Deck 13: Unsaturated Hydrocarbons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 13: Unsaturated Hydrocarbons
1
What is the IUPAC name of this compound? 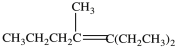
A)5-ethyl-4-methyl-4-heptene
B)3-ethyl-4-methyl-3-hexene
C)3-ethyl-4-methyl-4-heptene
D)3-ethyl-4-methyl-3-heptene
E)3-ethyl-4-propyl-2-pentene

A)5-ethyl-4-methyl-4-heptene
B)3-ethyl-4-methyl-3-hexene
C)3-ethyl-4-methyl-4-heptene
D)3-ethyl-4-methyl-3-heptene
E)3-ethyl-4-propyl-2-pentene
D
2
What is the IUPAC name for the compound CH3CH=CHCH2CH3?
A)pentene
B)2, 3-pentene
C)3-pentene
D)2-pentene
A)pentene
B)2, 3-pentene
C)3-pentene
D)2-pentene
D
3
The compound levonorgestrel, shown below, contains several different functional groups which allow it to be classified as more than one type of organic compound. Which compound classification type for levonorgestrel is incorrect? 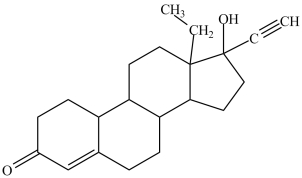
A)alcohol
B)alkene
C)alkyne
D)ketone
E)aromatic

A)alcohol
B)alkene
C)alkyne
D)ketone
E)aromatic
E
4
Which fatty acid has the lowest melting point?
A)CH3(CH2)12COOH
B)CH3(CH2)2CH=CH(CH2)8COOH
C)CH3(CH2)2CH=CH(CH2)2CH=CH(CH2)4COOH
D)CH3(CH2)14COOH
A)CH3(CH2)12COOH
B)CH3(CH2)2CH=CH(CH2)8COOH
C)CH3(CH2)2CH=CH(CH2)2CH=CH(CH2)4COOH
D)CH3(CH2)14COOH

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
Which name is a valid IUPAC name of an unsaturated hydrocarbon?
A)2, 3-dimethyl-3-hexyne
B)trans-1-hexene
C)trans-3-pentyne
D)5-butylcyclohexene
E)2-methyl-2-butene
A)2, 3-dimethyl-3-hexyne
B)trans-1-hexene
C)trans-3-pentyne
D)5-butylcyclohexene
E)2-methyl-2-butene

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
The molecules cis-2-pentene and trans-2-pentene are an example of a pair of
A)isotopes.
B)stereoisomers.
C)constitutional isomers.
D)identical molecules.
A)isotopes.
B)stereoisomers.
C)constitutional isomers.
D)identical molecules.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
Which is the molecular formula of an alkyne?
A)C10H18
B)C9H18
C)C8H18
D)C10H20
A)C10H18
B)C9H18
C)C8H18
D)C10H20

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
Which types of compounds are saturated hydrocarbons?
A)alkenes
B)aromatic hydrocarbons
C)alkanes
D)alkynes
E)All of the hydrocarbons above are unsaturated hydrocarbons.
A)alkenes
B)aromatic hydrocarbons
C)alkanes
D)alkynes
E)All of the hydrocarbons above are unsaturated hydrocarbons.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name of this compound? 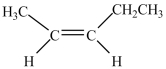
A)cis-3-pentene
B)cis-2-pentene
C)ethylpropene
D)1-methyl-2-ethylpentene
E)trans-3-pentene

A)cis-3-pentene
B)cis-2-pentene
C)ethylpropene
D)1-methyl-2-ethylpentene
E)trans-3-pentene

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
What is the structure of 3-methylcycloheptene?
A)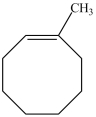
B)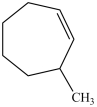
C)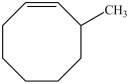
D)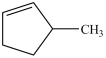
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
What product is formed from the reaction below? 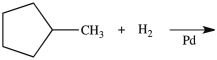
A)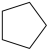
B)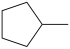
C)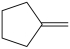
D)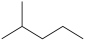
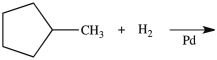
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name of this compound? 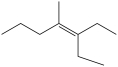
A)5-ethyl-4-methyl-4-heptene
B)3-ethyl-4-methyl-3-hexene
C)3-ethyl-4-methyl-4-heptene
D)3-ethyl-4-methyl-3-heptene
E)3-ethyl-4-propyl-2-pentene

A)5-ethyl-4-methyl-4-heptene
B)3-ethyl-4-methyl-3-hexene
C)3-ethyl-4-methyl-4-heptene
D)3-ethyl-4-methyl-3-heptene
E)3-ethyl-4-propyl-2-pentene

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
Which structure is trans-3-hexene?
A)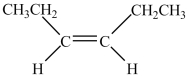
B)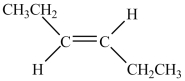
C)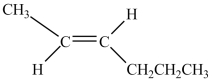
D)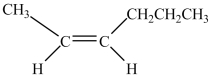
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
What configuration of a double bond is more common in naturally occurring fatty acids?
A)cis
B)trans
C)cis and trans are equally likely to occur.
A)cis
B)trans
C)cis and trans are equally likely to occur.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
Which fatty acid has the highest melting point?
A)CH3(CH2)12COOH
B)CH3(CH2)2CH=CH(CH2)8COOH
C)CH3(CH2)2CH=CH(CH2)2CH=CH(CH2)4COOH
D)CH3(CH2)14COOH
A)CH3(CH2)12COOH
B)CH3(CH2)2CH=CH(CH2)8COOH
C)CH3(CH2)2CH=CH(CH2)2CH=CH(CH2)4COOH
D)CH3(CH2)14COOH

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
What is the product of the reaction 2-pentene + H2  ?
?
A)pentane
B)2-pentane
C)ethane + propane
D)2-pentyne
 ?
?A)pentane
B)2-pentane
C)ethane + propane
D)2-pentyne

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
What is the IUPAC name of this compound? 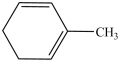
A)methylbenzene
B)2-methyl-1, 3-cyclohexadiene
C)3-methyl-1, 3-cyclohexadiene
D)1-methyl-1, 2-cyclohexadiene
E)toluene

A)methylbenzene
B)2-methyl-1, 3-cyclohexadiene
C)3-methyl-1, 3-cyclohexadiene
D)1-methyl-1, 2-cyclohexadiene
E)toluene

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
The compounds 1-pentene and cis-2-pentene are an example of a pair of
A)isotopes.
B)stereoisomers.
C)constitutional isomers.
D)identical molecules.
A)isotopes.
B)stereoisomers.
C)constitutional isomers.
D)identical molecules.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
What is the structure of 2-methyl-3-hexene?
A)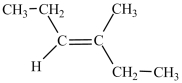
B)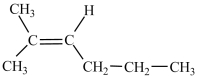
C)(CH3)2C=CHCH2CH2CH3
D)(CH3)2CHCH=CHCH2CH3
A)

B)

C)(CH3)2C=CHCH2CH2CH3
D)(CH3)2CHCH=CHCH2CH3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
Which is NOT a valid IUPAC name of an alkene?
A)2, 3-dimethyl-3-hexene
B)1-butene
C)1-ethylcyclopentene
D)2-butyl-3-methyl-2-nonene
A)2, 3-dimethyl-3-hexene
B)1-butene
C)1-ethylcyclopentene
D)2-butyl-3-methyl-2-nonene

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
What reagent (and catalyst, if necessary)is needed to convert 2-methyl-2-pentene to the compound below? 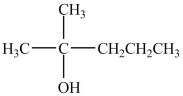
A)H2SO4
B)H2O, H2SO4
C)CH3OH, H2SO4
D)H2O
E)CH3OH

