Deck 11: Chemical Bonding II: Additional Aspects
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/97
Play
Full screen (f)
Deck 11: Chemical Bonding II: Additional Aspects
1
Resonance structures and delocalized orbitals are different ways to describe the same bonding.
True
2
Unhybridized p orbitals must be present for electron delocalization to occur.
True
3
A triple bond is two sigma bonds and one pi bond.
False
4
Which statement is correct for the structure shown? 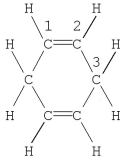
A) The molecule contains a total of 16 σ bonds.
B) Carbon no. 1 is described by sp hybridization.
C) The molecule contains a total of four π bonds.
D) Carbon no. 3 is described by sp3 hybridization.
E) The molecule contains a delocalized π bond system.

A) The molecule contains a total of 16 σ bonds.
B) Carbon no. 1 is described by sp hybridization.
C) The molecule contains a total of four π bonds.
D) Carbon no. 3 is described by sp3 hybridization.
E) The molecule contains a delocalized π bond system.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
5
The description of covalent bond formation as a region of high electronic charge density resulting from overlap of atomic orbitals between the two bonded atoms is referred to as:
A) atomic orbital theory
B) the electron-sea model
C) hybridization theory
D) valence-bond method
E) VSEPR method
A) atomic orbital theory
B) the electron-sea model
C) hybridization theory
D) valence-bond method
E) VSEPR method

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
6
Molecular orbitals are formed by adding and subtracting atomic orbitals.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
7
Band theory describes the properties of metals and insulators but not semiconductors.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements concerning the carbon dioxide molecule is correct?
A) The molecule contains two lone pairs of valence electrons.
B) The molecule contains two σ bonds.
C) The molecule contains four π bonds.
D) The carbon is described by sp2 hybridization.
E) Each oxygen is described by sp3 hybridization.
A) The molecule contains two lone pairs of valence electrons.
B) The molecule contains two σ bonds.
C) The molecule contains four π bonds.
D) The carbon is described by sp2 hybridization.
E) Each oxygen is described by sp3 hybridization.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
9
A double bond is two sigma bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
10
The electrical conductivity of semiconductors increases with increasing temperature.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
11
According to principles of VSEPR theory applied on AsCl52-, which of the following is INCORRECT?
A) VSEPR formula = AX5E
B) molecular geometry = square planar
C) electron pair geometry = octahedral
D) hybridization = sp3d2
E) one lone pair and 5 bonding pairs
A) VSEPR formula = AX5E
B) molecular geometry = square planar
C) electron pair geometry = octahedral
D) hybridization = sp3d2
E) one lone pair and 5 bonding pairs

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
12
Which statement regarding VB theory is INCORRECT?
A) d orbitals are not included in hybrid orbitals.
B) Hybrid orbitals use atomic orbitals from the outermost shell.
C) Sigma bonds are end-to-end overlaps.
D) Pi bonds are overlaps of parallel p orbitals.
E) A double bond is a sigma bond and a pi bond.
A) d orbitals are not included in hybrid orbitals.
B) Hybrid orbitals use atomic orbitals from the outermost shell.
C) Sigma bonds are end-to-end overlaps.
D) Pi bonds are overlaps of parallel p orbitals.
E) A double bond is a sigma bond and a pi bond.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
13
Which statement is INCORRECT?
A) The bond length is the internuclear distance.
B) The covalent bond is a region of high electron charge density between two atoms.
C) Hybrid orbitals are combinations of atomic orbitals.
D) There are the same number of hybrid orbitals produced as the number of atomic orbitals combined.
E) An sp hybridization produces a tetrahedral molecule.
A) The bond length is the internuclear distance.
B) The covalent bond is a region of high electron charge density between two atoms.
C) Hybrid orbitals are combinations of atomic orbitals.
D) There are the same number of hybrid orbitals produced as the number of atomic orbitals combined.
E) An sp hybridization produces a tetrahedral molecule.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
14
In extrinsic semiconductors, the band width is set by the element and cannot be changed.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
15
The valence-bond method describes covalent bonding as the overlap of partially filled orbitals.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
16
According to the principles of VSEPR applied on ICl5, which of the following is INCORRECT?
A) VSEPR formula = AX5E
B) molecular geometry = square pyramid
C) hybridization = sp3d
D) electron pair geometry = octahedral
E) one lone pair and 5 bonding pairs
A) VSEPR formula = AX5E
B) molecular geometry = square pyramid
C) hybridization = sp3d
D) electron pair geometry = octahedral
E) one lone pair and 5 bonding pairs

