × 10-3 M min-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
The rate of a specific chemical reaction is independent of the concentrations of the reactants. Thus the reaction is:
A) first order in A
B) second order
C) first order in the product
D) catalyzed
E) overall zero order
A) first order in A
B) second order
C) first order in the product
D) catalyzed
E) overall zero order

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
In a second order reaction:
I. the sum of the exponents in the rate law is equal to two.
II. at least one of the exponents in the rate law is a two.
III. the half-life is not constant.
IV. the half-life is constant.
V. k can be expressed as M-2s-1 or M-2min-1.
A) I and IV
B) II and IV
C) I, III, and V
D) I and III
E) II and III
I. the sum of the exponents in the rate law is equal to two.
II. at least one of the exponents in the rate law is a two.
III. the half-life is not constant.
IV. the half-life is constant.
V. k can be expressed as M-2s-1 or M-2min-1.
A) I and IV
B) II and IV
C) I, III, and V
D) I and III
E) II and III

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements is correct?
A) A zero order reaction depends on the concentration of reactants.
B) A reaction rate cannot be calculated from the collision frequency alone.
C) The activated complex is a chemical species that can be isolated and analysed.
D) The number of collisions has no effect on the rate constant.
E) The orientation of a collision does not affect the rate constant.
A) A zero order reaction depends on the concentration of reactants.
B) A reaction rate cannot be calculated from the collision frequency alone.
C) The activated complex is a chemical species that can be isolated and analysed.
D) The number of collisions has no effect on the rate constant.
E) The orientation of a collision does not affect the rate constant.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is FALSE for a second order reaction?
A) 1/[A]t - 1/[A]o = kt.
B) t1/2 = 1/k[A]o.
C) If 1/[A] versus time is a straight line, the reaction is second order.
D) Each successive half-life is 4 times as long as the previous.
E) The slope of 1/[A]t versus time is k.
A) 1/[A]t - 1/[A]o = kt.
B) t1/2 = 1/k[A]o.
C) If 1/[A] versus time is a straight line, the reaction is second order.
D) Each successive half-life is 4 times as long as the previous.
E) The slope of 1/[A]t versus time is k.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
For the reaction 2HgCl2 + C2O42- → products, data are: [HgCl2] 0.0836 0.0836 0.0418 M
[C2O42-] 0.202 0.404 0.404 M
Init. rate 0.26 1.04 0.53 M/hr
The rate law is Rate = [HgCl2]x[C2O42-]y. Thus:
A) x = 2, y = 1
B) x = 2, y = 2
C) x = 1, y = 2
D) x = 1, y = 1
E) x = 0, y = 2
[C2O42-] 0.202 0.404 0.404 M
Init. rate 0.26 1.04 0.53 M/hr
The rate law is Rate = [HgCl2]x[C2O42-]y. Thus:
A) x = 2, y = 1
B) x = 2, y = 2
C) x = 1, y = 2
D) x = 1, y = 1
E) x = 0, y = 2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
If increasing the concentration of A in a chemical reaction causes no increase in the rate of the reaction, then we may say:
A) A is a catalyst
B) the reaction rate is zero order in A
C) the reaction rate is zero order in [A]
D) the reaction rate is first order in [A]
E) A is not involved in the reaction
A) A is a catalyst
B) the reaction rate is zero order in A
C) the reaction rate is zero order in [A]
D) the reaction rate is first order in [A]
E) A is not involved in the reaction

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
In the first order reaction A → products, [A] = 0.400 M initially and 0.250 M after 15.0 min, what will [A] be after 175 min?
A) 1.67 × 10-3 M
B) 1.04 × 10-3 M
C) 3.70 × 10-2 M
D) 6.024 × 10-3 M
E) 2.31 × 10-1 M
A) 1.67 × 10-3 M
B) 1.04 × 10-3 M
C) 3.70 × 10-2 M
D) 6.024 × 10-3 M
E) 2.31 × 10-1 M