A)H2SO4
B)H2O, H2SO4
C)CH3OH, H2SO4
D)H2O
E)CH3OH

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
What is the structure of ortho-dichlorobenzene?
A)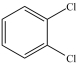
B)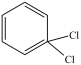
C)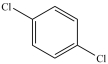
D)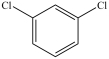
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
How many different products are formed in the reaction of p-dibromobenzene with Cl2, using FeCl3 as a catalyst?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
How many different products are formed in the hydration reaction of 2-pentene?
A)1
B)2
C)3
D)None; 2-pentene does not undergo hydration.
A)1
B)2
C)3
D)None; 2-pentene does not undergo hydration.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
What product is formed in the hydration reaction shown below? 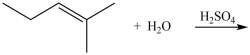
A)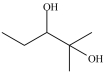
B)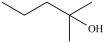
C)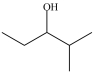
D)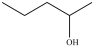
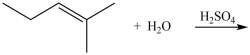
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
What monomer is used to form the polymer below? 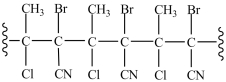
A)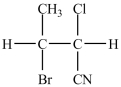
B)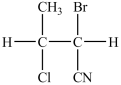
C)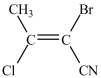
D)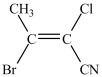

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
How many different products are formed in the reaction of m-dibromobenzene with Cl2, using FeCl3 as a catalyst?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
What is the structure of anthracene?
A)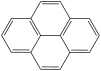
B)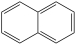
C)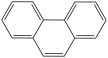
D)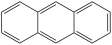
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
What product is formed in the hydration reaction shown below? 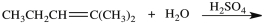
A)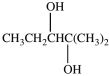
B)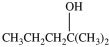
C)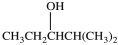
D)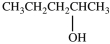
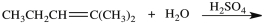
A)

B)
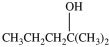
C)
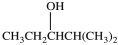
D)
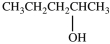

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
What starting material in a hydrochlorination reaction would produce the compound below? 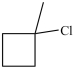
A)
B)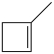
C)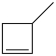
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
What product results when benzene undergoes the chlorination reaction below? 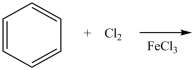
A)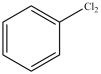
B)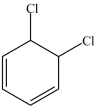
C)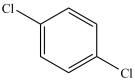
D)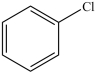
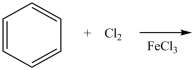
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
What is the product of the reaction below? 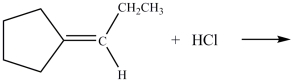
A)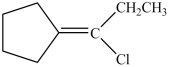
B)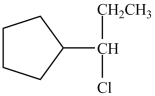
C)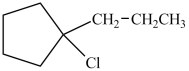
D)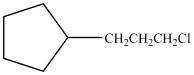

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
The compounds o-chlorophenol and m-chlorophenol are examples of
A)molecules that are identical.
B)molecules that are constitutional isomers.
C)molecules that are stereoisomers.
D)molecules that are isotopes.
A)molecules that are identical.
B)molecules that are constitutional isomers.
C)molecules that are stereoisomers.
D)molecules that are isotopes.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
What is the IUPAC name of the compound below? 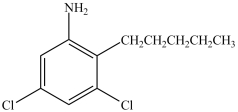
A)5-amino-1, 3-dichloro-4-pentylbenzene
B)3, 5-dichloro-2-pentylaniline
C)1, 3-dichloro-5-nitro-4-pentylbenzene
D)3, 5-dichloro-2-pentane aminobenzene
E)2, 4-dichloro-1-pentylaniline

A)5-amino-1, 3-dichloro-4-pentylbenzene
B)3, 5-dichloro-2-pentylaniline
C)1, 3-dichloro-5-nitro-4-pentylbenzene
D)3, 5-dichloro-2-pentane aminobenzene
E)2, 4-dichloro-1-pentylaniline

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
Which synthetic polymer is not one of the six compounds, called the "Big Six, " that account for 76% of the synthetic polymers produced in the U.S. each year?
A)polyethylene terephthalate (PET)
B)polystyrene (PS)
C)polytetrafluoroethylene (Teflon)
D)high-density polyethylene (HDPE)
E)polypropylene (PP)
A)polyethylene terephthalate (PET)
B)polystyrene (PS)
C)polytetrafluoroethylene (Teflon)
D)high-density polyethylene (HDPE)
E)polypropylene (PP)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
What product is formed in the reaction below? 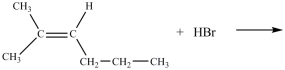
A)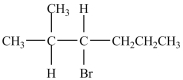
B)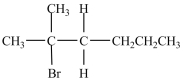
C)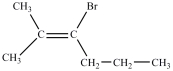
D)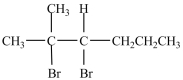
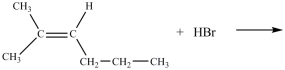
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name of the compound below? 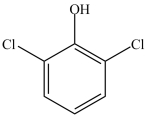
A)1, 3-dichloro-2-oxybenzene
B)1, 3-dichloro-2-hydroxylbenzene
C)2, 6-dichlorophenol
D)1, 5-dichlorophenol

A)1, 3-dichloro-2-oxybenzene
B)1, 3-dichloro-2-hydroxylbenzene
C)2, 6-dichlorophenol
D)1, 5-dichlorophenol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
What product is formed from the reaction below? 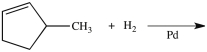
A)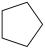
B)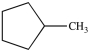
C)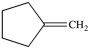
D)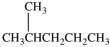
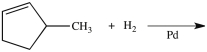
A)

B)

C)

D)
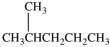

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
What starting material in a hydrochlorination reaction would produce the compound below? 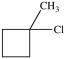
A)
B)
C)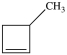
D)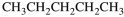

A)
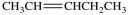
B)

C)

D)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
How many different products are formed in the hydrogenation reaction of 2-pentene?
A)1
B)2
C)3
D)None; 2-pentene does not undergo hydrogenation.
A)1
B)2
C)3
D)None; 2-pentene does not undergo hydrogenation.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
What product results when 1-heptene undergoes the halogenation reaction shown? 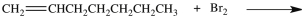
A)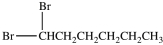
B)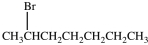
C)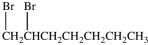
D)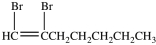
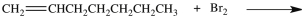
A)
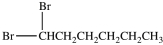
B)
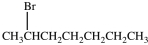
C)
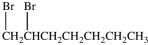
D)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
When an oil is partially hydrogenated, all of the double bonds react with H2, giving a product that has a higher melting point.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
Para-chlorotoluene is the name of an organic compound. Which of the following statements concerning the structure of this compound is false?
A)The compound is an alkene.
B)The compound contains a ring of carbon atoms.
C)A chlorine atom and a methyl group are substituents present in this compound.
D)The substituents that are present have a 1, 4 relationship.
A)The compound is an alkene.
B)The compound contains a ring of carbon atoms.
C)A chlorine atom and a methyl group are substituents present in this compound.
D)The substituents that are present have a 1, 4 relationship.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
When 4, 4-dimethyl-2-pentene adds H2 in the presence of a metal catalyst, the product is 4, 4-dimethylpentane.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
Essential fatty acids cannot be synthesized by the human body and therefore must be obtained in the diet.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
Fats are solids at room temperature and are generally formed from fatty acids having a large number of double bonds in their carbon chains.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
Which term correctly describes the relative position of the two substituents on the benzene ring in the compound below? 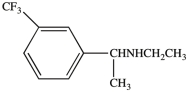
A)trans
B)syn
C)para
D)meta

A)trans
B)syn
C)para
D)meta

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
All commercially available sunscreens contain a benzene ring in the structure of the active ingredient. Which of the following compounds might be effective as an active ingredient in a commercial sunscreen?
A)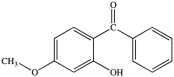
B)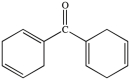
C)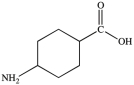
D)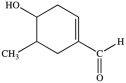
E)All would be effective.
A)

B)

C)

D)

E)All would be effective.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
What is the IUPAC name for the compound below? 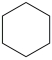
A)benzene
B)hexane
C)toluene
D)cyclohexane

A)benzene
B)hexane
C)toluene
D)cyclohexane

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
All commercially available sunscreens contain a benzene ring in the structure of the active ingredient. Which of the following compounds might be effective as an active ingredient in a commercial sunscreen?
A)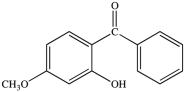
B)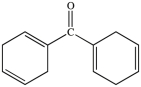
C)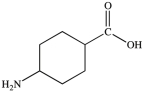
D)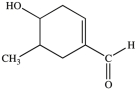
E)All would be effective.
A)

B)

C)

D)

E)All would be effective.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
Which term correctly describes the relative position of the two substituents on the benzene ring in the compound below? 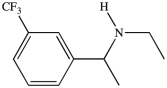
A)trans
B)syn
C)para
D)meta

A)trans
B)syn
C)para
D)meta

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
Which statement concerning the compound 1-ethylcyclohexene is false?
A)It contains a ring of six carbons.
B)It contains one C=C bond.
C)It contains a two carbon substituent on the parent carbon chain.
D)It is a saturated hydrocarbon.
A)It contains a ring of six carbons.
B)It contains one C=C bond.
C)It contains a two carbon substituent on the parent carbon chain.
D)It is a saturated hydrocarbon.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
The addition of water to an alkene is called a hydrogenation reaction.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
When an alkene undergoes an addition reaction, new atoms replace the hydrogen atoms on the carbons that are part of the double bond.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
Partial hydrogenation of soybean oil has the effect of decreasing the number of carbon-carbon double bonds in the oil.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
An organic compound is classified as aromatic if it is cyclic and has a fragrant odor.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
The double bonds in naturally occurring fatty acids are typically trans.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
What product results when 1-heptene undergoes the halogenation reaction shown? 
A)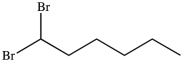
B)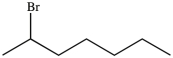
C)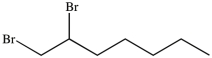
D)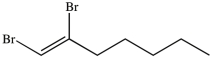

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
Saturated oils are more susceptible than unsaturated fats to oxidation, giving them a longer shelf life.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
Saturated and unsaturated fatty acids have a similar 3-D shape.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
Naphthalene, shown below, is a planar molecule. 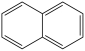


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
The compound 2, 3-dimethyl-2-butene has cis and trans stereoisomers.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
The nitration of benzene is illustrated in the reaction below. 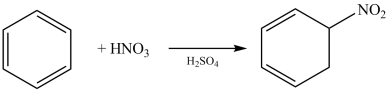


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
When benzo[a]pyrene is oxidized in the body, a potent carcinogen is formed.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
Aniline can be formed by the hydrogenation of nitrobenzene.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
The IUPAC name of this compound is 3, 4-dimethyl-2, 4, 6-octatriene. 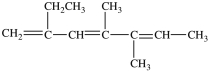


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Aromatic hydrocarbons and alkenes undergo the same kind of addition reactions.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Compounds containing two or more benzene rings that share carbon-carbon bonds are called polycyclic aromatic hydrocarbons (PAHs).

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
The reaction below illustrates the polymerization of tetrafluoroethylene. 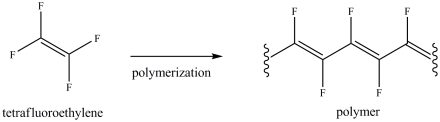


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
The common name for methylbenzene is aniline.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
When benzene undergoes a sulfonation reaction the product is the compound shown below. 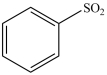


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
Cis and trans isomers are constitutional isomers that differ in the location of a C=C bond.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
The IUPAC name of this compound is 5-butyl-5-methyl-3-heptyne. 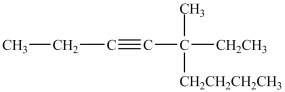


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
The IUPAC name of this compound is 3, 4-dimethyl-2, 4, 6-octatriene. 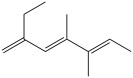


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
A common functional group in many antioxidants is a carbonyl group on a benzene ring.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Phenols are antioxidants.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
In the structure below, all of the carbon atoms have a trigonal planar shape. 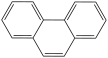


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
The reaction below illustrates the polymerization of tetrafluoroethylene. 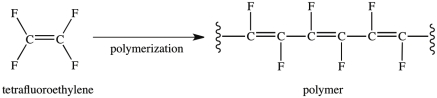


Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
Butter and lard are composed mostly of saturated fatty acids.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
Markovnikov's rule is used to predict the addition products of benzene.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


