Deck 10: Chemical Bonding I: Basic Concepts
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/97
Play
Full screen (f)
Deck 10: Chemical Bonding I: Basic Concepts
1
Which of the following is least likely to form multiple bonds?
A) phosphorus
B) oxygen
C) nitrogen
D) carbon
E) barium
A) phosphorus
B) oxygen
C) nitrogen
D) carbon
E) barium
barium
2
Which chloride should have the greatest covalent character?
A) NaCl
B) BeCl2
C) KCl
D) BaCl2
E) CaCl2
A) NaCl
B) BeCl2
C) KCl
D) BaCl2
E) CaCl2
BeCl2
3
In a Lewis structure, a terminal atom is bonded to two or more atoms.
False
4
Choose the INCORRECT statement.
A) The core electrons are called valence electrons.
B) Ionic bonds are formed by the attraction between cations and anions.
C) Ionic bonds are formed from atoms by a transfer of electrons.
D) Covalent bonds are formed by atoms sharing electrons.
E) An octet is 8 outer-shell electrons.
A) The core electrons are called valence electrons.
B) Ionic bonds are formed by the attraction between cations and anions.
C) Ionic bonds are formed from atoms by a transfer of electrons.
D) Covalent bonds are formed by atoms sharing electrons.
E) An octet is 8 outer-shell electrons.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following species contains a triple bond?
A) NH3
B) HCCl3
C) NO3-
D) CO32-
E) CN-
A) NH3
B) HCCl3
C) NO3-
D) CO32-
E) CN-

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
6
In a Lewis structure, usually each atom acquires an outer-shell octet of electrons; H has 2 outer shell electrons.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
7
Choose the INCORRECT statement.
A) In a Lewis structure, a covalent bond can be represented by a pair of electrons or a dash.
B) Pairs of electrons not involved in bonding are called lone pairs.
C) A molecule of two atoms is called a diatomic molecule.
D) Two electrons involved in a bond produce a double bond.
E) Three electron pairs involved in a bond produce a triple bond.
A) In a Lewis structure, a covalent bond can be represented by a pair of electrons or a dash.
B) Pairs of electrons not involved in bonding are called lone pairs.
C) A molecule of two atoms is called a diatomic molecule.
D) Two electrons involved in a bond produce a double bond.
E) Three electron pairs involved in a bond produce a triple bond.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
8
The central atom is typically the atom with the highest electronegativity.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
9
In a Lewis structure, the number of valence electrons shown is one less for each negative charge.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
10
Polar bonds are caused by the bonding pair of electrons being attracted more to one atom than the other.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
11
A chemical bond for which one of the bonded atoms provides both electrons for the bond is referred to as a:
A) double covalent bond
B) coordinate covalent bond
C) formal covalent bond
D) free radical bond
E) VSEPR bond
A) double covalent bond
B) coordinate covalent bond
C) formal covalent bond
D) free radical bond
E) VSEPR bond

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
12
In which of the following molecules would you expect the nitrogen-to-nitrogen bond to be the shortest?
A) N2H4
B) N2
C) N2O4
D) N2O
E) NH3
A) N2H4
B) N2
C) N2O4
D) N2O
E) NH3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
13
Choose the INCORRECT statement.
A) A Lewis symbol consists of a chemical symbol and the outer shell electrons.
B) In a Lewis symbol, the chemical symbol represents the nucleus of the atom.
C) A Lewis structure is a combination of Lewis symbols that represent the transfer or sharing of electrons in a chemical bond.
D) An ion written as a Lewis symbol has brackets outside the electrons.
E) An ion written as a Lewis symbol has the charge written as a superscript outside the closing bracket.
A) A Lewis symbol consists of a chemical symbol and the outer shell electrons.
B) In a Lewis symbol, the chemical symbol represents the nucleus of the atom.
C) A Lewis structure is a combination of Lewis symbols that represent the transfer or sharing of electrons in a chemical bond.
D) An ion written as a Lewis symbol has brackets outside the electrons.
E) An ion written as a Lewis symbol has the charge written as a superscript outside the closing bracket.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
14
The core electrons are called valence electrons.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
15
Valence electrons are:
A) all electrons of the last rare gas configuration
B) electrons in filled orbits
C) electrons in the shell of the highest principal quantum number
D) unpaired electrons
E) paired electrons
A) all electrons of the last rare gas configuration
B) electrons in filled orbits
C) electrons in the shell of the highest principal quantum number
D) unpaired electrons
E) paired electrons