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
Activation energy is:
A) energy at the bottom of the reaction curve
B) the heat energy in Joules required to break the bonds in one reactant
C) an energy that a catalyst brings to the system to activate one of the reactants
D) the kinetic energy of solution stirring that brings the reaction to start
E) minimum kinetic energy that molecules must bring to their collisions for a chemical reaction to occur
A) energy at the bottom of the reaction curve
B) the heat energy in Joules required to break the bonds in one reactant
C) an energy that a catalyst brings to the system to activate one of the reactants
D) the kinetic energy of solution stirring that brings the reaction to start
E) minimum kinetic energy that molecules must bring to their collisions for a chemical reaction to occur

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
Data for the reaction A + B → C are given below. Find the rate constant for this system. Experiment [A], M [B], M Initial rate, M/s
1 0)030 0.060 2.5 × 10-5
2 0)030 0.020 2.5 × 10-5
3 0)060 0.060 10.0 × 10-5
A) 2.8 × 10-2 M-1s-1
B) 2.8 × 10-2 Ms-1
C) 2.8 × 10-2 M2s-1
D) 1.7 × 10-3 M-1s-1
E) 1.7 × 10-3 Ms-1
1 0)030 0.060 2.5 × 10-5
2 0)030 0.020 2.5 × 10-5
3 0)060 0.060 10.0 × 10-5
A) 2.8 × 10-2 M-1s-1
B) 2.8 × 10-2 Ms-1
C) 2.8 × 10-2 M2s-1
D) 1.7 × 10-3 M-1s-1
E) 1.7 × 10-3 Ms-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate rate constant k for a first order reaction with a half-life of 75.0 min.
A) 52.0 min-1
B) 1.54 × 10-4 min-1
C) 1.33 × 10-2 min-1
D) 9.24 × 10-3 min-1
E) 2.67 × 10-2 min-1
A) 52.0 min-1
B) 1.54 × 10-4 min-1
C) 1.33 × 10-2 min-1
D) 9.24 × 10-3 min-1
E) 2.67 × 10-2 min-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
Choose the INCORRECT statement.
A) A reaction intermediate is produced and used up during the reaction.
B) A transition state and a reaction intermediate are the same.
C) An activated complex has partially formed bonds.
D) A reaction intermediates have fully formed bonds.
E) The rate-determining step is the slow-step.
A) A reaction intermediate is produced and used up during the reaction.
B) A transition state and a reaction intermediate are the same.
C) An activated complex has partially formed bonds.
D) A reaction intermediates have fully formed bonds.
E) The rate-determining step is the slow-step.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following has no effect on the rate of a reaction?
A) value of ΔH°
B) activation energy
C) presence of a catalyst
D) temperature of reactants
E) concentrations of reactants
A) value of ΔH°
B) activation energy
C) presence of a catalyst
D) temperature of reactants
E) concentrations of reactants

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Choose the INCORRECT answer. The rate of a chemical reaction:
A) usually is increased when the concentration of one of the reactants is increased
B) is dependent on temperature
C) may be increased by certain catalytic agents
D) will be very rapid if the activation energy is large
E) describes the change in concentration of a reactant or product with time
A) usually is increased when the concentration of one of the reactants is increased
B) is dependent on temperature
C) may be increased by certain catalytic agents
D) will be very rapid if the activation energy is large
E) describes the change in concentration of a reactant or product with time

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
For the second order reaction A → products, the following data are obtained: [A] = 1.512 M, t = 0 min
[A] = 1.490 M, t = 1.0 min
[A] = 1.469 M, t = 2.0 min
What is the rate constant, k, for the reaction?
A) 3.6 × 10-3 M-1 min-1
B) 1.4 × 10-2 M-1 min-1
C) 2.2 × 10-2 M-1 min-1
D) 1.0 × 10-2 M-1 min-1
E) 9.7 × 10-3 M-1 min-1
[A] = 1.490 M, t = 1.0 min
[A] = 1.469 M, t = 2.0 min
What is the rate constant, k, for the reaction?
A) 3.6 × 10-3 M-1 min-1
B) 1.4 × 10-2 M-1 min-1
C) 2.2 × 10-2 M-1 min-1
D) 1.0 × 10-2 M-1 min-1
E) 9.7 × 10-3 M-1 min-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
Define activation energy.
A) the difference between the energy of the products and reactants
B) the energy difference between the maximum energy of reaction and the energy of the products
C) the minimum total kinetic energy that molecules must bring to their collisions for a chemical reaction to occur
D) the total kinetic energy of molecules in collisions
E) the total kinetic energy of molecules in a system
A) the difference between the energy of the products and reactants
B) the energy difference between the maximum energy of reaction and the energy of the products
C) the minimum total kinetic energy that molecules must bring to their collisions for a chemical reaction to occur
D) the total kinetic energy of molecules in collisions
E) the total kinetic energy of molecules in a system

