Deck 23: Digestion and the Conversion of Food Into Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 23: Digestion and the Conversion of Food Into Energy
1
Considering the energy changes associated with the two individual reactions below, what is the energy change associated with the energetically favorable coupled reaction? 
A)- 11.2 kcal/mol
B)- 3.4 kcal/mol
C)- 7.3 kcal/mol
D)+ 3.4 kcal/mol
E)+ 11.2 kcal/mol

A)- 11.2 kcal/mol
B)- 3.4 kcal/mol
C)- 7.3 kcal/mol
D)+ 3.4 kcal/mol
E)+ 11.2 kcal/mol
B
2
Where does energy production occur in animal cells?
A)cell membrane
B)cytoplasm
C)mitochondria
D)nucleus
A)cell membrane
B)cytoplasm
C)mitochondria
D)nucleus
C
3
Where does the hydrolysis of carbohydrates to monosaccharides begin?
A)in the stomach
B)in the saliva
C)in the liver
D)in the small intestines
A)in the stomach
B)in the saliva
C)in the liver
D)in the small intestines
B
4
What is the classification of the reaction shown? 
A)redox
B)isomerization
C)decarboxylation
D)hydrolysis

A)redox
B)isomerization
C)decarboxylation
D)hydrolysis

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
How many reactions make up the citric acid cycle?
A)3
B)4
C)6
D)8
A)3
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
If the phosphorylation of GMP to form GDP requires 7.3 kcal/mol of energy, what is the energy change associated with the hydrolysis of GDP to form GMP?
A)7.3 kcal/mol
B)-7.3 kcal/mol
C)14.6 kcal/mol
D)Not enough information is given to determine the change in energy.
A)7.3 kcal/mol
B)-7.3 kcal/mol
C)14.6 kcal/mol
D)Not enough information is given to determine the change in energy.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
Which functional group is not contained in coenzyme A, whose structure is shown below? 
A)alcohol
B)sulfhydryl
C)phosphoester
D)amide
E)aldehyde

A)alcohol
B)sulfhydryl
C)phosphoester
D)amide
E)aldehyde

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
The addition of a phosphate group to ADP, forming ATP, is an example of what type of reaction?
A)hydration
B)hydrolysis
C)phosphorylation
D)reduction
E)decarboxylation
A)hydration
B)hydrolysis
C)phosphorylation
D)reduction
E)decarboxylation

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
How many molecules of reduced coenzymes are produced for each turn of the citric acid cycle?
A)2
B)4
C)16
D)32
A)2
B)4
C)16
D)32

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
Which is not true about coenzymes?
A)Many reactions in metabolic pathways involve coenzymes.
B)When a coenzyme gains hydrogen atoms the coenzyme is an oxidizing agent.
C)Many coenzymes are involved in oxidation and reduction reactions.
D)The coenzyme nicotinamide adenine dinucleotide, NAD+, is a common biological reducing agent.
A)Many reactions in metabolic pathways involve coenzymes.
B)When a coenzyme gains hydrogen atoms the coenzyme is an oxidizing agent.
C)Many coenzymes are involved in oxidation and reduction reactions.
D)The coenzyme nicotinamide adenine dinucleotide, NAD+, is a common biological reducing agent.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
Considering the energy changes associated with the individual reactions below, which reaction can be coupled with the hydrolysis of ATP to generate a coupled reaction that is energetically favorable? 
A)fructose 6-phosphate + HPO42- fructose 1, 6-bisphosphate + H2O
fructose 1, 6-bisphosphate + H2O
B)succinate + HSCoA + HPO42- succinyl CoA + H2O
succinyl CoA + H2O
C)3-phosphoglycerate + HPO42- 1, 3-bisphosphoglycerate + H2O
1, 3-bisphosphoglycerate + H2O
D)All of the reactions can be coupled with the hydrolysis of ATP to generate a coupled reaction that is energetically favorable.