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
16
A chemical bond for which one of the bonded atoms provides both electrons for the bond is referred to as a coordinate covalent bond

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
17
Covalent bonds are formed by atoms sharing electrons.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
18
Bonds between like atoms are nonpolar bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the INCORRECT statement.
A) Bonds between like atoms are nonpolar bonds.
B) Polar bonds are caused by the bonding pair of electrons being attracted more to one atom than the other.
C) Electronegativity is the ability of an atom to attract electrons when involved in a bond.
D) A difference in electronegativity between two atoms causes a polar bond.
E) If the ΔEN value is large the bond is polar covalent.
A) Bonds between like atoms are nonpolar bonds.
B) Polar bonds are caused by the bonding pair of electrons being attracted more to one atom than the other.
C) Electronegativity is the ability of an atom to attract electrons when involved in a bond.
D) A difference in electronegativity between two atoms causes a polar bond.
E) If the ΔEN value is large the bond is polar covalent.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
20
A Lewis structure is a combination of Lewis symbols representing either the transfer or the sharing of electrons in a chemical bond.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
21
As indicated by Lewis structures, which of the following molecules would probably be unstable?
A) NH3
B) N2H6
C) SF4
D) CH2F2
E) SiH4
A) NH3
B) N2H6
C) SF4
D) CH2F2
E) SiH4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
22
After drawing the Lewis dot structure of HC(O)OH, pick the INCORRECT statement from the following.
A) The oxygen not also bonded to hydrogen has a double bond to carbon.
B) The carbon has a lone pair.
C) Both oxygens have two lone pairs.
D) The O-H bond is a single bond.
E) The C-H bond is a single bond.
A) The oxygen not also bonded to hydrogen has a double bond to carbon.
B) The carbon has a lone pair.
C) Both oxygens have two lone pairs.
D) The O-H bond is a single bond.
E) The C-H bond is a single bond.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
23
Choose the INCORRECT statement.
A) In a Lewis structure, the number of valence electrons shown is one more for each negative charge.
B) The central atom is typically the atom with the highest electronegativity.
C) One atom supplies both electrons for a coordinate covalent bond.
D) Formal charges are apparent charges associated with atoms in a Lewis structure.
E) Resonance is when more than one plausible structure can be written but the "correct" structure cannot be written.
A) In a Lewis structure, the number of valence electrons shown is one more for each negative charge.
B) The central atom is typically the atom with the highest electronegativity.
C) One atom supplies both electrons for a coordinate covalent bond.
D) Formal charges are apparent charges associated with atoms in a Lewis structure.
E) Resonance is when more than one plausible structure can be written but the "correct" structure cannot be written.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following species is best represented by a resonance hybrid having two contributing structures?
A) nitric acid
B) nitrous acid
C) nitride ion
D) nitrate ion
E) nitrogen
A) nitric acid
B) nitrous acid
C) nitride ion
D) nitrate ion
E) nitrogen

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is diamagnetic?
A) N2
B) O2-
C) SO2+
D) O2
E) NO2
A) N2
B) O2-
C) SO2+
D) O2
E) NO2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
26
Based on the Lewis structures, which of the following molecules would you expect to exhibit resonance?
A) LiH
B) CH4
C) HNO2
D) OF2
E) none of these
A) LiH
B) CH4
C) HNO2
D) OF2
E) none of these

