Deck 3: Atomic Structure and the Periodic Table
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 3: Atomic Structure and the Periodic Table
1
Isotopes of a given element
A) have the same mass number but different numbers of protons
B) have different mass numbers but identical numbers of protons
C) have the same atomic number but different chemical properties
D) have the same mass number but different chemical properties
A) have the same mass number but different numbers of protons
B) have different mass numbers but identical numbers of protons
C) have the same atomic number but different chemical properties
D) have the same mass number but different chemical properties
have different mass numbers but identical numbers of protons
2
Chlorine, which exists in nature in two isotopic forms, has an atomic mass of 35.5 amu. This means that:
A) All chlorine atoms have masses of 35.5 amu.
B) More than half of all chlorine atoms have masses of 35.5.
C) 35.5 amu is the upper limit for the mass of a chlorine atom.
D) The mass of one isotope must be lighter than 35.5 amu while the mass of the other isotope is heavier than 35.5 amu.
A) All chlorine atoms have masses of 35.5 amu.
B) More than half of all chlorine atoms have masses of 35.5.
C) 35.5 amu is the upper limit for the mass of a chlorine atom.
D) The mass of one isotope must be lighter than 35.5 amu while the mass of the other isotope is heavier than 35.5 amu.
The mass of one isotope must be lighter than 35.5 amu while the mass of the other isotope is heavier than 35.5 amu.
3
The quantity "A + Z" gives the number of
A) electrons in an atom
B) neutrons in an atomic nucleus
C) nucleons in an atomic nucleus
D) all subatomic particles in an atom
A) electrons in an atom
B) neutrons in an atomic nucleus
C) nucleons in an atomic nucleus
D) all subatomic particles in an atom
all subatomic particles in an atom
4
The nuclear charge for an atom of 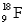 is
is
A) zero
B) + 9
C) + 18
D) + 28
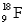 is
isA) zero
B) + 9
C) + 18
D) + 28

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about a d subshell is correct?
A) It is found only in shells greater than 2.
B) It contains 3 orbitals.
C) It is found only in shells 2 and 3.
D) It is found in all shells greater than 1.
A) It is found only in shells greater than 2.
B) It contains 3 orbitals.
C) It is found only in shells 2 and 3.
D) It is found in all shells greater than 1.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
The nucleus of an atom
A) contains neutrons and electrons
B) has no charge because of the presence of neutrons
C) repels the negatively charged electrons in an atom
D) does not account for a large amount of the total volume of an atom
A) contains neutrons and electrons
B) has no charge because of the presence of neutrons
C) repels the negatively charged electrons in an atom
D) does not account for a large amount of the total volume of an atom

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
The modern periodic table arranges the elements in order of
A) year of discovery
B) decreasing size of the nucleus
C) increasing reactivity with oxygen
D) increasing number of protons
A) year of discovery
B) decreasing size of the nucleus
C) increasing reactivity with oxygen
D) increasing number of protons

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
An atom contains 33 protons, 35 neutrons and 33 electrons. The atomic number and mass number for this atom are, respectively,
A) 35 and 33
B) 33 and 35
C) 33 and 68
D) 35 and 68
A) 35 and 33
B) 33 and 35
C) 33 and 68
D) 35 and 68

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
Two naturally occurring isotopes exist for the hypothetical element dippium. 25.0 percent of dippium atoms have a relative mass of 17.0 amu and 75.0 percent a relative mass of 21.3 amu. What is the atomic mass of dippium?
A) 18.1 amu
B) 19.1 amu
C) 20.2 amu
D) 38.3 amu
A) 18.1 amu
B) 19.1 amu
C) 20.2 amu
D) 38.3 amu

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
How many unpaired electrons are present in the orbital diagram for an atom whose electron configuration is 1s22s22p4?
A) 0
B) 1
C) 2
D) 4
A) 0
B) 1
C) 2
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following element classification pairings is incorrect?
A) O representative element
B) Xe noble gas element
C) Cr transition element
D) Os inner transition element
A) O representative element
B) Xe noble gas element
C) Cr transition element
D) Os inner transition element

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is correct for 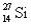 ?
?
A) contains more electrons than neutrons
B) contains no electrons
C) contains an equal number of neutrons and electrons
D) contains more neutrons than electrons
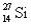 ?
?A) contains more electrons than neutrons
B) contains no electrons
C) contains an equal number of neutrons and electrons
D) contains more neutrons than electrons

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
The elements in group IVA of the periodic table all have electron configurations ending in
A) p2
B) p4
C) d4
D) s2
A) p2
B) p4
C) d4
D) s2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
In which of the following pairs of elements are both members of the pair metals?
A) P and O
B) Al and Cl
C) Nb and Ar
D) Ag and Bi
A) P and O
B) Al and Cl
C) Nb and Ar
D) Ag and Bi