A)fructose 6-phosphate + HPO42-
 fructose 1, 6-bisphosphate + H2O
fructose 1, 6-bisphosphate + H2OB)succinate + HSCoA + HPO42-
 succinyl CoA + H2O
succinyl CoA + H2OC)3-phosphoglycerate + HPO42-
 1, 3-bisphosphoglycerate + H2O
1, 3-bisphosphoglycerate + H2OD)All of the reactions can be coupled with the hydrolysis of ATP to generate a coupled reaction that is energetically favorable.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
Which coupled reaction properly indicates the role of the coenzyme as an oxidizing agent?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
Which is the first stage of catabolism?
A)digestion
B)citric acid cycle
C)fatty acid oxidation
D)glycolysis
E)formation of acetyl CoA
A)digestion
B)citric acid cycle
C)fatty acid oxidation
D)glycolysis
E)formation of acetyl CoA

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
Which is the abbreviated structure of acetyl CoA?
A)
B)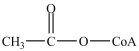
C)
D)
A)

B)
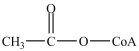
C)

D)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
Which is the sum of all of the chemical reactions that take place in an organism?
A)anabolism
B)metabolism
C)catabolism
D)citric acid cycle
A)anabolism
B)metabolism
C)catabolism
D)citric acid cycle

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
How many molecules of CO2 are produced for each turn of the citric acid cycle?
A)1
B)2
C)4
D)8
A)1
B)2
C)4
D)8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
Considering the energy changes associated with the two individual reactions below, what coupled reaction is an energetically favorable one? 
A)fructose 1, 6-bisphosphate + H2O fructose 6-phosphate + HPO42-
fructose 6-phosphate + HPO42-
B)ADP + fructose 1, 6-bisphosphate ATP + fructose 6-phosphate
ATP + fructose 6-phosphate
C)ATP + H2O ADP + HPO42-
ADP + HPO42-
D)ADP + fructose 6-phosphate ATP + fructose 1, 6-bisphosphate
ATP + fructose 1, 6-bisphosphate
E)ATP + fructose 6-phosphate ADP + fructose 1, 6-bisphosphate
ADP + fructose 1, 6-bisphosphate

A)fructose 1, 6-bisphosphate + H2O
 fructose 6-phosphate + HPO42-
fructose 6-phosphate + HPO42-B)ADP + fructose 1, 6-bisphosphate
 ATP + fructose 6-phosphate
ATP + fructose 6-phosphateC)ATP + H2O
 ADP + HPO42-
ADP + HPO42-D)ADP + fructose 6-phosphate
 ATP + fructose 1, 6-bisphosphate
ATP + fructose 1, 6-bisphosphateE)ATP + fructose 6-phosphate
 ADP + fructose 1, 6-bisphosphate
ADP + fructose 1, 6-bisphosphate
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
Which molecule contains the largest amount of stored energy?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
How many molecules of GTP are produced for each turn of the citric acid cycle?
A)1
B)2
C)4
D)8
A)1
B)2
C)4
D)8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
What is the classification of the reaction shown? 
A)oxidation
B)reduction
C)decarboxylation
D)hydrolysis
E)isomerization

A)oxidation
B)reduction
C)decarboxylation
D)hydrolysis
E)isomerization

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
How much ATP is generated for each acetyl CoA during stages [3] and [4] of catabolism?
A)4 molecules of ATP
B)5 molecules of ATP
C)8 molecules of ATP
D)10 molecules of ATP
E)16 molecules of ATP
A)4 molecules of ATP
B)5 molecules of ATP
C)8 molecules of ATP
D)10 molecules of ATP
E)16 molecules of ATP

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
How many reactions in the citric acid cycle generate FADH2?
A)0
B)1
C)3
D)8
A)0
B)1
C)3
D)8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
Why is hydrogen cyanide, HCN, poisonous?
A)HCN is a strong acid, dramatically altering the pH in cells.
B)Cyanide ions (-CN)irreversibly bind to the Fe3+ ion of cytochrome oxidase.
C)Cyanide ions (-CN)irreversibly bind to acetyl CoA.
D)Cyanide ions (-CN)irreversibly bind to NAD+.
A)HCN is a strong acid, dramatically altering the pH in cells.
B)Cyanide ions (-CN)irreversibly bind to the Fe3+ ion of cytochrome oxidase.
C)Cyanide ions (-CN)irreversibly bind to acetyl CoA.
D)Cyanide ions (-CN)irreversibly bind to NAD+.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
Where does the electron transport chain process take place?
A)in the inner membrane of mitochondria
B)in the outer membrane of mitochondria
C)in the intermembrane space of mitochondria
D)in the matrix of mitochondria
A)in the inner membrane of mitochondria
B)in the outer membrane of mitochondria
C)in the intermembrane space of mitochondria
D)in the matrix of mitochondria