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
17
Which statement is correct for the structure shown? 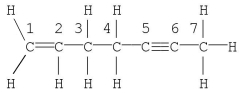
A) Carbon no. 1 is described by sp3 hybridization.
B) The molecule contains 19 σ bonds.
C) Carbon no. 2 is described by sp2 hybridization.
D) The molecule contains a total of five π bonds.
E) Carbon no. 7 is described by sp hybridization.

A) Carbon no. 1 is described by sp3 hybridization.
B) The molecule contains 19 σ bonds.
C) Carbon no. 2 is described by sp2 hybridization.
D) The molecule contains a total of five π bonds.
E) Carbon no. 7 is described by sp hybridization.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
18
The valence-bond method provides energy information about molecules.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
19
In a semiconductor, the energy gap between the valence band and the conduction band is large.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the pairs of molecules below have the same hybridization on the central atom? (The central atom is underlined.)
A) HOCl, ClF3
B) H2O, HNO
C) HCN, CO2
D) BeH2, NH3
E) H3COH, CH2O
A) HOCl, ClF3
B) H2O, HNO
C) HCN, CO2
D) BeH2, NH3
E) H3COH, CH2O

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the pairs of molecules below have the same hybridization on the central atom? (The central atom is underlined in each molecule.)
A) CO2, CH4
B) H2CO, BeH2
C) BCl3, HNO
D) H2O, HF
E) NH3, HNO
A) CO2, CH4
B) H2CO, BeH2
C) BCl3, HNO
D) H2O, HF
E) NH3, HNO

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
22
According to VB theory, sigma bonds are:
A) always present in double bonds
B) formed only by p orbitals
C) formed only by hybrid orbitals
D) formed only by s orbitals
E) formed only by unhybridized orbitals
A) always present in double bonds
B) formed only by p orbitals
C) formed only by hybrid orbitals
D) formed only by s orbitals
E) formed only by unhybridized orbitals

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
23
A pi bond is:
A) formed from two s orbitals
B) formed from two p orbitals
C) formed from hybridized orbitals
D) formed from one s and one p orbital
E) stronger than a sigma bond
A) formed from two s orbitals
B) formed from two p orbitals
C) formed from hybridized orbitals
D) formed from one s and one p orbital
E) stronger than a sigma bond

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
24
Choose the INCORRECT statement about NH2-.
A) There are no π bonds.
B) There are two σ bonds.
C) N is sp3 hybridization.
D) The molecule is bent.
E) There is one lone pair on N.
A) There are no π bonds.
B) There are two σ bonds.
C) N is sp3 hybridization.
D) The molecule is bent.
E) There is one lone pair on N.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the INCORRECT statement about H3O+.
A) There is one π bond.
B) There are 3 σ bonds.
C) There is one lone pair on O.
D) The hybridization on O is sp3.
E) The OH bonds are O(sp3) - H(1s).
A) There is one π bond.
B) There are 3 σ bonds.
C) There is one lone pair on O.
D) The hybridization on O is sp3.
E) The OH bonds are O(sp3) - H(1s).