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following bonds is probably the most polar?
A) NH in NH3
B) OH in H2O
C) PH in PH3
D) SeH in SeH2
E) CH in CH4
A) NH in NH3
B) OH in H2O
C) PH in PH3
D) SeH in SeH2
E) CH in CH4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
28
After drawing the Lewis dot structure of CH2CCl2, pick the INCORRECT statement of the following.
A) The CC bond is a double bond.
B) The HC bonds are single bonds.
C) The ClC bonds are single bonds.
D) Each carbon has a lone pair.
E) Each chlorine has three lone pairs.
A) The CC bond is a double bond.
B) The HC bonds are single bonds.
C) The ClC bonds are single bonds.
D) Each carbon has a lone pair.
E) Each chlorine has three lone pairs.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the INCORRECT statement.
A) In a Lewis structure, a terminal atom is bonded to two or more atoms.
B) In a Lewis structure all valence electrons must appear.
C) In a Lewis structure, usually all electrons are paired.
D) In a Lewis structure, usually each atom acquires an outer-shell octet of electrons; H has 2 outer shell electrons.
E) In Lewis structures, most multiple covalent bonds are formed by C, N, O, P, and S.
A) In a Lewis structure, a terminal atom is bonded to two or more atoms.
B) In a Lewis structure all valence electrons must appear.
C) In a Lewis structure, usually all electrons are paired.
D) In a Lewis structure, usually each atom acquires an outer-shell octet of electrons; H has 2 outer shell electrons.
E) In Lewis structures, most multiple covalent bonds are formed by C, N, O, P, and S.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
30
After drawing the Lewis dot structure of HOClO2, pick the INCORRECT statement of the following.
A) The oxygen bonded to the hydrogen has two lone pairs.
B) The oxygens not bonded to hydrogen have three lone pairs.
C) The OCl bonds are all double bonds.
D) The HO bond is a single bond.
E) Chlorine has a full octet.
A) The oxygen bonded to the hydrogen has two lone pairs.
B) The oxygens not bonded to hydrogen have three lone pairs.
C) The OCl bonds are all double bonds.
D) The HO bond is a single bond.
E) Chlorine has a full octet.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the INCORRECT statement.
A) Molecules with all paired electrons are diamagnetic.
B) Highly reactive molecular fragments with unpaired electrons are free radicals.
C) An expanded octet has larger electron clouds.
D) Electron pairs repel each other.
E) VSEPR stands for valence-shell electron pair repulsion.
A) Molecules with all paired electrons are diamagnetic.
B) Highly reactive molecular fragments with unpaired electrons are free radicals.
C) An expanded octet has larger electron clouds.
D) Electron pairs repel each other.
E) VSEPR stands for valence-shell electron pair repulsion.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
32
After drawing the Lewis dot structure of HCN, pick the INCORRECT statement from the following.
A) The C-N bond is a double bond.
B) There are no lone pairs on C.
C) The C-H bond is a single bond.
D) There is a lone pair of electrons on N.
E) There are no lone pairs on H.
A) The C-N bond is a double bond.
B) There are no lone pairs on C.
C) The C-H bond is a single bond.
D) There is a lone pair of electrons on N.
E) There are no lone pairs on H.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following exhibits covalent bonding?
A) NaF
B) SrO
C) LiH
D) OF2
E) K2S
A) NaF
B) SrO
C) LiH
D) OF2
E) K2S

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following would be properly classified as a set of covalent molecules?
A) NaClO4, C4H10, NH3
B) NaCl, CH4, S8
C) CO2, HCN, O2
D) CO2, NH4Cl, C2H6
E) AgCl, ScF3, P4
A) NaClO4, C4H10, NH3
B) NaCl, CH4, S8
C) CO2, HCN, O2
D) CO2, NH4Cl, C2H6
E) AgCl, ScF3, P4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is diamagnetic?
A) ClO2
B) NO
C) CO
D) NO2
E) CH3
A) ClO2
B) NO
C) CO
D) NO2
E) CH3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following must be paramagnetic?
A) F2
B) N2
C) SO2+
D) H2
E) ClO2-
A) F2
B) N2
C) SO2+
D) H2
E) ClO2-

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
37
With the representations of "I" for ionic bond, "PC" for polar covalent bond, and "NP" for nonpolar covalent bond, which of the following descriptive combinations is correct?
A) CsF/PC
B) F2/I
C) NO/NP
D) HCl/PC
E) BrCl/NP
A) CsF/PC
B) F2/I
C) NO/NP
D) HCl/PC
E) BrCl/NP

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
38
An expanded octet may occur:
A) in the 1st and 2nd period only
B) in families IA, IIA, and IIIA only
C) anywhere except period 1 and 2
D) in all families except IA
E) in the 3rd and 4th period only
A) in the 1st and 2nd period only
B) in families IA, IIA, and IIIA only
C) anywhere except period 1 and 2
D) in all families except IA
E) in the 3rd and 4th period only

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following exhibits ionic bonding?
A) C2H6
B) Na2S
C) H2CO
D) SiCl4
E) SF4
A) C2H6
B) Na2S
C) H2CO
D) SiCl4
E) SF4

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following species exhibits resonance?
A) OF2
B) ClO3-
C) N2
D) PCl5
E) BrF3
A) OF2
B) ClO3-
C) N2
D) PCl5
E) BrF3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
41
How many "dots" should be shown in the Lewis symbol for phosphorus?
A) 1
B) 3
C) 5
D) 8
E) 10
A) 1
B) 3
C) 5
D) 8
E) 10

