Deck 15: The Three-Dimensional Shape of Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 15: The Three-Dimensional Shape of Molecules
1
Which molecule is chiral?
A)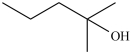
B)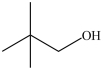
C)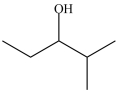
D)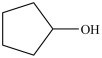
A)

B)

C)

D)

C
2
How many chirality centers does the following molecule contain? 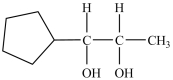
A)1
B)2
C)3
D)8

A)1
B)2
C)3
D)8
B
3
How many chirality centers does the following molecule contain? 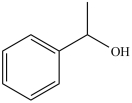
A)0
B)1
C)2
D)3

A)0
B)1
C)2
D)3
B
4
Consider the stereoisomers (A-E)drawn below: 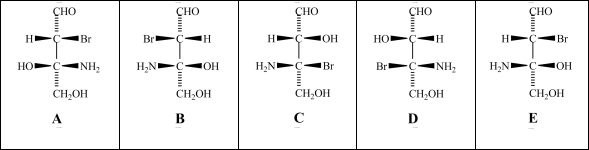 Which structure is an enantiomer of structure A?
Which structure is an enantiomer of structure A?
A)structure B
B)structure C
C)structure D
D)structure E
 Which structure is an enantiomer of structure A?
Which structure is an enantiomer of structure A?A)structure B
B)structure C
C)structure D
D)structure E

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the stereoisomers (A-E)drawn below: 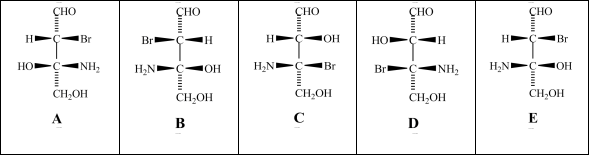 Which structure(s)is/are a diastereomer of structure C?
Which structure(s)is/are a diastereomer of structure C?
A)structure A
B)structure B
C)structure D
D)structure E
E)None of the structures is a diastereomer of structure C.
 Which structure(s)is/are a diastereomer of structure C?
Which structure(s)is/are a diastereomer of structure C?A)structure A
B)structure B
C)structure D
D)structure E
E)None of the structures is a diastereomer of structure C.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
Which molecule is not an enantiomer of the molecule whose structure is shown below? 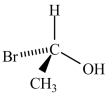
A)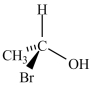
B)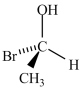
C)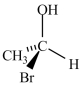
D)All the molecules above are enantiomers of the original structure.

A)

B)

C)

D)All the molecules above are enantiomers of the original structure.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Which molecule is an enantiomer of the one shown below? 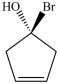
A)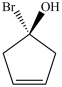
B)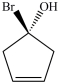
C)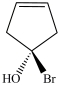
D)None of the molecules above is an enantiomer of the original molecule.

A)

B)

C)

D)None of the molecules above is an enantiomer of the original molecule.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
What is always true about compounds related as isomers?
A)They differ in the way that atoms are connected to one another.
B)They have different functional groups.
C)They have the same molecular formula.
D)They are mirror images of each other.
E)All of the statements above are correct.
A)They differ in the way that atoms are connected to one another.
B)They have different functional groups.
C)They have the same molecular formula.
D)They are mirror images of each other.
E)All of the statements above are correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
How many chirality centers does the following molecule contain? 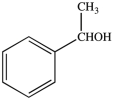
A)0
B)1
C)2
D)3

A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
How are the two compounds below related to each other? 
A)They are identical molecules.
B)They are constitutional isomers.
C)They are enantiomers.
D)They are diastereomers.

A)They are identical molecules.
B)They are constitutional isomers.
C)They are enantiomers.
D)They are diastereomers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
The Fischer projection formula for D-mannose, a hexose sugar, is shown below. How many chirality centers are in D-mannose? 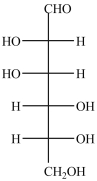
A)2
B)4
C)5
D)6

A)2
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
Diastereomers are stereoisomers that are
A)mirror images of each other.
B)not mirror images of each other.
C)nonsuperimposable mirror images of each other.
D)superimposable on each other.
A)mirror images of each other.
B)not mirror images of each other.
C)nonsuperimposable mirror images of each other.
D)superimposable on each other.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
Which object is chiral?
A)a sock
B)a shoe
C)a baseball
D)a fork
E)More than one of the objects above is chiral.
A)a sock
B)a shoe
C)a baseball
D)a fork
E)More than one of the objects above is chiral.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
Which molecule is chiral?
A)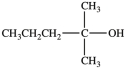
B)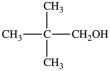
C)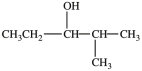
D)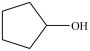
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
How many chirality centers are present in the compound below? 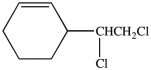
A)0
B)1
C)2
D)3
E)5