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
26
Which statement regarding VB theory is INCORRECT?
A) A single bond is a sigma bond.
B) A triple bond is two sigma bonds and a pi bond.
C) A double bond requires each atom to have an unhybridized p orbital.
D) Both atoms in a triple bond have linear geometry.
E) sp3 hybridization produces tetrahedral geometry.
A) A single bond is a sigma bond.
B) A triple bond is two sigma bonds and a pi bond.
C) A double bond requires each atom to have an unhybridized p orbital.
D) Both atoms in a triple bond have linear geometry.
E) sp3 hybridization produces tetrahedral geometry.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
27
A σ bond:
A) can only be formed by s orbitals
B) is weaker than a pi bond
C) has high electron probability along the axis between atoms
D) cannot be formed by two p orbitals
E) is formed through side-on overlap of two atomic orbitals
A) can only be formed by s orbitals
B) is weaker than a pi bond
C) has high electron probability along the axis between atoms
D) cannot be formed by two p orbitals
E) is formed through side-on overlap of two atomic orbitals

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following carbon molecules has sp hybridization?
A) CH4
B) C2H2
C) Cl2CS
D) CH3-
E) CO2
A) CH4
B) C2H2
C) Cl2CS
D) CH3-
E) CO2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
29
Find the correct statements about the bonding in methane, CH4. I) The carbon s and p orbitals combine to form four equivalent sp3 orbitals
II) All C-H bonds have the same strength
III) Molecular geometry is different from electron group geometry
IV) Four C sp3 orbitals combine with the s orbitals of the hydrogens to form bonds
A) I), II), III)
B) I), II), IV)
C) II), III), IV)
D) I), III) IV)
E) I), II)
II) All C-H bonds have the same strength
III) Molecular geometry is different from electron group geometry
IV) Four C sp3 orbitals combine with the s orbitals of the hydrogens to form bonds
A) I), II), III)
B) I), II), IV)
C) II), III), IV)
D) I), III) IV)
E) I), II)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
30
Choose the INCORRECT statement about SnCl2.
A) The hybridization of Sn is sp2.
B) The molecule is linear.
C) There is one lone pair of electrons on Sn.
D) There are no π bonds.
E) There are two σ bonds.
A) The hybridization of Sn is sp2.
B) The molecule is linear.
C) There is one lone pair of electrons on Sn.
D) There are no π bonds.
E) There are two σ bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the INCORRECT statement about HCN.
A) There are two π bonds.
B) There are two σ bonds.
C) N has a lone pair.
D) The molecule is bent.
E) C has sp hybridization.
A) There are two π bonds.
B) There are two σ bonds.
C) N has a lone pair.
D) The molecule is bent.
E) C has sp hybridization.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
32
For the molecule 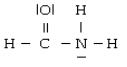
A) the hybridization for C is sp3
B) the hybridization for N is sp2
C) the hybridization for O is sp3
D) N is not hybridized
E) the hybridization for C is sp2
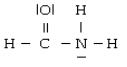
A) the hybridization for C is sp3
B) the hybridization for N is sp2
C) the hybridization for O is sp3
D) N is not hybridized
E) the hybridization for C is sp2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
33
Which combination of hybrid orbital descriptions and electronic geometry descriptions is INCORRECT?
A) sp/linear
B) sp2/trigonal planar
C) sp3/tetrahedral
D) sp3d/square planar
E) sp3d2/octahedral
A) sp/linear
B) sp2/trigonal planar
C) sp3/tetrahedral
D) sp3d/square planar
E) sp3d2/octahedral

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
34
For the molecule 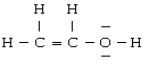
A) the geometry about O is linear
B) the hybridization on O is sp
C) O is not hybridized
D) both carbons are sp2 hybridized
E) there are two π bonds between the two carbons
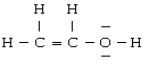
A) the geometry about O is linear
B) the hybridization on O is sp
C) O is not hybridized
D) both carbons are sp2 hybridized
E) there are two π bonds between the two carbons

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
35
The double covalent bond between two carbon atoms in ethylene (C2H4):
A) is free to rotate because of delocalized electrons
B) is a sigma bond
C) consists of one sigma and one pi bond
D) has a low electron density
E) includes a "lone pair" of electrons on one of the carbons
A) is free to rotate because of delocalized electrons
B) is a sigma bond
C) consists of one sigma and one pi bond
D) has a low electron density
E) includes a "lone pair" of electrons on one of the carbons