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
42
Write a Lewis structure for BaO.
A)![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0daf_abb0_737ceb4cf307_TB2799_11.jpg)
B)![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0db0_abb0_c10dce74800b_TB2799_11.jpg)
C)![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0db1_abb0_d33153dc04a0_TB2799_11.jpg)
D) [BaO]
E)![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0db2_abb0_b519d406690b_TB2799_11.jpg)
A)
![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0daf_abb0_737ceb4cf307_TB2799_11.jpg)
B)
![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0db0_abb0_c10dce74800b_TB2799_11.jpg)
C)
![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0db1_abb0_d33153dc04a0_TB2799_11.jpg)
D) [BaO]
E)
![<strong>Write a Lewis structure for BaO.</strong> A) B) C) D) [BaO] E)](https://storage.examlex.com/TB2799/11eaaed0_3e90_0db2_abb0_b519d406690b_TB2799_11.jpg)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following sets contains only linear molecules?
A) CO2, HCN, O2
B) H2S, HCN, CO2
C) H2O, CO, Cl2
D) H2S, CO, CO2
E) BF3, Cl2, O2
A) CO2, HCN, O2
B) H2S, HCN, CO2
C) H2O, CO, Cl2
D) H2S, CO, CO2
E) BF3, Cl2, O2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the INCORRECT statement.
A) Molecules with unpaired electrons are paramagnetic.
B) The bond order describes whether a covalent bond is single, double or triple.
C) A larger bond order means a shorter bond length.
D) Polar covalent bonds involve equal sharing of electrons in a bond.
E) Bonds between like atoms share electrons equally.
A) Molecules with unpaired electrons are paramagnetic.
B) The bond order describes whether a covalent bond is single, double or triple.
C) A larger bond order means a shorter bond length.
D) Polar covalent bonds involve equal sharing of electrons in a bond.
E) Bonds between like atoms share electrons equally.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following molecules is polar?
A) NBr3
B) CH4
C) CS2
D) NH4+
E) PCl5
A) NBr3
B) CH4
C) CS2
D) NH4+
E) PCl5

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
46
Given the bond enthalpies CC (348), CO (707), OO (498), HO (464), CH (414) all in kJ/mol, compute ΔH° kJ/mol for the complete combustion of C7H16.
A) -3.13 × 103 kJ/mol
B) -2.93 × 103 kJ/mol
C) -2.57 × 103 kJ/mol
D) -2.43 × 103 kJ/mol
E) +2.57 × 103 kJ/mol
A) -3.13 × 103 kJ/mol
B) -2.93 × 103 kJ/mol
C) -2.57 × 103 kJ/mol
D) -2.43 × 103 kJ/mol
E) +2.57 × 103 kJ/mol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
47
Choose the correct statement about the compound SO2.
A) The S atom has an unshared electron pair.
B) The SO bonds are ionic in character.
C) The two SO bonds have different lengths since one is a single bond and the other a double bond.
D) The molecule has a linear structure.
E) The O atoms have no unshared electron pairs.
A) The S atom has an unshared electron pair.
B) The SO bonds are ionic in character.
C) The two SO bonds have different lengths since one is a single bond and the other a double bond.
D) The molecule has a linear structure.
E) The O atoms have no unshared electron pairs.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
48
How many "dots" should be shown for the oxygen symbol in lithium oxide?
A) 2
B) 4
C) 6
D) 8
E) 10
A) 2
B) 4
C) 6
D) 8
E) 10

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following molecules has both an electron group geometry and a molecular geometry described as trigonal planar?
A) SiH4
B) PF3
C) OF2
D) CHF3
E) BF3
A) SiH4
B) PF3
C) OF2
D) CHF3
E) BF3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
50
Which compound would be expected to have the shortest carbon-carbon bond?
A) H3CCH3
B) HCCH
C) H2CCH2
D) F3CCF3
E) Cl2CCCl2
A) H3CCH3
B) HCCH
C) H2CCH2
D) F3CCF3
E) Cl2CCCl2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following has a molecular structure best described as involving an "incomplete octet"?
A) OF2
B) BF3
C) CF4
D) NF3
E) F2
A) OF2
B) BF3
C) CF4
D) NF3
E) F2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements correctly describe the basic concepts and uses of VSEPR theory:
I. The VSEPR theory is used for estimating bond angles.
II. The VSEPR theory is used for predicting electronegativities.
III. The VSEPR theory is helpful in predicting polarity.
IV. The VSEPR theory states that electron pairs repel each other.
V.The VSEPR theory uses valence electron counting for structure prediction.
A) I), II), III), IV)
B) I), II), IV), V)
C) I), II), III), V)
D) II), III), IV), V)
E) I), III), IV), V)
I. The VSEPR theory is used for estimating bond angles.
II. The VSEPR theory is used for predicting electronegativities.
III. The VSEPR theory is helpful in predicting polarity.
IV. The VSEPR theory states that electron pairs repel each other.
V.The VSEPR theory uses valence electron counting for structure prediction.
A) I), II), III), IV)
B) I), II), IV), V)
C) I), II), III), V)
D) II), III), IV), V)
E) I), III), IV), V)