A)0
B)1
C)2
D)3
E)5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Which, if any, of the labeled carbon atoms in the compound below is a chirality center? 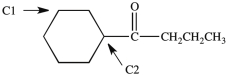
A)C1 is a chirality center and C2 is not a chirality center.
B)C1 is not a chirality center and C2 is a chirality center.
C)C1 and C2 are both chirality centers.
D)Neither C1 nor C2 are chirality centers.

A)C1 is a chirality center and C2 is not a chirality center.
B)C1 is not a chirality center and C2 is a chirality center.
C)C1 and C2 are both chirality centers.
D)Neither C1 nor C2 are chirality centers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
Which molecule is chiral?
A)1-pentanol
B)cyclopentanol
C)2-methylcyclopentanol
D)2-methylpentane
E)More than one of the molecules above is chiral.
A)1-pentanol
B)cyclopentanol
C)2-methylcyclopentanol
D)2-methylpentane
E)More than one of the molecules above is chiral.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
Which, if any, of the labeled carbon atoms in the compound below is a chirality center? 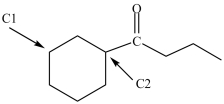
A)C1 is a chirality center and C2 is not a chirality center.
B)C1 is not a chirality center and C2 is a chirality center.
C)C1 and C2 are both chirality centers.
D)Neither C1 nor C2 are chirality centers.

A)C1 is a chirality center and C2 is not a chirality center.
B)C1 is not a chirality center and C2 is a chirality center.
C)C1 and C2 are both chirality centers.
D)Neither C1 nor C2 are chirality centers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the stereoisomers (A-E)drawn below: 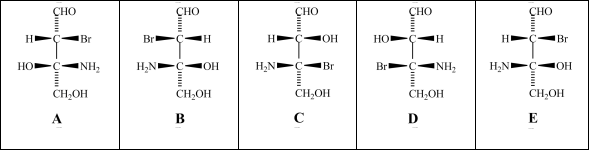 Structures A and C are related as _____.
Structures A and C are related as _____.
A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers
 Structures A and C are related as _____.
Structures A and C are related as _____.A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the stereoisomers (A-E)drawn below: 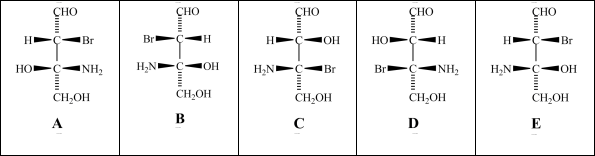 Which structure(s)is/are diastereomers of structure E?
Which structure(s)is/are diastereomers of structure E?
A)structure A
B)structure B
C)structure C
D)structure D
E)structures A and B
 Which structure(s)is/are diastereomers of structure E?
Which structure(s)is/are diastereomers of structure E?A)structure A
B)structure B
C)structure C
D)structure D
E)structures A and B

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
Which is NOT another name for a chirality center?
A)stereogenic center
B)chiral carbon
C)asymmetric carbon
D)tetravalent carbon
A)stereogenic center
B)chiral carbon
C)asymmetric carbon
D)tetravalent carbon

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Which nonsteroidal anti-inflammatory drug(s)(NSAID)is/are sold as a racemic mixture?
A)Naproxen
B)Ibuprofen
C)Both Naproxen and Ibuprofen are sold as a racemic mixture.
D)Neither Naproxen nor Ibuprofen are sold as a racemic mixture.
A)Naproxen
B)Ibuprofen
C)Both Naproxen and Ibuprofen are sold as a racemic mixture.
D)Neither Naproxen nor Ibuprofen are sold as a racemic mixture.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
How many enantiomers can a chiral stereoisomer have?
A)0
B)1
C)2
D)The number of enantiomers depends on the number of chirality centers.
A)0
B)1
C)2
D)The number of enantiomers depends on the number of chirality centers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement is not true?
A)A molecule that is not superimposable on its mirror image is a chiral molecule.
B)Enantiomers are mirror images that are not superimposable.
C)A carbon atom surrounded by four different groups is a chirality center.
D)A racemic mixture contains an equal mixture of two diastereomers.
A)A molecule that is not superimposable on its mirror image is a chiral molecule.
B)Enantiomers are mirror images that are not superimposable.
C)A carbon atom surrounded by four different groups is a chirality center.
D)A racemic mixture contains an equal mixture of two diastereomers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
How are the Fischer projections below related? 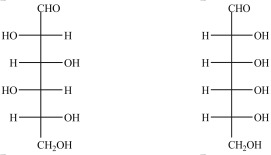
A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers