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
36
Three of the molecular shapes which a sp3d hybridized molecule can have are:
A) triangular, trigonal bipyramid, linear
B) linear, square planar, T-shaped
C) irregular tetrahedron, T-shaped, bent
D) T-shaped, linear, trigonal bipyramid
E) linear, tetragonal pyramid, octahedral
A) triangular, trigonal bipyramid, linear
B) linear, square planar, T-shaped
C) irregular tetrahedron, T-shaped, bent
D) T-shaped, linear, trigonal bipyramid
E) linear, tetragonal pyramid, octahedral

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
37
For BeCl2, the dipole moment of the molecule, hybridization on the central atom, and number of lone pairs on the central atom are:
A) toward Be, sp2, none
B) away from Be, sp2, one
C) none, sp, none
D) toward Be, sp, one
E) none, sp3, two
A) toward Be, sp2, none
B) away from Be, sp2, one
C) none, sp, none
D) toward Be, sp, one
E) none, sp3, two

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
38
Choose the INCORRECT statement about PCl5.
A) There are no π bonds.
B) P has one lone pair.
C) P has sp3d hybridization.
D) Each Cl has 3 lone pairs.
E) There are 5 σ bonds.
A) There are no π bonds.
B) P has one lone pair.
C) P has sp3d hybridization.
D) Each Cl has 3 lone pairs.
E) There are 5 σ bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
39
π bonds:
A) are the only kind of bonds present in double bonds
B) have very little electron density along the internuclear axis
C) are formed by endwise overlap of p orbits
D) are formed from hybrid orbitals
E) are formed from s orbitals
A) are the only kind of bonds present in double bonds
B) have very little electron density along the internuclear axis
C) are formed by endwise overlap of p orbits
D) are formed from hybrid orbitals
E) are formed from s orbitals

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
40
Which hybrid orbitals are impossible in electrically neutral molecules containing only the elements shown?
A) sp3/Al and H
B) sp2/B and F
C) sp/C and H
D) sp2/N and O
E) sp3/O and F
A) sp3/Al and H
B) sp2/B and F
C) sp/C and H
D) sp2/N and O
E) sp3/O and F

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
41
According to MO theory, all of the following have a bond order of 2 EXCEPT:
A) NO
B) CN+
C) C2
D) O2
A) NO
B) CN+
C) C2
D) O2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
42
According to the molecular orbital (MO) theory, when two oxygen atoms bond together, their 2p orbitals combine to form:
A) two sigma molecular orbitals (MOs) and four pi MOs
B) two sigma MOs and two pi MOs
C) four pi MOs only
D) one sigma and one pi MO
E) a delocalized set of MO
A) two sigma molecular orbitals (MOs) and four pi MOs
B) two sigma MOs and two pi MOs
C) four pi MOs only
D) one sigma and one pi MO
E) a delocalized set of MO

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
43
Choose the INCORRECT statement about AsF6-.
A) The hybridization is sp3d2.
B) The molecule is octahedral.
C) There are no π bonds.
D) There is one lone pair of electrons.
E) There are six σ bonds.
A) The hybridization is sp3d2.
B) The molecule is octahedral.
C) There are no π bonds.
D) There is one lone pair of electrons.
E) There are six σ bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is INCORRECT?
A) A double bond is stronger than a single bond for the same atoms.
B) A double bond is less than twice as strong as a single bond for the same atoms.
C) Bonded atoms cannot rotate freely around a double bond.
D) Molecular geometry depends on the σ bond framework.
E) There are no hybridized orbitals in multiple bonds.
A) A double bond is stronger than a single bond for the same atoms.
B) A double bond is less than twice as strong as a single bond for the same atoms.
C) Bonded atoms cannot rotate freely around a double bond.
D) Molecular geometry depends on the σ bond framework.
E) There are no hybridized orbitals in multiple bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
45
According to M.O. theory, which is an INCORRECT statement for H2-?
A) The B.O. is 1/2.
B) There are no unpaired electrons.
C) The σ1s* orbital has one electron.
D) The molecule is paramagnetic.
E) There are 3 electrons in the molecular orbitals.
A) The B.O. is 1/2.
B) There are no unpaired electrons.
C) The σ1s* orbital has one electron.
D) The molecule is paramagnetic.
E) There are 3 electrons in the molecular orbitals.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements concerning the relative energy levels of molecular orbitals for the O2 molecule is INCORRECT?
A) σ1s < σ1s*
B) σ2s < σ2p
C) π2p < σ2p
D) π2p* < σ2p*
E) σ1s* < σ2s
A) σ1s < σ1s*
B) σ2s < σ2p
C) π2p < σ2p
D) π2p* < σ2p*
E) σ1s* < σ2s