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is consistent with the electron configuration 1s22s22p63s23p6?
A) There are six electrons present in a 3p orbital.
B) There are six electrons present in a 3p subshell.
C) There are two electrons present in a 2s shell.
D) There are two electrons total in the second shell.
A) There are six electrons present in a 3p orbital.
B) There are six electrons present in a 3p subshell.
C) There are two electrons present in a 2s shell.
D) There are two electrons total in the second shell.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
After the 4s subshell of an atom is filled with electrons, the next electron added will enter the
A) 3d subshell
B) 4p subshell
C) 4d subshell
D) 5s subshell
A) 3d subshell
B) 4p subshell
C) 4d subshell
D) 5s subshell

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements concerning subatomic particles is correct?
A) Four fundamental types exist, all of which are charged.
B) Four fundamental types exist, two of which are charged.
C) Three fundamental types exist, none of which are charged.
D) Three fundamental types exist, two of which are charged.
A) Four fundamental types exist, all of which are charged.
B) Four fundamental types exist, two of which are charged.
C) Three fundamental types exist, none of which are charged.
D) Three fundamental types exist, two of which are charged.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements correctly characterizes the subatomic particle called a neutron?
A) Its mass is less than that of an electron.
B) it has a negative charge
C) its mass is about that same as that of a proton
D) it contributes 25% of the mass of an atom
A) Its mass is less than that of an electron.
B) it has a negative charge
C) its mass is about that same as that of a proton
D) it contributes 25% of the mass of an atom

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
In which of the following pairs of elements are both members of the pair in the same period in the periodic table?
A) Ne and Mg
B) Ni and Pd
C) Ca and Se
D) K and Rb
A) Ne and Mg
B) Ni and Pd
C) Ca and Se
D) K and Rb