A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
The drug L-Dopa
A)is marketed as a racemic mixture for the treatment of Parkinson's disease.
B)was first isolated from the leaves of the broad bean Vicia faba.
C)is a chiral molecule with two chirality centers.
D)has only one enantiomer that is active against Parkinson's disease.
A)is marketed as a racemic mixture for the treatment of Parkinson's disease.
B)was first isolated from the leaves of the broad bean Vicia faba.
C)is a chiral molecule with two chirality centers.
D)has only one enantiomer that is active against Parkinson's disease.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
In a Fischer projection,
A)horizontal lines represent bonds that come out of the plane on wedges, and the vertical lines represent bonds that go back on dashed lines.
B)vertical lines represent bonds that come out of the plane on wedges, and the horizontal lines represent bonds that go back on dashed lines.
C)horizontal lines represent bonds that are in the plane of the paper, and the vertical lines represent bonds that go back on dashed lines.
D)vertical lines represent bonds that are in the plane of the paper, and the horizontal lines represent bonds that go back on dashed lines.
A)horizontal lines represent bonds that come out of the plane on wedges, and the vertical lines represent bonds that go back on dashed lines.
B)vertical lines represent bonds that come out of the plane on wedges, and the horizontal lines represent bonds that go back on dashed lines.
C)horizontal lines represent bonds that are in the plane of the paper, and the vertical lines represent bonds that go back on dashed lines.
D)vertical lines represent bonds that are in the plane of the paper, and the horizontal lines represent bonds that go back on dashed lines.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Penicillamine is used to treat arthritis. How many chirality centers are present in penicillamine? 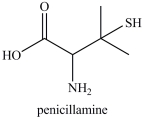
A)0
B)1
C)2
D)3
E)more than 3

A)0
B)1
C)2
D)3
E)more than 3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the stereoisomers (A-E)drawn below: 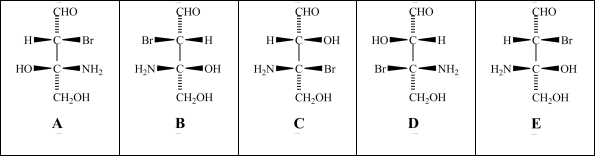 Structures C and D are _____.
Structures C and D are _____.
A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers
 Structures C and D are _____.
Structures C and D are _____.A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
What property is the odor of a molecule most dependent on?
A)the functional groups present in the molecule
B)the shape of the molecule
C)whether a molecule can form hydrogen bonds
D)the number of carbons in the molecule
A)the functional groups present in the molecule
B)the shape of the molecule
C)whether a molecule can form hydrogen bonds
D)the number of carbons in the molecule

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
Compounds related as constitutional isomers have
A)the same number of chirality centers.
B)the same functional groups.
C)the same molecular formulas.
D)the same smell.
E)All of the statements above are true.
A)the same number of chirality centers.
B)the same functional groups.
C)the same molecular formulas.
D)the same smell.
E)All of the statements above are true.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Alanine, shown below, is a naturally occurring chiral amino acid. Which pair represents the two enantiomers of alanine? 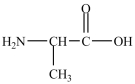
A)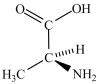 and
and 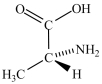
B)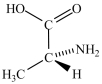 and
and 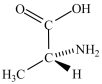
C)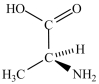 and
and 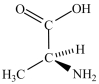

A)
 and
and 
B)
 and
and 
C)
 and
and 

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
Which structure represents the three-dimensional structure of the Fischer projection shown? 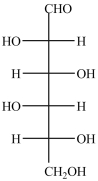
A)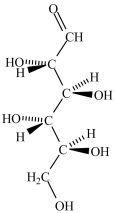
B)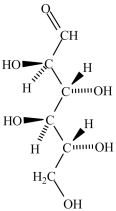
C)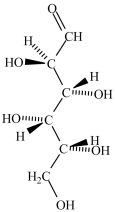
D)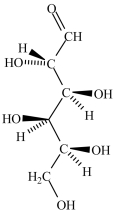