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following molecules is nonpolar?
A) HCN
B) CHCl3
C) H2O
D) HClO4
E) BCl3
A) HCN
B) CHCl3
C) H2O
D) HClO4
E) BCl3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
54
Choose the groups of molecules below in which all the molecules have a net dipole moment.
A) SiHCl3, O2, H2O
B) HF, H2C=CH2, H2O
C) HF, CH3Cl, H2O
D) CCl4, HCl, NH3
E) HF, H2O, N2
A) SiHCl3, O2, H2O
B) HF, H2C=CH2, H2O
C) HF, CH3Cl, H2O
D) CCl4, HCl, NH3
E) HF, H2O, N2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following molecules contains polar bonds but has a zero dipole moment?
A) N2
B) NH3
C) CO2
D) CH3Cl
E) BrCl
A) N2
B) NH3
C) CO2
D) CH3Cl
E) BrCl

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
56
Which compound would be expected to have the shortest nitrogen-nitrogen bond?
A) H2NNH2
B) HNNH
C) N2
D) O2NNO2
E) (CH3)2NNH2
A) H2NNH2
B) HNNH
C) N2
D) O2NNO2
E) (CH3)2NNH2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is a bent (nonlinear) molecule?
A) CO2
B) CS2
C) BeF2
D) OF2
E) XeF2
A) CO2
B) CS2
C) BeF2
D) OF2
E) XeF2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following has a molecular structure that can be represented without use of an "expanded octet"?
A) Fe(CN)63-
B) C2Cl6
C) SF6
D) PF5
E) PtCl5-
A) Fe(CN)63-
B) C2Cl6
C) SF6
D) PF5
E) PtCl5-

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following species has a pyramidal structure?
A) ClO3-
B) ClO4-
C) ClO2-
D) ClO2
A) ClO3-
B) ClO4-
C) ClO2-
D) ClO2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
60
Choose the INCORRECT statement.
A) The geometrical distribution of electron groups is the electron-group geometry.
B) The geometrical arrangement of the atomic nuclei is the molecular geometry.
C) A polar molecule has a dipole moment.
D) A dipole moment is the product of the number of atoms and the charge.
E) A nonpolar molecule can have polar bonds.
A) The geometrical distribution of electron groups is the electron-group geometry.
B) The geometrical arrangement of the atomic nuclei is the molecular geometry.
C) A polar molecule has a dipole moment.
D) A dipole moment is the product of the number of atoms and the charge.
E) A nonpolar molecule can have polar bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
61
After drawing the Lewis dot structure for CH3Cl, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 4, 1, 0
B) 4, 0, 1
C) 3, 0, 1
D) 4, 0, 0
E) 3, 1, 0
A) 4, 1, 0
B) 4, 0, 1
C) 3, 0, 1
D) 4, 0, 0
E) 3, 1, 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
62
How many bond pairs ("bp") and how many lone pairs ("lp") should be shown in the Lewis structure for carbon monoxide?
A) 1 bp/4 lp
B) 2 bp/3 lp
C) 3 bp/2 lp
D) 4 bp/1 lp
E) 5 bp/0 lp
A) 1 bp/4 lp
B) 2 bp/3 lp
C) 3 bp/2 lp
D) 4 bp/1 lp
E) 5 bp/0 lp

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
63
What is the formal charge on N in NO3-?
A) +2
B) -2
C) +1
D) -1
E) 0
A) +2
B) -2
C) +1
D) -1
E) 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
64
After drawing the Lewis dot structure for PCl3, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 2, 1, 1
B) 3, 0, 1
C) 2, 0, 2
D) 3, 1, 0
E) 3, 1, 1
A) 2, 1, 1
B) 3, 0, 1
C) 2, 0, 2
D) 3, 1, 0
E) 3, 1, 1