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
The correct electron configuration for 17Cl is
A) 1s22s22p5
B) 1s22s22p63s2
C) 1s22s22p63s23p5
D) 1s22s22p63s23p64s23d5
A) 1s22s22p5
B) 1s22s22p63s2
C) 1s22s22p63s23p5
D) 1s22s22p63s23p64s23d5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following pairs of atoms, specified in terms of subatomic particle composition, are isotopes?
A) (24p, 24e, 24n) and (25p, 25e, 25n)
B) (24p, 24e, 24n) and (24p, 24e, 28n)
C) (24p, 24e, 28n) and (25p, 25e, 28n)
D) more than one correct response
E) no correct response
A) (24p, 24e, 24n) and (25p, 25e, 25n)
B) (24p, 24e, 24n) and (24p, 24e, 28n)
C) (24p, 24e, 28n) and (25p, 25e, 28n)
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements is correct for 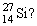
A) atomic number = 27 and mass number = 14
B) contains 14 protons and 27 neutrons
C) contains an equal number of protons and electrons
D) more than one correct response
E) no correct response
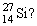
A) atomic number = 27 and mass number = 14
B) contains 14 protons and 27 neutrons
C) contains an equal number of protons and electrons
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
Element A exists in three isotopic forms with masses of 21.0, 25.0 and 26.0 amu, respectively. Element Q also exists in three isotopic forms with masses of 20, 24.0 and 25.0 amu, respectively. It is true that:
A) Element A has a lower atomic mass than element Q since it has the lightest isotope.
B) Elements A and Q have identical atomic masses since the sums of their isotopic masses are equal.
C) Element Q has a higher atomic mass than element A since its atomic masses are closer together.
D) More than one correct response.
E) No correct response.
A) Element A has a lower atomic mass than element Q since it has the lightest isotope.
B) Elements A and Q have identical atomic masses since the sums of their isotopic masses are equal.
C) Element Q has a higher atomic mass than element A since its atomic masses are closer together.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements concerning the nucleus of an atom is correct?
A) It accounts for almost all of the mass of an atom.
B) It is always positively charged.
C) It contains only neutrons.
D) More than one correct response.
E) No correct response.
A) It accounts for almost all of the mass of an atom.
B) It is always positively charged.
C) It contains only neutrons.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following characterizations for the element 52Te is correct?
A) both a representative element and a metal
B) electron configuration involves a partially filled s subshell
C) electron configuration involves a total of 52 electrons
D) more than one correct response
E) no correct response
A) both a representative element and a metal
B) electron configuration involves a partially filled s subshell
C) electron configuration involves a total of 52 electrons
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) The shape of an s orbital is always spherical.
(2) All naturally occurring atoms of an element must be identical to each other in terms of subatomic particle makeup.
(3) The alkaline earth metals are the elements in group IA of the periodic table.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) All naturally occurring atoms of an element must be identical to each other in terms of subatomic particle makeup.
(3) The alkaline earth metals are the elements in group IA of the periodic table.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) Electrons occupy the orbitals of a subshell such that each orbital acquires one electron before any orbital acquires a second electron.
(2) The nucleus of an atom is always positively charged.
(3) Both electrons and protons are massive particles when compared with neutrons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The nucleus of an atom is always positively charged.
(3) Both electrons and protons are massive particles when compared with neutrons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) The number of elements that are metals and nonmetals is approximately equal.
(2) The number of orbitals present in an electron subshell is always six.
(3) An atom as a whole is electrically neutral because all charged subatomic particles reside in the nucleus.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The number of orbitals present in an electron subshell is always six.
(3) An atom as a whole is electrically neutral because all charged subatomic particles reside in the nucleus.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
Period number and group number in the periodic table are numerically equal for which of the following elements?
A) (13Al)
B) (33As)
C) (51Sb)
D) more than one correct response
E) no correct response
A) (13Al)
B) (33As)
C) (51Sb)
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following collections of subatomic particles possesses a net charge of +2?
A) two neutrons and two electrons
B) three protons and one electron
C) three electrons and one proton
D) more than one correct response
E) no correct response
A) two neutrons and two electrons
B) three protons and one electron
C) three electrons and one proton
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following periodic table groups contains three or more nonmetallic elements?
A) IIIA
B) IVA
C) VA
D) more than one correct response
E) no correct response
A) IIIA
B) IVA
C) VA
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements concerning types of elements is correct?
A) There are more noble gas elements than transition elements.
B) There are more s-block elements than d-block elements.
C) There are more nonmetals than metals.
D) More than one correct response.
E) No correct response.
A) There are more noble gas elements than transition elements.
B) There are more s-block elements than d-block elements.
C) There are more nonmetals than metals.
D) More than one correct response.
E) No correct response.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) The mass of an atom and the mass of its nucleus are essentially the same.
(2) Two of the six electrons present in an atom of carbon are unpaired.
(3) The element aluminum is located in the d area of the periodic table.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Two of the six electrons present in an atom of carbon are unpaired.
(3) The element aluminum is located in the d area of the periodic table.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
Two unpaired electrons are present in the orbital diagram of which of the following elements?
A) (6C)
B) (10Ne)
C) (12Mg)
D) more than one correct response
E) no correct response
A) (6C)
B) (10Ne)
C) (12Mg)
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements correctly describes the subatomic particle called a neutron?
A) has a mass slightly less than that of a proton
B) is not found in the nucleus
C) has a positive charge
D) more than one correct response
E) no correct response
A) has a mass slightly less than that of a proton
B) is not found in the nucleus
C) has a positive charge
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following electron configurations corresponds to an element in the same group of the periodic table as the element whose electron configuration is 1s22s22p5?
A) 1s22s22p4
B) 1s22s22p6
C) 1s22s22p63s23p64s23d5
D) more than one correct response
E) no correct response
A) 1s22s22p4
B) 1s22s22p6
C) 1s22s22p63s23p64s23d5
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following characterizations for the element 23V is correct?
A) a period 4 element
B) electron configuration ends in d3
C) an inner-transition element
D) more than one correct response
E) no correct response
A) a period 4 element
B) electron configuration ends in d3
C) an inner-transition element
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
Both a number and a letter are used in designating an electron subshell. The letter
A) indicates the shell to which the subshell belongs
B) may be s, p, d, or f
C) gives information about the maximum number of electrons the subshell can contain
D) more than one correct response
E) no correct response
A) indicates the shell to which the subshell belongs
B) may be s, p, d, or f
C) gives information about the maximum number of electrons the subshell can contain
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
Tin is element number 50. This means that all tin atoms have
A) an atomic number of 50
B) 50 neutrons in the nucleus
C) a total of 50 subatomic particles in the nucleus
D) more than one correct response
E) no correct response
A) an atomic number of 50
B) 50 neutrons in the nucleus
C) a total of 50 subatomic particles in the nucleus
D) more than one correct response
E) no correct response

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.
(2) The element sulfur is both a nonmetal and a representative element.
(3) Elements with electron configurations that end in p4 would be found in group VIA of the periodic table.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The element sulfur is both a nonmetal and a representative element.
(3) Elements with electron configurations that end in p4 would be found in group VIA of the periodic table.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Choose the correct atom that has a mass number of 17.
A)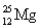
B)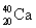
C)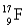
D)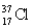
A)
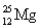
B)
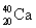
C)
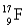
D)
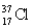