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
Which compound has the most stereoisomers?
A)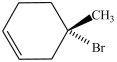
B)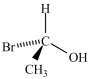
C)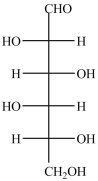
D)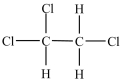
E)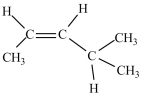
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Penicillamine is used to treat arthritis. How many chirality centers are present in penicillamine? 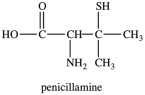
A)0
B)1
C)2
D)3
E)more than 3

A)0
B)1
C)2
D)3
E)more than 3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
Enantiomers are stereoisomers that are
A)mirror images of each other that are identical.
B)not mirror images of each other.
C)nonsuperimposable mirror images of each other.
D)cis-trans isomers of each other.
A)mirror images of each other that are identical.
B)not mirror images of each other.
C)nonsuperimposable mirror images of each other.
D)cis-trans isomers of each other.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
How many diastereomers can a chiral stereoisomer have?
A)1
B)2
C)3
D)The number of diastereomers depends on the number of chirality centers.
A)1
B)2
C)3
D)The number of diastereomers depends on the number of chirality centers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Which statement is true?
A)A carbon that is part of a multiple is always a chirality center.
B)The carbon atom in a CH2 group is a chirality center only when it is part of a ring.
C)Stereoisomers always have the same functional groups.
D)Diastereomers are mirror image isomers that are not superimposable.
A)A carbon that is part of a multiple is always a chirality center.
B)The carbon atom in a CH2 group is a chirality center only when it is part of a ring.
C)Stereoisomers always have the same functional groups.
D)Diastereomers are mirror image isomers that are not superimposable.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
How are the structures below related? 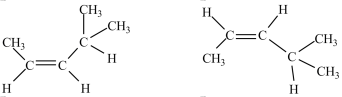
A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers

A)identical molecules
B)enantiomers
C)constitutional isomers
D)diastereomers

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
The drug Thalidomide was
A)invented by Frances Oldham Kelsey.
B)sold as a mixture of its two enantiomers, and each of these stereoisomers has a different biological activity.
C)was approved for use in the United States.
D)All of the statements above are true.
A)invented by Frances Oldham Kelsey.
B)sold as a mixture of its two enantiomers, and each of these stereoisomers has a different biological activity.
C)was approved for use in the United States.
D)All of the statements above are true.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Two enantiomers typically have the same biological activity.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
The compound 3, 6-diethyl-6-methyl-3-decanethiol is a chiral molecule with more than one chirality center.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement concerning stereochemistry is incorrect?
A)An object or molecule is chiral if it is nonsuperimposable on its mirror image.
B)A chiral molecule is optically active and rotates plane polarized light either clockwise or counterclockwise.
C)Enantiomers are superimposable mirror image isomers.
D)Diastereomers are stereoisomers that are not related as mirror images.
A)An object or molecule is chiral if it is nonsuperimposable on its mirror image.
B)A chiral molecule is optically active and rotates plane polarized light either clockwise or counterclockwise.
C)Enantiomers are superimposable mirror image isomers.
D)Diastereomers are stereoisomers that are not related as mirror images.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
Any molecule with one chirality center is a chiral compound.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
Hexane (CH3CH2CH2CH2CH2CH3)has a mirror image, but it does not have an enantiomer.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
Recent rulings by the Food and Drug Administration have encouraged the development of so-called racemic switches, the patenting and marketing of a single enantiomer that was originally sold as a racemic mixture.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
The specific rotation of pure cholesterol was measured to be -32. Based on this information, which statement concerning cholesterol is NOT true?
A)Cholesterol is a chiral compound.
B)Cholesterol is optically inactive.
C)Cholesterol is levorotatory.
D)Cholesterol rotates plane polarized light in a counterclockwise direction.
A)Cholesterol is a chiral compound.
B)Cholesterol is optically inactive.
C)Cholesterol is levorotatory.
D)Cholesterol rotates plane polarized light in a counterclockwise direction.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
cis-2-butene and trans-2-butene are enantiomers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Another term in common use for a chirality center is an asymmetric carbon.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
The Fischer projection shown on the right properly represents the three-dimensional compound shown on the left. 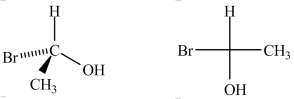
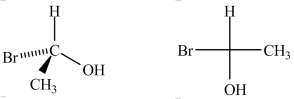