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
47
According to Molecular Orbital (MO) theory, which statement below is true?
A) A molecule with an even number of electrons must be diamagnetic.
B) There are as many sigma bonds as pi bonds in a molecule.
C) There are as many molecular orbitals as there are atomic orbitals.
D) There are as many bonding as antibonding electrons in a molecule.
E) All molecules contain pi bonds.
A) A molecule with an even number of electrons must be diamagnetic.
B) There are as many sigma bonds as pi bonds in a molecule.
C) There are as many molecular orbitals as there are atomic orbitals.
D) There are as many bonding as antibonding electrons in a molecule.
E) All molecules contain pi bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
48
Choose the INCORRECT statement about CO2.
A) There are two π bonds.
B) There are two σ bonds.
C) C has one lone pair.
D) The hybridization on C is sp.
E) The molecule is linear.
A) There are two π bonds.
B) There are two σ bonds.
C) C has one lone pair.
D) The hybridization on C is sp.
E) The molecule is linear.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
49
The structure of aspirin is given below. 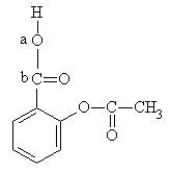 Which set of hybrid orbitals best describes the O-C bond, labeled "a", "b", in aspirin?
Which set of hybrid orbitals best describes the O-C bond, labeled "a", "b", in aspirin?
A) sp3-sp2
B) sp3-sp3
C) sp2-sp2
D) sp-sp2
 Which set of hybrid orbitals best describes the O-C bond, labeled "a", "b", in aspirin?
Which set of hybrid orbitals best describes the O-C bond, labeled "a", "b", in aspirin?A) sp3-sp2
B) sp3-sp3
C) sp2-sp2
D) sp-sp2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
50
According to MO theory, which is the INCORRECT statement for N2+?
A) The BO is 2.5.
B) There is one unpaired electron.
C) The σ2p orbital has one electron in it.
D) The molecule is diamagnetic.
E) There are 9 electrons in the molecular orbitals.
A) The BO is 2.5.
B) There is one unpaired electron.
C) The σ2p orbital has one electron in it.
D) The molecule is diamagnetic.
E) There are 9 electrons in the molecular orbitals.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
51
Which statement is INCORRECT about molecular orbital theory?
A) The number of molecular orbitals produced is equal to the number of atomic orbitals combined.
B) Each pair of sigma molecular orbitals is a bonding orbital and an antibonding orbital.
C) The antibonding orbital is at a lower energy than the bonding orbital.
D) The bond order (BO) is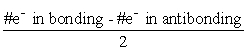 .
.
E) Hund's rule says that each orbital of identical energy has one electron before pairs are formed.
A) The number of molecular orbitals produced is equal to the number of atomic orbitals combined.
B) Each pair of sigma molecular orbitals is a bonding orbital and an antibonding orbital.
C) The antibonding orbital is at a lower energy than the bonding orbital.
D) The bond order (BO) is
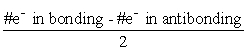 .
.E) Hund's rule says that each orbital of identical energy has one electron before pairs are formed.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
52
According to VB theory, a double bond that results from the sharing of four electrons consists of:
A) 2 sigma bonds
B) one sigma and one pi bond
C) one sigma bond
D) two pi bonds
E) one sigma bond and one coordinate bond
A) 2 sigma bonds
B) one sigma and one pi bond
C) one sigma bond
D) two pi bonds
E) one sigma bond and one coordinate bond