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement is INCORRECT?
A) Activation energy is the same for forward and reverse reaction.
B) If the forward reaction is endothermic, the reverse will be exothermic.
C) In an endothermic reaction, activation energy is usually greater than the enthalpy.
D) An activated complex has higher energy than any molecule contributing to it.
E) The activated complex will be the highest on the energy profile.
A) Activation energy is the same for forward and reverse reaction.
B) If the forward reaction is endothermic, the reverse will be exothermic.
C) In an endothermic reaction, activation energy is usually greater than the enthalpy.
D) An activated complex has higher energy than any molecule contributing to it.
E) The activated complex will be the highest on the energy profile.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following lowers the activation energy of a reaction?
A) adding reactants
B) lowering the temperature
C) removing products
D) adding a catalyst
E) raising the temperature
A) adding reactants
B) lowering the temperature
C) removing products
D) adding a catalyst
E) raising the temperature

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
What is the rate constant at 305 K for the reaction: 2N2O5 → 2N2O4 + O2,
If k = 3.46 × 10-5 s-1 at 298 K and Ea = 106 kJ/mol?
A) 2.4 × 10-5 s-1
B) 4.8 × 10-5 s-1
C) 6.0 × 10-5 s-1
D) 1.2 × 10-5 s-1
E) 9.2 × 10-5 s-1
If k = 3.46 × 10-5 s-1 at 298 K and Ea = 106 kJ/mol?
A) 2.4 × 10-5 s-1
B) 4.8 × 10-5 s-1
C) 6.0 × 10-5 s-1
D) 1.2 × 10-5 s-1
E) 9.2 × 10-5 s-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
What is the rate law for the following mechanism? N2O + NO → N2ONO (Slow)
N2ONO → N2 + NO2 (Fast)
A) Rate = k[N2O]
B) Rate = k[NO]
C) Rate = k[N2O][NO]
D) Rate = k[N2][NO2]
E) Rate = k[N2ONO]
N2ONO → N2 + NO2 (Fast)
A) Rate = k[N2O]
B) Rate = k[NO]
C) Rate = k[N2O][NO]
D) Rate = k[N2][NO2]
E) Rate = k[N2ONO]

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
In the Arrhenius equation, ln k = -Ea/RT + ln A, the symbol A denotes:
A) the initial concentration of A
B) the activation energy
C) the rate constant
D) a constant that represents the frequency of collisions with the proper orientation and other steric conditions favorable for a reaction
E) the absolute temperature
A) the initial concentration of A
B) the activation energy
C) the rate constant
D) a constant that represents the frequency of collisions with the proper orientation and other steric conditions favorable for a reaction
E) the absolute temperature

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: ![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the half-life at 310 K?</strong> A) 2.49 × 104 s B) 9.51 × 104 s C) 9.51 × 106 s D) 6.57 × 103 s E) 1.87× 10-1 s](https://storage.examlex.com/TB2799/11eaaed0_3e87_0ceb_abb0_fbb87d9d1830_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the half-life at 310 K?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the half-life at 310 K?
A) 2.49 × 104 s
B) 9.51 × 104 s
C) 9.51 × 106 s
D) 6.57 × 103 s
E) 1.87× 10-1 s
![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the half-life at 310 K?</strong> A) 2.49 × 104 s B) 9.51 × 104 s C) 9.51 × 106 s D) 6.57 × 103 s E) 1.87× 10-1 s](https://storage.examlex.com/TB2799/11eaaed0_3e87_0ceb_abb0_fbb87d9d1830_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the half-life at 310 K?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the half-life at 310 K?A) 2.49 × 104 s
B) 9.51 × 104 s
C) 9.51 × 106 s
D) 6.57 × 103 s
E) 1.87× 10-1 s