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
The molecule below is achiral. 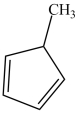


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
Enantiomers are mirror images that are not superimposable.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
The Fischer projection shown on the right properly represents the ball and stick model of the compound shown on the left. 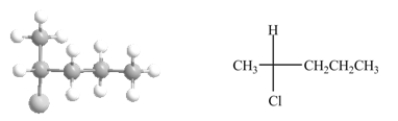


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
Any molecule with two chirality centers is an achiral compound.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
Propranol, shown below, is a chiral compound that exists as a pair of enantiomers. One enantiomer is used to treat irregular heartbeats, and the other enantiomer is used as a contraceptive. Identify the chirality center(s)in Propranol. 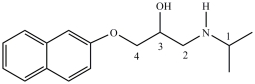
A)C1
B)C2
C)C3
D)C4
E)C1 and C3

A)C1
B)C2
C)C3
D)C4
E)C1 and C3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
A carbonyl carbon is a common example of a chirality center.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
Which property of enantiomers is not identical?
A)Melting point
B)Boiling point
C)Solubility
D)Specific rotation
E)All of the above properties are identical for a pair of enantiomers.
A)Melting point
B)Boiling point
C)Solubility
D)Specific rotation
E)All of the above properties are identical for a pair of enantiomers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
Propranol, shown below, is a chiral compound that exists as a pair of enantiomers. One enantiomer is used to treat irregular heartbeats, and the other enantiomer is used as a contraceptive. Identify the chirality center(s)in Propranol. 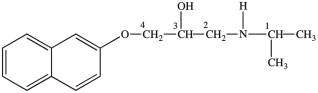
A)C1
B)C2
C)C3
D)C4
E)C1 and C3

A)C1
B)C2
C)C3
D)C4
E)C1 and C3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
Carbohydrates, lipids, proteins, and nucleic acids are all chiral biomolecules. What does the term chiral indicate about a molecule?
A)The molecule can be synthesized by the body.
B)The molecule can be digested by the body.
C)The molecule has a mirror image.
D)The molecule and its mirror image are not identical.
A)The molecule can be synthesized by the body.
B)The molecule can be digested by the body.
C)The molecule has a mirror image.
D)The molecule and its mirror image are not identical.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
The amino acid alanine found in the proteins of the body has a melting point of 297 °C and a specific rotation of +8.5. The enantiomer of alanine would have a melting point of -297 °C and a specific rotation of -8.5.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
Aspartame contains two chirality centers. 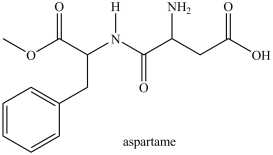


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
The compounds shown below are related as constitutional isomers. 


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
There are six chirality centers in the simple sugar D-glucose, shown below. 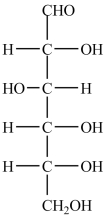


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
The major product of the dehydration of the alcohol below has one chirality center. 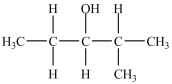


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
A nail is an example of a chiral object.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
The alkyl bromide below contains one chirality center. 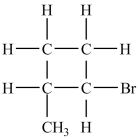


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
The Fischer projection shown to the right represents the enantiomer of the three-dimensional structure to the left. 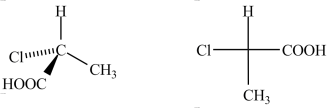


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
A chiral molecule has at least one carbon atom that has four different substituents bonded to it.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
The compound 4-methyl-2-hexene is not a chiral molecule.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
In organic molecules, oxygen atoms cannot be chirality centers.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
If a stereoisomer has an enantiomer, it does not have a diastereomer.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
All tertiary alcohols (3°)are chiral.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
Only chiral molecules have a mirror image.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
The compounds shown below are related as constitutional isomers. 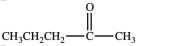


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Enantiomers may have different odors because they bind with chiral receptors and each enantiomer fits the chiral receptors in a different way.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
The Fischer projection to the right represents the compound to the left. 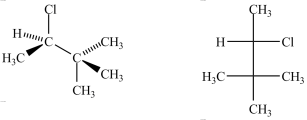


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
Aspartame contains two chirality centers. 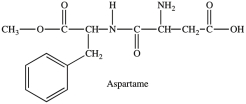


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
A human foot is a chiral object.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
For an odor to be perceived, a molecule must bind to an olfactory receptor, resulting in a nerve impulse that travels to the brain.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
The compound 3-methylcyclopentanol contains one chirality center.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