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
53
The concept of an anti-bonding orbital is unique to the:
A) theory of bond hybridization
B) valence bond theory
C) molecular orbital theory
D) concept of resonance
E) electrostatic repulsion theory
A) theory of bond hybridization
B) valence bond theory
C) molecular orbital theory
D) concept of resonance
E) electrostatic repulsion theory

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following species is paramagnetic?
A) B2
B) C2
C) N2
D) CO
A) B2
B) C2
C) N2
D) CO

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
55
According to MO theory, which is the INCORRECT statement for C2-?
A) The BO is 2.5.
B) There is one unpaired electron.
C) The σ2p orbital has two electrons.
D) The molecule is paramagnetic.
E) There are 9 electrons in the molecular orbitals.
A) The BO is 2.5.
B) There is one unpaired electron.
C) The σ2p orbital has two electrons.
D) The molecule is paramagnetic.
E) There are 9 electrons in the molecular orbitals.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
56
Choose the INCORRECT statement about NH4+.
A) The hybridization of N is sp3.
B) The molecule is tetrahedral.
C) There is one lone pair of electrons on N.
D) There are no π bonds.
E) There are four σ bonds.
A) The hybridization of N is sp3.
B) The molecule is tetrahedral.
C) There is one lone pair of electrons on N.
D) There are no π bonds.
E) There are four σ bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
57
According to MO theory, assuming that the molecular orbits are the same as a diatomic molecule, which is an INCORRECT statement for CN-?
A) The BO is 2.
B) There are no unpaired electrons.
C) The σ2p is the highest filled level.
D) The molecule is diamagnetic.
E) There are 10 electrons in the n=2 level orbitals.
A) The BO is 2.
B) There are no unpaired electrons.
C) The σ2p is the highest filled level.
D) The molecule is diamagnetic.
E) There are 10 electrons in the n=2 level orbitals.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
58
Choose the INCORRECT statement about NO2+.
A) There is one π bond.
B) There are two σ bonds.
C) The hybridization is sp.
D) There are no lone pairs on N.
E) There are two lone pairs on each O.
A) There is one π bond.
B) There are two σ bonds.
C) The hybridization is sp.
D) There are no lone pairs on N.
E) There are two lone pairs on each O.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements concerning molecular orbital (MO) theory is FALSE?
A) The number of MOs formed equals the number of atomic orbitals combined.
B) Any set of MOs from 2 atomic orbitals involves 1 bonding and 1 antibonding orbital.
C) Electrons normally enter the lowest energy MO available to them.
D) No more than two electrons can enter a particular MO.
E) Electrons enter MOs of identical energies in pairs before any enter singly.
A) The number of MOs formed equals the number of atomic orbitals combined.
B) Any set of MOs from 2 atomic orbitals involves 1 bonding and 1 antibonding orbital.
C) Electrons normally enter the lowest energy MO available to them.
D) No more than two electrons can enter a particular MO.
E) Electrons enter MOs of identical energies in pairs before any enter singly.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
60
The extra stability that a molecule gains due to electron delocalizations (the electrons being free to move throughout large parts of the molecule) is called:
A) ionization energy
B) lattice energy
C) covalent strength
D) resonance energy
E) bond energy
A) ionization energy
B) lattice energy
C) covalent strength
D) resonance energy
E) bond energy