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Which is a plausible mechanism for the following reaction if the rate law is Rate = k[O3]2/[O2]? 2O3 → 3O2
A) 2O3 → O2 + O4 (Slow) O4 → 2O2 (Fast)
B) 2O3 → O6 (Slow) O6 → O4 + O2 (Fast)
O4 → 2O2 (Slow)
C) 2O3 → O4- + O2+ (Slow) O4- → O2 + O2- (Fast)
O2- + O2+ → 2O2 (Slow)
D) O3 ⇌ O2 + O∙ (Fast) O∙ + O3 → 2O2 (Slow)
E) O3 ⇌ O2+ + O- (Fast) O- + O3 → O2 + O2- (Slow)
O2- + O2+ → 2O2 (Fast)
A) 2O3 → O2 + O4 (Slow) O4 → 2O2 (Fast)
B) 2O3 → O6 (Slow) O6 → O4 + O2 (Fast)
O4 → 2O2 (Slow)
C) 2O3 → O4- + O2+ (Slow) O4- → O2 + O2- (Fast)
O2- + O2+ → 2O2 (Slow)
D) O3 ⇌ O2 + O∙ (Fast) O∙ + O3 → 2O2 (Slow)
E) O3 ⇌ O2+ + O- (Fast) O- + O3 → O2 + O2- (Slow)
O2- + O2+ → 2O2 (Fast)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
What is the rate law for the following reaction and its mechanism? 2O3 → 3O2 (overall reaction)
O3 → O2 + Oσ (Slow)
O∙ + O3 → 2O2 (Fast)
A) Rate = k[O3]
B) Rate = k[O3]2
C) Rate = k[O3]2/[O2]
D) Rate = k[O3]/[O2]
E) Rate = k[O3][O2]
O3 → O2 + Oσ (Slow)
O∙ + O3 → 2O2 (Fast)
A) Rate = k[O3]
B) Rate = k[O3]2
C) Rate = k[O3]2/[O2]
D) Rate = k[O3]/[O2]
E) Rate = k[O3][O2]

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
What is the rate law for the following reaction and its mechanism? 2HgCl2 + C2O42- → 2Cl- + 2CO2 + Hg2Cl2 (overall reaction)
HgCl2 + C2O42- ⇌ HgCl2C2O42- (Fast)
HgCl2C2O42- + C2O42- → Hg + 2C2O4Cl2- (Slow)
Hg + HgCl2 → Hg2Cl2 (Fast)
2C2O4Cl2- → C2O42- + 2Cl- + 2CO2 (Fast)
A) Rate = k[HgCl2][C2O42-]
B) Rate = k[HgCl2]2[C2O42-]
C) Rate = k[Hg2Cl2]
D) Rate = k[HgCl2][C2O42-]2
E) Rate = k[HgCl2]2[C2O42-]2
HgCl2 + C2O42- ⇌ HgCl2C2O42- (Fast)
HgCl2C2O42- + C2O42- → Hg + 2C2O4Cl2- (Slow)
Hg + HgCl2 → Hg2Cl2 (Fast)
2C2O4Cl2- → C2O42- + 2Cl- + 2CO2 (Fast)
A) Rate = k[HgCl2][C2O42-]
B) Rate = k[HgCl2]2[C2O42-]
C) Rate = k[Hg2Cl2]
D) Rate = k[HgCl2][C2O42-]2
E) Rate = k[HgCl2]2[C2O42-]2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Activation energy is: I) the minimum kinetic energy required for each molecule in a collision to produce a reaction.
II) the minimum total kinetic energy required for the molecules in a collision to produce a reaction.
III) a factor in determining the rate of a reaction.
IV) high for fast reactions.
A) I and III
B) I, III, and IV
C) II, III
D) II and IV
E) II, III, and IV
II) the minimum total kinetic energy required for the molecules in a collision to produce a reaction.
III) a factor in determining the rate of a reaction.
IV) high for fast reactions.
A) I and III
B) I, III, and IV
C) II, III
D) II and IV
E) II, III, and IV