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
In which stage of metabolism are biomolecules degraded into two-carbon acetyl units?
A)Stage [1]
B)Stage [2]
C)Stage [3]
D)Stage [4]
A)Stage [1]
B)Stage [2]
C)Stage [3]
D)Stage [4]

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
Which is the primary energy-carrying molecule in metabolic pathways?
A)AMP
B)ATP
C)NADH
D)Acetyl CoA
E)FADH2
A)AMP
B)ATP
C)NADH
D)Acetyl CoA
E)FADH2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
Which intermediate in the citric acid cycle contains two chirality centers?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
What is the primary function of the citric acid cycle in metabolism?
A)to break down food molecules into smaller components so they can be absorbed by the blood
B)to synthesize ATP from the energy produced in the hydrolysis of citric acid
C)to provide the enzymes necessary to aid in the hydrolysis of carbohydrates, proteins, and lipids
D)to convert acetyl groups to CO2 molecules and provide reduced coenzymes for the electron transport chain
A)to break down food molecules into smaller components so they can be absorbed by the blood
B)to synthesize ATP from the energy produced in the hydrolysis of citric acid
C)to provide the enzymes necessary to aid in the hydrolysis of carbohydrates, proteins, and lipids
D)to convert acetyl groups to CO2 molecules and provide reduced coenzymes for the electron transport chain

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
In which region of a mitochondrion would the pH be lower?
A)the matrix
B)the intermembrane space
C)the inner mitochondrial membrane
D)all regions of a mitochondrion have the same pH
A)the matrix
B)the intermembrane space
C)the inner mitochondrial membrane
D)all regions of a mitochondrion have the same pH

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
Which product(s)of the citric acid cycle are funneled into the electron transport chain?
A)ATP
B)NAD+ and FAD
C)NADH, FADH2, and H+
D)NADH, FADH2, H+, and ATP
A)ATP
B)NAD+ and FAD
C)NADH, FADH2, and H+
D)NADH, FADH2, H+, and ATP

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
Which intermediate in the citric acid cycle is a secondary alcohol?
A)
B)
C)
D)
E)Two of the intermediates above are secondary alcohols.
A)

B)

C)

D)

E)Two of the intermediates above are secondary alcohols.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
Which is the enzyme that hydrolyzes triacylglycerols?
A)acetyl CoA
B)protease pepsin
C)amylase
D)lipase
A)acetyl CoA
B)protease pepsin
C)amylase
D)lipase

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
Which is not a reaction found in stage [2] of catabolism?
A)fatty acid oxidation
B)glycolysis
C)oxidative phosphorylation
D)amino acid catabolism
A)fatty acid oxidation
B)glycolysis
C)oxidative phosphorylation
D)amino acid catabolism

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
In which stage of catabolism is starch hydrolyzed to glucose with the aid of the enzyme amylase?
A)Stage [1]
B)Stage [2]
C)Stage [3]
D)Stage [4]
A)Stage [1]
B)Stage [2]
C)Stage [3]
D)Stage [4]

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
How many reactions in the citric acid cycle generate NAD+?
A)0
B)1
C)3
D)8
A)0
B)1
C)3
D)8

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
Which is an oxidizing agent?
A)Fe2+
B)NAD+
C)FADH2
D)ATP
A)Fe2+
B)NAD+
C)FADH2
D)ATP

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
Which best describes the function of the coenzymes NAD+ and FAD in catabolic pathways?
A)competitive inhibitors of the enzymes present in each pathway
B)non-competitive inhibitors of the enzymes present in each pathway
C)oxidizing agents that accept electrons and hydrogen ions from molecules undergoing oxidation
D)transport molecules that carry acetyl units to or from the different stages of metabolism
A)competitive inhibitors of the enzymes present in each pathway
B)non-competitive inhibitors of the enzymes present in each pathway
C)oxidizing agents that accept electrons and hydrogen ions from molecules undergoing oxidation
D)transport molecules that carry acetyl units to or from the different stages of metabolism