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
65
What is the formal charge on B in BF4-?
A) +1
B) -1
C) +2
D) 0
E) -2
A) +1
B) -1
C) +2
D) 0
E) -2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
66
After drawing the Lewis dot structure for XeCl4, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 4, 0, 0
B) 4, 0, 2
C) 4, 0, 1
D) 3, 1, 0
E) 1, 3, 0
A) 4, 0, 0
B) 4, 0, 2
C) 4, 0, 1
D) 3, 1, 0
E) 1, 3, 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
67
After drawing the Lewis dot structure for IF7, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 3, 2, 1
B) 7, 0, 1
C) 7, 0, 0
D) 1, 0, 0
E) 4, 0, 0
A) 3, 2, 1
B) 7, 0, 1
C) 7, 0, 0
D) 1, 0, 0
E) 4, 0, 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
68
Arrange the following in order of increasing electronegativity: Cs, F, As, Cl.
A) As < Cl < Cs < F
B) Cl < As < F < Cs
C) As < F < Cs < Cl
D) Cl < Cs < F < As
E) Cs < As < Cl < F
A) As < Cl < Cs < F
B) Cl < As < F < Cs
C) As < F < Cs < Cl
D) Cl < Cs < F < As
E) Cs < As < Cl < F

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
69
After drawing the Lewis dot structure for BeCl2, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 2, 0, 0
B) 2, 0, 2
C) 2, 0,1
D) 0, 2, 0
E) 1, 1, 0
A) 2, 0, 0
B) 2, 0, 2
C) 2, 0,1
D) 0, 2, 0
E) 1, 1, 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
70
Write a Lewis structure for calcium hydride.
A)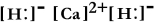
B)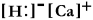
C)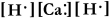
D) HCaH
E) CaH
A)
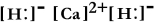
B)
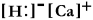
C)
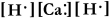
D) HCaH
E) CaH

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
71
After drawing the Lewis dot structure for CO2, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 2, 2, 0
B) 2, 0, 2
C) 0, 2, 0
D) 2, 0, 1
E) 0, 2, 2
A) 2, 2, 0
B) 2, 0, 2
C) 0, 2, 0
D) 2, 0, 1
E) 0, 2, 2

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
72
What is the formal charge on P in PCl5?
A) +5
B) +2
C) +1
D) 0
E) -1
A) +5
B) +2
C) +1
D) 0
E) -1

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
73
Write a Lewis structure for barium fluoride.
A)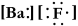
B) FBF
C)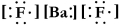
D)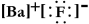
E)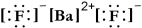
A)
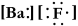
B) FBF
C)
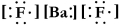
D)
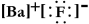
E)
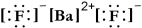

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
74
What is the formal charge on carbon in the bicarbonate ion (HOCO2)?
A) +2
B) -2
C) +1
D) -1
E) 0
A) +2
B) -2
C) +1
D) -1
E) 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
75
After drawing the Lewis dot structure for XeCl2, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 2, 0, 2
B) 0, 2, 0
C) 0, 2, 1
D) 2, 0, 3
E) 2, 0, 0
A) 2, 0, 2
B) 0, 2, 0
C) 0, 2, 1
D) 2, 0, 3
E) 2, 0, 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
76
What is the molecular shape of BrF5?
A) linear
B) square pyramid
C) square planar
D) octahedral
E) trigonal bipyramid
A) linear
B) square pyramid
C) square planar
D) octahedral
E) trigonal bipyramid

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
77
Write a Lewis structure for sodium iodide.
A)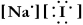
B)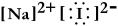
C)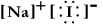
D) NaI
E)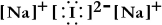
A)
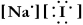
B)
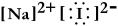
C)
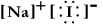
D) NaI
E)
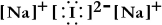

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
78
The electron-pair geometry of H2O is ________.
A) tetrahedral
B) linear
C) trigonal planar
D) bent
E) V-shaped
A) tetrahedral
B) linear
C) trigonal planar
D) bent
E) V-shaped

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
79
After drawing the Lewis dot structure for CH2O, determine the number of single bonds, double bonds, and lone pairs on the central atom.
A) 1, 2, 0
B) 2, 1, 1
C) 3, 0, 1
D) 3, 1, 0
E) 2, 1, 0
A) 1, 2, 0
B) 2, 1, 1
C) 3, 0, 1
D) 3, 1, 0
E) 2, 1, 0

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
80
Write a Lewis structure for potassium oxide.
A)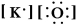
B) KO
C) KOK
D)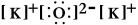
E)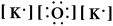
A)
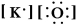
B) KO
C) KOK
D)
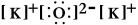
E)
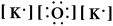

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck