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
What is the main difference between a Pt catalyst and an enzyme catalyst?
A) An enzyme has greater substrate specificity.
B) The Pt catalyst causes a faster reaction.
C) The enzyme causes a faster reaction.
D) A Pt catalyst is always a homogeneous catalyst.
E) A Pt catalyst is an enzyme.
A) An enzyme has greater substrate specificity.
B) The Pt catalyst causes a faster reaction.
C) The enzyme causes a faster reaction.
D) A Pt catalyst is always a homogeneous catalyst.
E) A Pt catalyst is an enzyme.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
Choose the INCORRECT statement.
A) The rate-determining step is always the first step.
B) A unimolecular process is one in which a single molecule dissociates.
C) A bimolecular process is one involving a collision of two molecules.
D) A reaction mechanism is a step-by-step detailed description of a chemical reaction.
E) An elementary process is a step in the mechanism.
A) The rate-determining step is always the first step.
B) A unimolecular process is one in which a single molecule dissociates.
C) A bimolecular process is one involving a collision of two molecules.
D) A reaction mechanism is a step-by-step detailed description of a chemical reaction.
E) An elementary process is a step in the mechanism.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
What is the rate law for the following mechanism? CH3COOC2H5 + H2O → CH3COOC2H6+ + OH- (Slow)
CH3COOC2H6+ → CH3COOH + C2H5+ (Fast)
C2H5+ + OH- → C2H5OH (Fast)
A) Rate = k[CH3COOC2H5][H2O]2
B) Rate = k[C2H5OH]
C) Rate = k[CH3COOH]
D) Rate = k[CH3COOC2H5]
E) Rate = k[CH3COOC2H5][H2O]
CH3COOC2H6+ → CH3COOH + C2H5+ (Fast)
C2H5+ + OH- → C2H5OH (Fast)
A) Rate = k[CH3COOC2H5][H2O]2
B) Rate = k[C2H5OH]
C) Rate = k[CH3COOH]
D) Rate = k[CH3COOC2H5]
E) Rate = k[CH3COOC2H5][H2O]

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
Why is rate = k[HgCl2] 2[C2O42-] not the rate law for the following mechanism? 2HgCl2 + C2O42- → 2Cl- + 2CO2 + Hg2Cl2 (overall reaction)
HgCl2 + C2O42- ⇌ HgCl2C2O42- (Fast)
HgCl2C2O42- + C2O42- → Hg + 2C2O4Cl2- (Slow)
Hg + HgCl2 → Hg2Cl2 (Fast)
2C2O4Cl2- → C2O42- + 2Cl- + 2CO2 (Fast)
A) The rate law is not based on the slow step of the proposed mechanism.
B) The steps do not add to the overall reaction.
C) The rate law does not agree with the overall reaction.
D) The exponents of HgCl2 and C2O42- are not equal.
E) The first step is not the slow step.
HgCl2 + C2O42- ⇌ HgCl2C2O42- (Fast)
HgCl2C2O42- + C2O42- → Hg + 2C2O4Cl2- (Slow)
Hg + HgCl2 → Hg2Cl2 (Fast)
2C2O4Cl2- → C2O42- + 2Cl- + 2CO2 (Fast)
A) The rate law is not based on the slow step of the proposed mechanism.
B) The steps do not add to the overall reaction.
C) The rate law does not agree with the overall reaction.
D) The exponents of HgCl2 and C2O42- are not equal.
E) The first step is not the slow step.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: ![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 350 K?</strong> A) 4.78 s-1 B) 2.79 × 10-5 s-1 C) 6.38 × 1016 s-1 D) 7.47 × 10-8 s-1 E) 1.03 × 10-2 s-1](https://storage.examlex.com/TB2799/11eaaed0_3e86_e5da_abb0_1765c2e97a73_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 350 K?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 350 K?
A) 4.78 s-1
B) 2.79 × 10-5 s-1
C) 6.38 × 1016 s-1
D) 7.47 × 10-8 s-1
E) 1.03 × 10-2 s-1
![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 350 K?</strong> A) 4.78 s-1 B) 2.79 × 10-5 s-1 C) 6.38 × 1016 s-1 D) 7.47 × 10-8 s-1 E) 1.03 × 10-2 s-1](https://storage.examlex.com/TB2799/11eaaed0_3e86_e5da_abb0_1765c2e97a73_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 350 K?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 350 K?A) 4.78 s-1
B) 2.79 × 10-5 s-1
C) 6.38 × 1016 s-1
D) 7.47 × 10-8 s-1
E) 1.03 × 10-2 s-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
A catalyst alters the rate of a chemical reaction by:
A) always providing a surface on which molecules react
B) changing the products formed in the reaction
C) inducing an alternate pathway for the reaction with generally lower activation energy
D) changing the frequency of collisions between molecules
E) increasing the number of collisions of molecules
A) always providing a surface on which molecules react
B) changing the products formed in the reaction
C) inducing an alternate pathway for the reaction with generally lower activation energy
D) changing the frequency of collisions between molecules
E) increasing the number of collisions of molecules