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
Which is the major product of stage [2] of catabolism?
A)NADH
B)acetyl CoA
C)GTP
D)CO2
A)NADH
B)acetyl CoA
C)GTP
D)CO2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Which statement concerning the digestion of food groups is incorrect?
A)Carbohydrates are ultimately broken down to glucose and other monosaccharides.
B)Triacylglycerols are ultimately broken down by lipases into glycerol and fatty acids.
C)Proteins are ultimately broken down by proteases into amino acids.
D)Polysaccharides are ultimately broken down to nucleotides.
A)Carbohydrates are ultimately broken down to glucose and other monosaccharides.
B)Triacylglycerols are ultimately broken down by lipases into glycerol and fatty acids.
C)Proteins are ultimately broken down by proteases into amino acids.
D)Polysaccharides are ultimately broken down to nucleotides.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
At which stage of metabolism is the most energy in the form of ATP produced?
A)electron transport chain and oxidative phosphorylation
B)digestion
C)citric acid cycle
D)transcription
A)electron transport chain and oxidative phosphorylation
B)digestion
C)citric acid cycle
D)transcription

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
The primary function of the electron transport chain and oxidative phosphorylation is which of the following?
A)to provide electrons and energy for the phosphorylation of ATP
B)to synthesize the reduced coenzymes necessary for digestion
C)to oxidize the reduced coenzymes NADH and FADH2, and provide energy for the synthesis of ATP
D)to transport electrons from ATP to ADP, and synthesize acetyl CoA molecules
A)to provide electrons and energy for the phosphorylation of ATP
B)to synthesize the reduced coenzymes necessary for digestion
C)to oxidize the reduced coenzymes NADH and FADH2, and provide energy for the synthesis of ATP
D)to transport electrons from ATP to ADP, and synthesize acetyl CoA molecules

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Coenzyme A is a biological oxidizing agent used to convert alcohols to carbonyl-containing compounds.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
Step 7 of the citric acid cycle is shown. Which statement best describes what occurs in this step? 
A)Fumarate undergoes hydrolysis with the aid of the enzyme fumarase.
B)Fumarate undergoes reduction with the aid of the cofactor fumarase.
C)Fumarate undergoes hydrogenation with hydrogens and electrons provided by the enzyme fumarase.
D)Fumarate undergoes hydration with the aid of the enzyme fumarase.

A)Fumarate undergoes hydrolysis with the aid of the enzyme fumarase.
B)Fumarate undergoes reduction with the aid of the cofactor fumarase.
C)Fumarate undergoes hydrogenation with hydrogens and electrons provided by the enzyme fumarase.
D)Fumarate undergoes hydration with the aid of the enzyme fumarase.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
Step 7 of the citric acid cycle is shown. Which statement best describes what occurs in this step? 
A)Fumarate undergoes hydrolysis with the aid of the enzyme fumarase.
B)Fumarate undergoes reduction with the aid of the cofactor fumarase.
C)Fumarate undergoes hydrogenation with hydrogens and electrons provided by the enzyme fumarase.
D)Fumarate undergoes hydration with the aid of the enzyme fumarase.

A)Fumarate undergoes hydrolysis with the aid of the enzyme fumarase.
B)Fumarate undergoes reduction with the aid of the cofactor fumarase.
C)Fumarate undergoes hydrogenation with hydrogens and electrons provided by the enzyme fumarase.
D)Fumarate undergoes hydration with the aid of the enzyme fumarase.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
The final stage of the electron transport chain forms water in an anaerobic process.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
Energy released from H+ movement in the electron transport chain fuels the phosphorylation of ADP.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
Oxaloacetate is the starting material in the first step of the citric acid cycle and the product of the last step. The last step of the citric acid cycle, the oxidation of malate to oxaloacetate, is shown. What is the structure of oxaloacetate? 
A)
B)
C)
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Which statement best describes how the interconversion of ATP and ADP is responsible for storing and providing energy for cellular reactions? 
A)The energy stored in ADP is released when ATP is synthesized.
B)The energy required to phosphorylate ADP is stored in ATP, and released when ATP undergoes hydrolysis.
C)The energy released in the phosphorylation of ADP can be coupled with unfavorable reactions within the cell.
D)The energy required to hydrolyze ATP comes from the energy producing reactions in the cell; the energy lost is then stored in ATP.