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following statements about extrinsic semiconductors is INCORRECT?
A) They are generally made from pure Si or Ge with small amounts of impurities.
B) n-type are those in which the impurity is an element of Group 15.
C) p-type are those in which the impurity is an element of Group 13.
D) The crystal need not be very pure before the impurity is added to produce the semiconductor.
E) Boron is a common impurity in n-type extrinsic semiconductors.
A) They are generally made from pure Si or Ge with small amounts of impurities.
B) n-type are those in which the impurity is an element of Group 15.
C) p-type are those in which the impurity is an element of Group 13.
D) The crystal need not be very pure before the impurity is added to produce the semiconductor.
E) Boron is a common impurity in n-type extrinsic semiconductors.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
62
If the HCOO- ion is described using delocalized electrons, why can the oxygen atoms not have sp3 hybrids?
A) There must be an unhybridized p orbital to form the pi bond system.
B) The s orbital must be unhybridized to form the pi bond system.
C) There is no hybridization in systems with delocalized electrons.
D) Molecular orbital theory does not include hybridization.
E) The four lobes repel each other too strongly.
A) There must be an unhybridized p orbital to form the pi bond system.
B) The s orbital must be unhybridized to form the pi bond system.
C) There is no hybridization in systems with delocalized electrons.
D) Molecular orbital theory does not include hybridization.
E) The four lobes repel each other too strongly.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
63
According to the band theory, why is a material such as diamond an insulator?
A) All of the electrons are in the conduction band.
B) All of the electrons are tied to nuclei.
C) Very few electrons can make the transition over the large energy gap between the valence band and the conduction band.
D) The bonds in diamond are too strong to allow atoms to move so no current flows.
E) There are no electrons in the valence band.
A) All of the electrons are in the conduction band.
B) All of the electrons are tied to nuclei.
C) Very few electrons can make the transition over the large energy gap between the valence band and the conduction band.
D) The bonds in diamond are too strong to allow atoms to move so no current flows.
E) There are no electrons in the valence band.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is INCORRECT about silicon doped with phosphorus?
A) It would form a p-type semiconductor.
B) Phosphorus is a donor atom.
C) Phosphorus has five valence electrons.
D) Each phosphorus is bonded to four neighboring silicon atoms.
E) The extra phosphorus electron is promoted to the conduction band.
A) It would form a p-type semiconductor.
B) Phosphorus is a donor atom.
C) Phosphorus has five valence electrons.
D) Each phosphorus is bonded to four neighboring silicon atoms.
E) The extra phosphorus electron is promoted to the conduction band.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
65
The octahedral hybrid configuration is composed of which orbital combination?
A) sp3d2
B) sp2d3
C) spd2
D) sp3d
E) sp3
A) sp3d2
B) sp2d3
C) spd2
D) sp3d
E) sp3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following could act as a p-type semiconductor?
A) aluminum with traces of scandium
B) beryllium with traces of barium
C) germanium with traces of arsenic
D) silicon with traces of boron
E) tin with traces of lead
A) aluminum with traces of scandium
B) beryllium with traces of barium
C) germanium with traces of arsenic
D) silicon with traces of boron
E) tin with traces of lead

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
67
The inclusion of a small amount of aluminum in a crystal of pure germanium yields what kind of semiconductor?
A) an insulator
B) an amorphous solid
C) n-type, in which extra electrons carry the current
D) p-type, in which a deficiency of electrons carries the current
E) p-n-p type
A) an insulator
B) an amorphous solid
C) n-type, in which extra electrons carry the current
D) p-type, in which a deficiency of electrons carries the current
E) p-n-p type

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
68
The hybridization of N in NO2- is ________.
A) sp
B) sp2
C) spd
D) sp3
E) dsp
A) sp
B) sp2
C) spd
D) sp3
E) dsp

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
69
According to the band theory of bonding, the smallest energy difference between a conduction band and a valence band typically occurs in substances such as:
A) crystalline rock salt
B) crystalline rubidium
C) crystalline silicon
D) crystalline iodine
E) crystalline arsenic
A) crystalline rock salt
B) crystalline rubidium
C) crystalline silicon
D) crystalline iodine
E) crystalline arsenic

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following does NOT involve any delocalized π bonds?
A) acetate ion
B) carbonate ion
C) nitric acid
D) nitrous acid
E) ozone
A) acetate ion
B) carbonate ion
C) nitric acid
D) nitrous acid
E) ozone

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
71
All of the following are p-type semiconductors EXCEPT:
A) As in Si.
B) Al in Si.
C) B in Ge.
D) In in Se.
A) As in Si.
B) Al in Si.
C) B in Ge.
D) In in Se.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
72
A junction in a photovoltaic (solar) cell is which of the following?
A) a thin layer of n-type semiconductor in contact with a p-type semiconductor
B) three pieces of semiconductor that form a p-n-p connection
C) wires that carry electricity that are attached to the cell
D) pure germanium that is attached to a p-type semiconductor
E) a thin layer of p-type semiconductor in contact with an n-type semiconductor
A) a thin layer of n-type semiconductor in contact with a p-type semiconductor
B) three pieces of semiconductor that form a p-n-p connection
C) wires that carry electricity that are attached to the cell
D) pure germanium that is attached to a p-type semiconductor
E) a thin layer of p-type semiconductor in contact with an n-type semiconductor