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
A factor that decreases the activation energy for a reaction:
I. decreases the rate constant
II. increases the rate constant
III. has no effect on the rate constant
IV. makes the product yield increase
V. might be a catalyst
A) I and IV
B) II and IV
C) I, IV, and V
D) IV and III
E) II and V
I. decreases the rate constant
II. increases the rate constant
III. has no effect on the rate constant
IV. makes the product yield increase
V. might be a catalyst
A) I and IV
B) II and IV
C) I, IV, and V
D) IV and III
E) II and V

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
According to the collision theory in gaseous molecules, collision frequency is ________ and reaction is ________ because ________.
A) low, low, molecules are so far apart
B) high, high, each collision results in a reaction
C) low, low, molecules must collide before they can react
D) high, relatively low, only a fraction of the collisions lead to a reaction
E) low, high, molecules are moving so fast that each reaction causes many others
A) low, low, molecules are so far apart
B) high, high, each collision results in a reaction
C) low, low, molecules must collide before they can react
D) high, relatively low, only a fraction of the collisions lead to a reaction
E) low, high, molecules are moving so fast that each reaction causes many others

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: ![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 310 K?</strong> A) 2.78 × 10-5 s-1 B) 7.29 × 10-6 s-1 C) 7.29 × 10-8 s-1 D) 3.70 × 10-5 s-1 E) 1.05 × 10-4 s-1](https://storage.examlex.com/TB2799/11eaaed0_3e86_e5d9_abb0_b5100ff474de_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 310 K?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 310 K?
A) 2.78 × 10-5 s-1
B) 7.29 × 10-6 s-1
C) 7.29 × 10-8 s-1
D) 3.70 × 10-5 s-1
E) 1.05 × 10-4 s-1
![<strong>For the reaction: 2N2O5(g) → 4NO2(g) + O2(g) the rate law is: = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 310 K?</strong> A) 2.78 × 10-5 s-1 B) 7.29 × 10-6 s-1 C) 7.29 × 10-8 s-1 D) 3.70 × 10-5 s-1 E) 1.05 × 10-4 s-1](https://storage.examlex.com/TB2799/11eaaed0_3e86_e5d9_abb0_b5100ff474de_TB2799_11.jpg) = k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 310 K?
= k[N2O5] At 300 K, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol. What is the rate constant at 310 K?A) 2.78 × 10-5 s-1
B) 7.29 × 10-6 s-1
C) 7.29 × 10-8 s-1
D) 3.70 × 10-5 s-1
E) 1.05 × 10-4 s-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
Given the following initial rate data, calculate the specific rate constant. 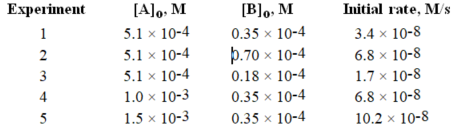
A) 1.9 M2/s2
B) 1.9 M-1∙ s-1
C) 3.6 M2s-1
D) 1.1 × 108 M2/s2
E) 3.6 M2s-1.

A) 1.9 M2/s2
B) 1.9 M-1∙ s-1
C) 3.6 M2s-1
D) 1.1 × 108 M2/s2
E) 3.6 M2s-1.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Given the following initial rate data, write the rate law expression. Experiment [A]o, M [B]o, M Initial rate, M/s
1 5)1 × 10-4 0.35 × 10-4 3.4 × 10-8
2 5)1 × 10-4 0.70 × 10-4 6.8 × 10-8
3 5)1 × 10-4 0.18 × 10-4 1.7 × 10-8
4 1)0 × 10-3 0.35 × 10-4 6.8 × 10-8
5 1)5 × 10-3 0.35 × 10-4 10.2 × 10-8
A) k [A] [B]
B) k [A]2 [B]2
C) k [A]2 [B]
D) k [B]2
E) k [A]2
1 5)1 × 10-4 0.35 × 10-4 3.4 × 10-8
2 5)1 × 10-4 0.70 × 10-4 6.8 × 10-8
3 5)1 × 10-4 0.18 × 10-4 1.7 × 10-8
4 1)0 × 10-3 0.35 × 10-4 6.8 × 10-8
5 1)5 × 10-3 0.35 × 10-4 10.2 × 10-8
A) k [A] [B]
B) k [A]2 [B]2
C) k [A]2 [B]
D) k [B]2
E) k [A]2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
A catalyst:
I. lowers activation energy
II. provides an alternate reaction pathway
III. is consumed in the reaction and therefore does not appear
In the chemical equation of each mechanism
IV. speeds a reaction
V. is heterogeneous if it is in a different phase than the reactants
A) I, III, and IV
B) I, IV, and V
C) II, III, and IV
D) II and IV
E) I, II, IV, and V
I. lowers activation energy
II. provides an alternate reaction pathway
III. is consumed in the reaction and therefore does not appear
In the chemical equation of each mechanism
IV. speeds a reaction
V. is heterogeneous if it is in a different phase than the reactants
A) I, III, and IV
B) I, IV, and V
C) II, III, and IV
D) II and IV
E) I, II, IV, and V

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
If a reaction is first order with a rate constant of 5.48 × 10-2/sec, how long is required for 3/4 of the initial concentration of reactant to be used up?
A) 25.3 sec
B) 36.5 sec
C) 6.3 sec
D) 18.2 sec
E) 50.6 sec
A) 25.3 sec
B) 36.5 sec
C) 6.3 sec
D) 18.2 sec
E) 50.6 sec

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
The first order reaction A → products has t1/2 = 150 sec. What percent of the sample remains unreacted after 300 sec?
A) 25%
B) 50%
C) 12.5%
D) 0.0%
E) 100%
A) 25%
B) 50%
C) 12.5%
D) 0.0%
E) 100%

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
The rate constant at 160 °C for the first order decomposition of ore is 0.032/min. The half-life of the reaction is ________.
A) 62.5 sec
B) 31.25 sec
C) 5000 sec
D) 111 sec
E) 1300 sec
A) 62.5 sec
B) 31.25 sec
C) 5000 sec
D) 111 sec
E) 1300 sec

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
The rate constant for a first-order reaction is k = 0.00073 s-1. Determine the percent of reactant that has decomposed after 500 s.
A) 69%
B) 57%
C) 37%
D) 31%
E) 43%
A) 69%
B) 57%
C) 37%
D) 31%
E) 43%

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
For the reaction: C2H4Br2 + 3KI → C2H4 + 2KBr + KI3 Initial rate data at 60°C are:
[C2H4Br2], M [KI], M Δ[KI3]/Δt (M min)
0)500 1.80 0.269
0)500 7.20 1.08
1)50 1.80 0.807
The rate law is ________.
A) rate = k[KI]
B) rate = k[C2H4Br2]
C) rate = k[KI]2
D) rate = k[KI][C2H4Br2]
E) rate = k[KI][C2H4Br2]2
[C2H4Br2], M [KI], M Δ[KI3]/Δt (M min)
0)500 1.80 0.269
0)500 7.20 1.08
1)50 1.80 0.807
The rate law is ________.
A) rate = k[KI]
B) rate = k[C2H4Br2]
C) rate = k[KI]2
D) rate = k[KI][C2H4Br2]
E) rate = k[KI][C2H4Br2]2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
What is the order of reaction for the following reaction: Rate = k[A]-1/2 [B]1/2 ?
A) first order
B) zero order
C) 1/2 order
D) -1/2 order
E) second order
A) first order
B) zero order
C) 1/2 order
D) -1/2 order
E) second order