A)The energy stored in ADP is released when ATP is synthesized.
B)The energy required to phosphorylate ADP is stored in ATP, and released when ATP undergoes hydrolysis.
C)The energy released in the phosphorylation of ADP can be coupled with unfavorable reactions within the cell.
D)The energy required to hydrolyze ATP comes from the energy producing reactions in the cell; the energy lost is then stored in ATP.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
Oxaloacetate is the starting material in the first step of the citric acid cycle and the product of the last step. The last step of the citric acid cycle, the oxidation of malate to oxaloacetate, is shown. What is the structure of oxaloacetate? 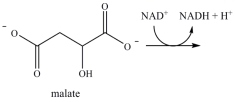
A)
B)
C)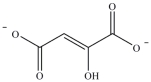
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
The terms phosphorylation and oxidative phosphorylation may be used interchangeably.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Coenzyme A is synthesized in cells from pantothenic acid, vitamin B5.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
The conversion of Fe3+ to Fe2+ in the electron transport chain is an example of Fe3+ acting as an oxidizing agent.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
The citric acid cycle is also called the tricarboxylic acid cycle (TCA cycle).

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
In the electron transport chain, H+ ions are pumped across the inner membrane of the mitochondrion, forming a high concentration of H+ ions in the intermembrane space, thus creating a potential energy gradient.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
All steps of the citric acid cycle are enzyme catalyzed.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
NAD+ is the abbreviation for nicotinamide adenine dinucleotide.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
The electron transport chain is a multistep process that relies on five enzyme systems as well as mobile electron carriers.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
Since more energy is released from the hydrolysis of creatine phosphate than is needed for the phosphorylation of ADP, the coupling of these two reactions results in the formation of ATP from ADP.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
The citric acid cycle comprises stage [4] of metabolism.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
H+ ions generated by reactions in the electron transport chain, as well as H+ ions present in the matrix of the mitochondria, are pumped across the inner mitochondrial membrane into the intermembrane space at three different sites.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
The rate of the citric acid cycle depends on the body's need for energy. When energy demands are low and NADH concentration is high, the cycle is inhibited.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Stage [3] of catabolism is sometimes called aerobic respiration.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
The structure of flavin adenine dinucleotide (FAD)is shown here. 


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
Coenzyme A, NADH, and FAD all contain phosphate groups.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
Two electrons are donated by each NADH in the electron transport chain.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
The cleavage of a protein with chymotrypsin occurs in stage [1] of catabolism.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
The structure of coenzyme A contains amine and amide functional groups.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
Within the cell, energy production occurs in the matrix of the mitochondrion.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
The reaction shown below requires the overall input of energy. 


Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
Any energy requiring reaction can be coupled with ATP hydrolysis to create an energetically favorable coupled reaction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
Acetyl CoA contains an acetyl group bonded to coenzyme A by a thioester bond.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
Each NADH enters the electron transport chain at complex I in the inner mitochondrial membrane and the resulting cascade of reactions produces enough energy to synthesize 4 ATPs.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
The product of the catabolic pathways is different for proteins, triacylglycerols, and carbohydrates.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
Part [2] of the citric acid cycle includes two separate decarboxylation reactions and a hydrolysis reaction.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
GTP is a high energy compound.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
The primary function of the citric acid cycle in metabolism is to synthesize ATP from the energy produced in the hydrolysis of citric acid.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
The primary function of the electron transport chain and oxidative phosphorylation is to provide electrons for the hydrolysis of ATP.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
Anabolism is an energy releasing process that involves the synthesis of large molecules from smaller ones.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
The cells in heart tissue have more mitochondria than the cells in bone tissue.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
The energy produced by the citric acid cycle is stored in the bonds of a nucleoside triphosphate and reduced coenzymes.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck