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Choose the correct atom that has a nuclear charge of +17
A)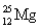
B)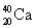
C)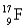
D)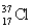
A)
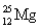
B)
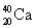
C)
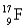
D)
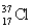

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
Choose the appropriate pair of atoms that contain the same number of nucleons.
A)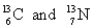
B)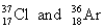
C)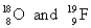
D)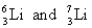
A)
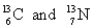
B)
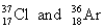
C)
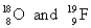
D)
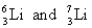

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the appropriate pair of atoms that contain the same number of electrons.
A)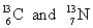
B)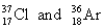
C)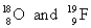
D)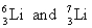
A)
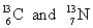
B)
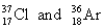
C)
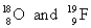
D)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) All naturally occurring atoms of an element must have the same mass number.
(2) Protons and electrons carry the same amount of charge.
(3) An isotope of chlorine with A = 35 and Z = 17 contains more neutrons than protons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Protons and electrons carry the same amount of charge.
(3) An isotope of chlorine with A = 35 and Z = 17 contains more neutrons than protons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
Choose the appropriate electron configuration for an element that has electrons in six orbitals.
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Choose the appropriate electron configuration for an element that has five completely filled subshells.
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
Has fewer neutrons than electrons.
A)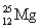
B)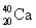
C)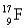
D)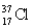
A)
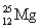
B)
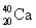
C)
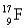
D)
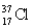

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
Choose the correct atom that has an equal number of protons, neutrons, and electrons.
A)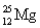
B)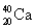
C)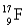
D)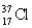
A)
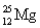
B)
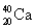
C)
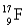
D)
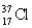

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) The maximum number of electrons that can occupy an electron shell is the same as the shell number.
(2) A nucleus is the very small dense uncharged center of an atom.
(3) The number of inner transition elements is greater than the number of transition elements.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) A nucleus is the very small dense uncharged center of an atom.
(3) The number of inner transition elements is greater than the number of transition elements.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
Choose the appropriate electron configuration for an element whose third shell contains six electrons.
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
Choose the appropriate electron configuration for an element with no unpaired electrons.
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
Choose the appropriate electron configuration for an element that has two unpaired electrons.
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
Choose the appropriate pair of atoms that contain the same number of neutrons.
A)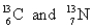
B)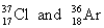
C)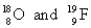
D)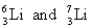
A)
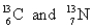
B)
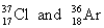
C)
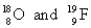
D)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) Most of the mass of an atom is concentrated in its nucleus.
(2) Sufficient information is present on a "standard" periodic table to determine the number of neutrons present in atoms of an element.
(3) An atom whose electron configuration is 1s22s22p63s1 contains 12 electrons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Sufficient information is present on a "standard" periodic table to determine the number of neutrons present in atoms of an element.
(3) An atom whose electron configuration is 1s22s22p63s1 contains 12 electrons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) An electron configuration specifies subshell occupancy for electrons.
(2) The atomic number is double the mass number for atoms that contain an equal number of protons and neutrons.
(3) All occupied orbitals are completely filled in atoms of the element neon, which contains a total of 12 electrons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) The atomic number is double the mass number for atoms that contain an equal number of protons and neutrons.
(3) All occupied orbitals are completely filled in atoms of the element neon, which contains a total of 12 electrons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) All of the major minerals in the human body are metals.
(2) Iron is the most abundant transition metal in the human body.
(3) Deuterium is an isotope of hydrogen in which two protons are present.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
(2) Iron is the most abundant transition metal in the human body.
(3) Deuterium is an isotope of hydrogen in which two protons are present.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
Choose the appropriate pair of atoms that contain the same number of subatomic particles.
A)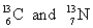
B)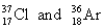
C)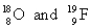
D)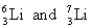
A)
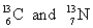
B)
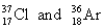
C)
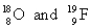
D)
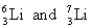

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
Choose the correct atom that has an atomic number of 17.
A)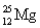
B)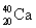
C)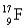
D)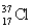
A)
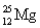
B)
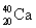
C)
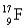
D)
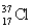

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
Choose the appropriate pair of atoms that contain the same number of protons.
A)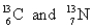
B)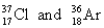
C)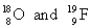
D)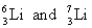
A)
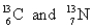
B)
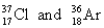
C)
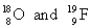
D)
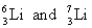

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
Choose an appropriate pair of elements that are in the d area of the periodic table.
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
Choose an appropriate pair of elements that have similar chemical properties.
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following elements is a transition element?
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following elements is both a metal and a representative element?
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following elements is both a nonmetal and a representative element?
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following elements is an inner-transition element?
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Choose an appropriate pair of elements that have electron configurations ending in p5.
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
Choose an appropriate pair of elements that are in the same period of the periodic table.
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
Choose an appropriate pair of elements that are in the same group of the periodic table.
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following elements is a halogen?
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