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
The reaction 2H2 + NO → H2O + 1/2N2 is first order in H2 and second order in NO. The rate law is ________.
A) k[H2]2[NO]
B) k[H2][NO]2
C) k[H2]
D) k[H2][NO]
E) k[H2][NO]-2
A) k[H2]2[NO]
B) k[H2][NO]2
C) k[H2]
D) k[H2][NO]
E) k[H2][NO]-2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
The correct units of the specific rate constant for a zero order reaction are ________.
A) L/mol-sec
B) sec-1
C) sec
D) L2/mol2sec
E) rate is a constant, so it has no units
A) L/mol-sec
B) sec-1
C) sec
D) L2/mol2sec
E) rate is a constant, so it has no units

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
In the first-order, reaction A → products, [A] = 0.400 M initially and 0.250 M after 15.0 min, what is the value of the rate constant, k?
A) 3.06 min-1
B) 0.0136 min-1
C) -0.0313 min-1
D) 7.05 min-1
E) 0.0313 min-1
A) 3.06 min-1
B) 0.0136 min-1
C) -0.0313 min-1
D) 7.05 min-1
E) 0.0313 min-1

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
If the half-life of a reactant is independent of its initial concentration, the reaction order is ________.
A) 0
B) 0.5
C) 1
D) 2
E) 3
A) 0
B) 0.5
C) 1
D) 2
E) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
Substance A decomposes by a first-order reaction. Starting initially with [A] = 2.00 M, after 150 min [A] = 0.50 M. For this reaction what is t1/2?
A) 150 min
B) 37.5 min
C) 75.0 min
D) 15.0 min
E) 300 min
A) 150 min
B) 37.5 min
C) 75.0 min
D) 15.0 min
E) 300 min

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
If a catalyst is added to a reaction:
I. the value of k is increased
II. the value of k is decreased
III. the rate is increased
IV. the rate is decreased
V. neither rate nor the constant are changed, only the order
A) I and IV
B) II and IV
C) II and III
D) I and III
E) V only
I. the value of k is increased
II. the value of k is decreased
III. the rate is increased
IV. the rate is decreased
V. neither rate nor the constant are changed, only the order
A) I and IV
B) II and IV
C) II and III
D) I and III
E) V only

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
If the half-life of a reaction depends on the concentration of the reactant, then the reaction cannot be ________ order.
A) second
B) zero
C) first
D) third
A) second
B) zero
C) first
D) third

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
The rate data from a chemical reaction shows that doubling the concentration of A with the concentration of B remaining constant causes the rate to increase by a factor of four. What is the reaction order for [A]?
A) 0
B) 0.5
C) 1
D) 2
E) 3
A) 0
B) 0.5
C) 1
D) 2
E) 3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following situations involves a heterogeneous catalysis?
A) The catalyst is present in a different phase of matter than are the reactants and products.
B) The catalyst is in two different phases of matter.
C) The reactants and products are different phases of matter in a catalyzed reaction.
D) The catalyst, reactants, and products are all different phases of matter.
E) The catalyst changes phases during the reaction.
A) The catalyst is present in a different phase of matter than are the reactants and products.
B) The catalyst is in two different phases of matter.
C) The reactants and products are different phases of matter in a catalyzed reaction.
D) The catalyst, reactants, and products are all different phases of matter.
E) The catalyst changes phases during the reaction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
Data for the reaction A + B → C are given below. What is the correct rate law? Experiment [A], M [B], M Initial rate, M/s
1 0)030 0.060 2.5 × 10-5
2 0)030 0.020 2.5 × 10-5
3 0)060 0.060 10.0 × 10-5
A) k [A] [B]
B) k [A]2 [B]
C) k [A]2 [B]2
D) k [B]2
E) k[A]2
1 0)030 0.060 2.5 × 10-5
2 0)030 0.020 2.5 × 10-5
3 0)060 0.060 10.0 × 10-5
A) k [A] [B]
B) k [A]2 [B]
C) k [A]2 [B]2
D) k [B]2
E) k[A]2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
A reaction is first order. If its initial rate is 0.0200 M/s and 25.0 days later its rate is 6.25 × 10-4 M/s, then its half-life is ________.
A) 12.5 days
B) 5.0 days
C) 25.0 days
D) 50.0 days
E) 2.5 days
A) 12.5 days
B) 5.0 days
C) 25.0 days
D) 50.0 days
E) 2.5 days

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck