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is INCORRECT?
A) Doping is adding a trace of donor or acceptor atoms to pure semiconductor.
B) Aluminum would be a donor atom in silicon.
C) Extrinsic semiconductors have the band gap controlled by the impurities added.
D) Intrinsic semiconductors have a fixed band gap.
E) In a p-type semiconductor, the electrical conductivity is due to positive hole migration.
A) Doping is adding a trace of donor or acceptor atoms to pure semiconductor.
B) Aluminum would be a donor atom in silicon.
C) Extrinsic semiconductors have the band gap controlled by the impurities added.
D) Intrinsic semiconductors have a fixed band gap.
E) In a p-type semiconductor, the electrical conductivity is due to positive hole migration.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
74
What are the ideal bond angles for H2O?
A) 120°
B) 109.5°
C) 90°
D) 180°
E) 75°
A) 120°
B) 109.5°
C) 90°
D) 180°
E) 75°

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
75
The inclusion of a small amount of phosphorus in a crystal of pure germanium yields what kind of semiconductor?
A) an insulator
B) an amorphous solid
C) n-type, in which extra electrons carry the current
D) p-type, in which a deficiency of electrons carries the current
E) p-n-p type
A) an insulator
B) an amorphous solid
C) n-type, in which extra electrons carry the current
D) p-type, in which a deficiency of electrons carries the current
E) p-n-p type

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following involves delocalized π bonds?
A) acetylene (C2H2)
B) benzene (C6H6)
C) carbon tetrachloride (CCl4)
D) dichlorodifluoromethane (CF2Cl2)
E) ethylene (C2H4)
A) acetylene (C2H2)
B) benzene (C6H6)
C) carbon tetrachloride (CCl4)
D) dichlorodifluoromethane (CF2Cl2)
E) ethylene (C2H4)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following statements is INCORRECT?
A) Delocalized pi orbitals are formed when electrons are shared by unhybridized p orbitals in more than two atoms.
B) The band theory is a form of molecular orbital theory.
C) In band theory, there are two bands.
D) The valence band is at higher energy than the conduction band.
E) An energy band partially filled with electrons is a conduction band.
A) Delocalized pi orbitals are formed when electrons are shared by unhybridized p orbitals in more than two atoms.
B) The band theory is a form of molecular orbital theory.
C) In band theory, there are two bands.
D) The valence band is at higher energy than the conduction band.
E) An energy band partially filled with electrons is a conduction band.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
78
The "electron-sea" model for bonding in metals, although an oversimplification, offers a reasonable explanation of all of the following metallic properties EXCEPT:
A) ductility
B) electrical conductivity
C) gaseous diatomic metal molecules
D) thermal conductivity
E) malleability
A) ductility
B) electrical conductivity
C) gaseous diatomic metal molecules
D) thermal conductivity
E) malleability

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
79
If the wave functions describing the 2s and two of the 2p orbitals are combined, the identical orbitals derived form bond angles of ________.
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
80
How would replacing one of benzene's C atoms and the H atom attached to it with an N atom (C5H5N) affect the delocalized electrons?
A) The molecule would not be stable.
B) The N can have delocalized electrons like C.
C) Only the five C atoms would be included in the delocalized electrons.
D) There would be no delocalized electrons but a double bond between the N and a C.
E) There would be no delocalized electrons but a double bond between the N and the C on each side of the N.
A) The molecule would not be stable.
B) The N can have delocalized electrons like C.
C) Only the five C atoms would be included in the delocalized electrons.
D) There would be no delocalized electrons but a double bond between the N and a C.
E) There would be no delocalized electrons but a double bond between the N and the C on each side of the N.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck


