Deck 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/111
Play
Full screen (f)
Deck 14: Organic Compounds That Contain Oxygen, Halogen, or Sulfur
1
What is the IUPAC name of the compound below? 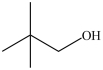
A)2, 2-dimethyl-3-propanol
B)2, 2-dimethyl-1-propanol
C)3, 3-dimethyl-1-butanol
D)2, 2-dimethyl-4-butanol

A)2, 2-dimethyl-3-propanol
B)2, 2-dimethyl-1-propanol
C)3, 3-dimethyl-1-butanol
D)2, 2-dimethyl-4-butanol
B
2
What is the IUPAC name of the compound below? 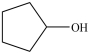
A)1-pentanol
B)phenol
C)cyclo-1-pentol
D)cyclopentanol

A)1-pentanol
B)phenol
C)cyclo-1-pentol
D)cyclopentanol
D
3
What is the structure of 1-chloro-3-ethyl-2-heptanol?
A)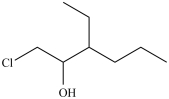
B)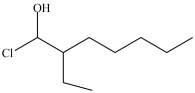
C)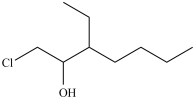
D)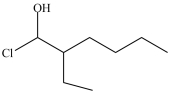
A)

B)

C)

D)

C
4
What is the functional group in thiols?
A)sulfide group
B)sulfate group
C)sulfhydryl group
D)sulfite group
A)sulfide group
B)sulfate group
C)sulfhydryl group
D)sulfite group

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
5
What is the structure of 1-chloro-3-ethyl-2-heptanol?
A)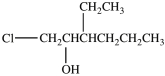
B)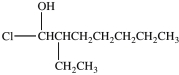
C)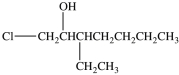
D)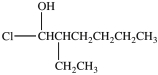
A)

B)
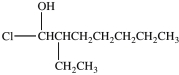
C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
6
What is/are the carbonyl product(s)formed when the alcohol below is oxidized with K2Cr2O7? 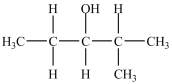
A)CH3(CH2)4COOH
B)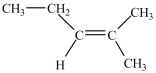
C)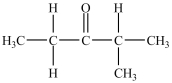
D)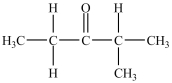 then
then 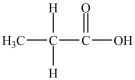
E)No reaction occurs.

A)CH3(CH2)4COOH
B)

C)

D)
 then
then 
E)No reaction occurs.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
7
What is the shape around the oxygen atom in an alcohol?
A)tetrahedral
B)trigonal pyramidal
C)trigonal planar
D)bent
E)linear
A)tetrahedral
B)trigonal pyramidal
C)trigonal planar
D)bent
E)linear

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
8
Which is a primary alcohol?
A)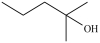
B)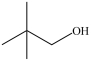
C)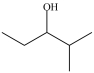
D)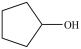
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
9
What reagent is commonly used for alcohol dehydration?
A)K2Cr2O7
B)H2SO4
C)Cl2
D)H2O
A)K2Cr2O7
B)H2SO4
C)Cl2
D)H2O

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
10
Which is a primary alcohol?
A)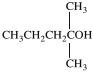
B)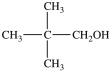
C)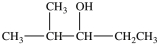
D)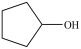
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
11
How many products are possible from the dehydration of the alcohol below? 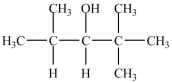
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
12
Which is a secondary alcohol?
A)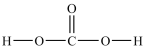
B)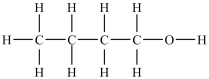
C)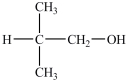
D)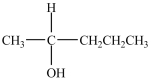
E)More than one of the compounds above is a secondary alcohol.
A)
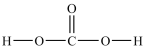
B)

C)

D)

E)More than one of the compounds above is a secondary alcohol.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
13
Which alcohol is most soluble in water?
A)(CH3)2CHCH2OH
B)CH3CH2CH2CH2CH2CH2CH2OH
C)CH3(CH2)10CH2OH
D)All alcohols are soluble in water.
A)(CH3)2CHCH2OH
B)CH3CH2CH2CH2CH2CH2CH2OH
C)CH3(CH2)10CH2OH
D)All alcohols are soluble in water.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
14
The molecule below is classified as what type of alcohol? 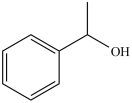
A)secondary alcohol
B)primary alcohol
C)tertiary alcohol

A)secondary alcohol
B)primary alcohol
C)tertiary alcohol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
15
What alcohol would be oxidized to form the compound below? 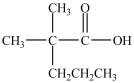
A)CH3(CH2)5CH2OH
B)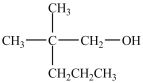
C)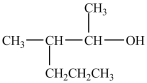
D)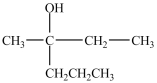

A)CH3(CH2)5CH2OH
B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
16
What is the major product of the dehydration of the compound below? 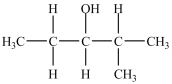
A)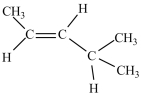
B)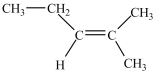
C)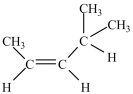
D)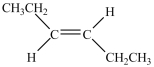

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
17
How many products, including stereoisomers, are possible from the dehydration of the alcohol below? 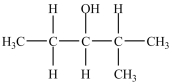
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
18
The molecule below is classified as what type of alcohol? 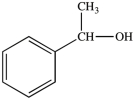
A)secondary alcohol
B)primary alcohol
C)tertiary alcohol

A)secondary alcohol
B)primary alcohol
C)tertiary alcohol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
19
What is/are the carbonyl product(s)formed when the alcohol below is oxidized with K2Cr2O7? 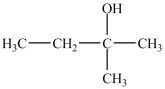
A)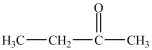
B)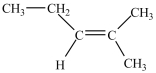
C)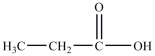
D)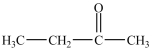 then
then 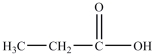
E)No reaction occurs.

A)
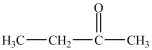
B)

C)
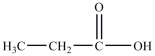
D)
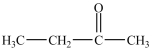 then
then 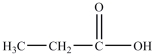
E)No reaction occurs.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the compound below? 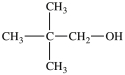
A)2, 2-dimethyl-3-propanol
B)2, 2-dimethyl-1-propanol
C)3, 3-dimethyl-1-butanol
D)2, 2-dimethyl-4-butanol

A)2, 2-dimethyl-3-propanol
B)2, 2-dimethyl-1-propanol
C)3, 3-dimethyl-1-butanol
D)2, 2-dimethyl-4-butanol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
21
What is the common name of CH3(CH2)2-O-(CH2)2CH3?
A)propyl propyl ether
B)dipropyl ether
C)dibutyl ether
D)butyl butyl ether
E)butyl propyl ether
A)propyl propyl ether
B)dipropyl ether
C)dibutyl ether
D)butyl butyl ether
E)butyl propyl ether

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
22
Which compound has the highest boiling point?
A)BrCH2CH2CH2CH3
B)FCH2CH2CH2CH2CH3
C)FCH2CH2CH2CH3
D)ClCH2CH2CH2CH2CH3
E)ICH2CH2CH2CH2CH3
A)BrCH2CH2CH2CH3
B)FCH2CH2CH2CH2CH3
C)FCH2CH2CH2CH3
D)ClCH2CH2CH2CH2CH3
E)ICH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
23
What type of product results from the reaction shown below? 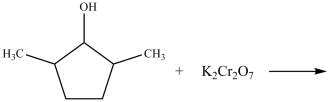
A)a cyclic carboxylic acid
B)a primary alcohol
C)a cyclic ketone
D)an ether

A)a cyclic carboxylic acid
B)a primary alcohol
C)a cyclic ketone
D)an ether

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
24
Which compound has the highest boiling point?
A)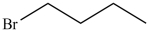
B)
C)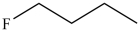
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
25
Which compound is a secondary alkyl halide?
A)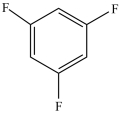
B)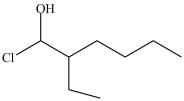
C)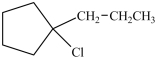
D)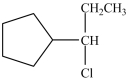
E)More than one of the above compounds is a secondary alkyl halide.
A)

B)

C)

D)

E)More than one of the above compounds is a secondary alkyl halide.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound has the highest boiling point?
A)CH3(CH2)4CH2OH
B)CH3(CH2)2O(CH2)2CH3
C)CH3(CH2)5CH3
D)All of the compounds above have the same boiling point.
A)CH3(CH2)4CH2OH
B)CH3(CH2)2O(CH2)2CH3
C)CH3(CH2)5CH3
D)All of the compounds above have the same boiling point.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name of the compound shown below? 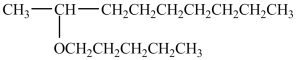
A)heptyl methyl pentyl ether
B)nonyl pentyl ether
C)8-pentoxynonane
D)2-nonyl pentyl ether
E)2-pentoxynonane
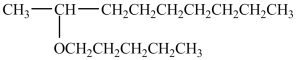
A)heptyl methyl pentyl ether
B)nonyl pentyl ether
C)8-pentoxynonane
D)2-nonyl pentyl ether
E)2-pentoxynonane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
28
What is the common name of the compound below? CH3CH2CH2CH2CH2I
A)pentyl iodide
B)5-iodopentane
C)hexyl iodide
D)iodyl pentane
A)pentyl iodide
B)5-iodopentane
C)hexyl iodide
D)iodyl pentane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
29
Which is an example of an ether?
A)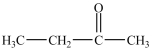
B)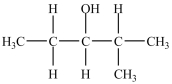
C)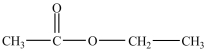
D)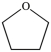
E)None of the above structures is an ether.
A)
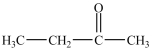
B)

C)
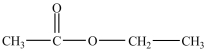
D)

E)None of the above structures is an ether.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement concerning chlorofluorocarbons (CFCs)is not true?
A)CFCs are simple halogen-containing compounds having the general molecular formula CFxCl4 - x.
B)CFCs are manufactured under the trade name Freons.
C)CFCs are inert and nontoxic.
D)CFCs are currently used as refrigerants, solvents, and aerosol propellants in the United States.
E)CFCs are decomposed by sunlight, forming highly reactive intermediates that have been shown to destroy the ozone layer.
A)CFCs are simple halogen-containing compounds having the general molecular formula CFxCl4 - x.
B)CFCs are manufactured under the trade name Freons.
C)CFCs are inert and nontoxic.
D)CFCs are currently used as refrigerants, solvents, and aerosol propellants in the United States.
E)CFCs are decomposed by sunlight, forming highly reactive intermediates that have been shown to destroy the ozone layer.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
31
What is the common name of the compound below? 
A)pentyl iodide
B)5-iodopentane
C)hexyl iodide
D)iodyl pentane

A)pentyl iodide
B)5-iodopentane
C)hexyl iodide
D)iodyl pentane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
32
Which compound has the lowest boiling point?
A)BrCH2CH2CH2CH3
B)FCH2CH2CH2CH2CH3
C)FCH2CH2CH2CH3
D)ClCH2CH2CH2CH2CH3
E)ICH2CH2CH2CH2CH3
A)BrCH2CH2CH2CH3
B)FCH2CH2CH2CH2CH3
C)FCH2CH2CH2CH3
D)ClCH2CH2CH2CH2CH3
E)ICH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
33
What is the structure of methoxycyclobutane?
A)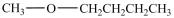
B)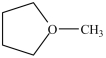
C)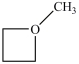
D)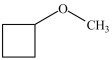
A)
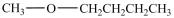
B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
34
What is the structure of 1, 1, 2-trichloroethane?
A)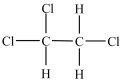
B)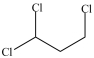
C)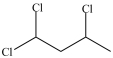
D)More than one of the structures is 1, 1, 2-trichloroethane.
A)

B)

C)

D)More than one of the structures is 1, 1, 2-trichloroethane.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
35
Which compound has the lowest boiling point?
A)CH3(CH2)4CH2OH
B)CH3(CH2)4CH3
C)CH3(CH2)4CH2SH
D)CH3(CH2)4CH2OH and CH3(CH2)4CH2SH have the same boiling point and the boiling point is lower than that of CH3(CH2)4CH3.
A)CH3(CH2)4CH2OH
B)CH3(CH2)4CH3
C)CH3(CH2)4CH2SH
D)CH3(CH2)4CH2OH and CH3(CH2)4CH2SH have the same boiling point and the boiling point is lower than that of CH3(CH2)4CH3.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
36
What is the structure of 1, 1, 2-trichloroethane?
A)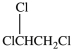
B)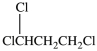
C)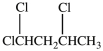
D)More than one of the structures is 1, 1, 2-trichloroethane.
A)
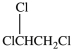
B)
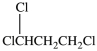
C)
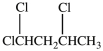
D)More than one of the structures is 1, 1, 2-trichloroethane.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
37
Which compound has the lowest boiling point?
A)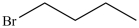
B)
C)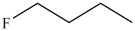
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
38
What product is formed when CH3CH2CH2CH2-SH is oxidized?
A)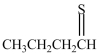
B)CH3CH2CH2CH2-S-S-CH2CH2CH2CH3
C)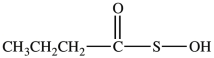
D)CH3CH2CH2CH2-S-CH2CH2CH2CH3
A)
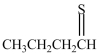
B)CH3CH2CH2CH2-S-S-CH2CH2CH2CH3
C)
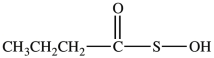
D)CH3CH2CH2CH2-S-CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name of the compound below? 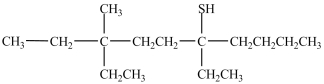
A)3, 6-diethyl-3-methyl-8-decathiol
B)3, 6-diethyl-3-methyl-8-decanethiol
C)5, 8-diethyl-8-methyl-5-decanethiol
D)5, 8-diethyl-8-methyl-5-decathiol
E)3-butyl-6-ethyl-6-methyl-3-octanethiol

A)3, 6-diethyl-3-methyl-8-decathiol
B)3, 6-diethyl-3-methyl-8-decanethiol
C)5, 8-diethyl-8-methyl-5-decanethiol
D)5, 8-diethyl-8-methyl-5-decathiol
E)3-butyl-6-ethyl-6-methyl-3-octanethiol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
40
Which alcohol can be dehydrated with sulfuric acid to form the alkene below? 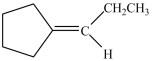
A)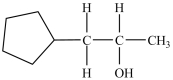
B)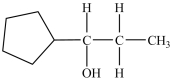
C)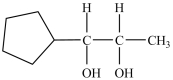
D)Dehydration of more than one of the compounds above produces the desired product.

A)

B)

C)

D)Dehydration of more than one of the compounds above produces the desired product.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
41
Which compound is a secondary alkyl halide?
A)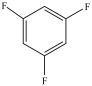
B)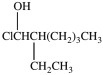
C)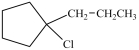
D)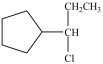
E)More than one of the above compounds is a secondary alkyl halide.
A)

B)
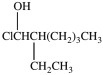
C)

D)

E)More than one of the above compounds is a secondary alkyl halide.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement concerning the compound shown below is incorrect? 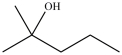
A)It is a tertiary alcohol.
B)Its molecular formula is C6H14O.
C)Its name is 2-methyl-2-pentanol.
D)It can be oxidized to give a ketone.

A)It is a tertiary alcohol.
B)Its molecular formula is C6H14O.
C)Its name is 2-methyl-2-pentanol.
D)It can be oxidized to give a ketone.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
43
Which compound can be oxidized to a carboxylic acid?
A)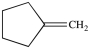
B)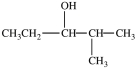
C)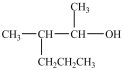
D)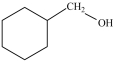
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
44
Alcohol dehydration reactions are important biological reactions. For example, 2-phosphogylcerate undergoes dehydration in the glycolysis pathway to produce phosphoenolpyruvate. Which structure represents the product that results when 2-phosphoglycerate is dehydrated? 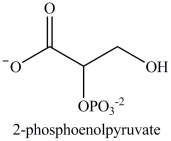
A)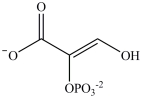
B)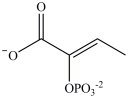
C)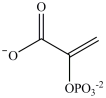
D)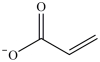

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
45
What is the IUPAC name of the compound below? 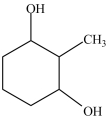
A)2, 6-dihydroxytoluene
B)1-methyl-2, 6-cyclohexanediol
C)2-methyl-1, 3-cyclohexanediol
D)2-methyl-3-hydroxyphenol

A)2, 6-dihydroxytoluene
B)1-methyl-2, 6-cyclohexanediol
C)2-methyl-1, 3-cyclohexanediol
D)2-methyl-3-hydroxyphenol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
46
Primary (1°)alcohols are first oxidized to aldehydes (RCHO), which are further oxidized to carboxylic acids (RCOOH).

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
47
What dithiol is formed when the cyclic disulfide shown below is reduced? 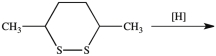
A)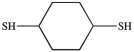
B)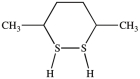
C)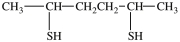
D)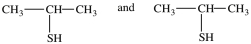

A)

B)

C)
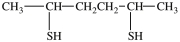
D)
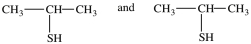

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
48
Lactic acid gives milk its sour taste. What product is formed when lactic acid is oxidized? 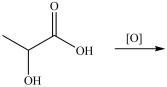
A)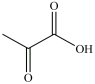
B)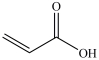
C)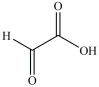
D)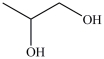

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
49
When ethanol (CH3CH2OH)is ingested, it is oxidized in the liver first to acetaldehyde by alcohol dehydrogenase, and then to acetic acid by aldehyde dehydrogenase.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
50
The oxidation of CH3CH2OH with K2Cr2O7 was the first method used for the routine testing of alcohol concentration in exhaled air.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
51
Which statement best describes the changes that occurred in the reactant in forming the alkene product? 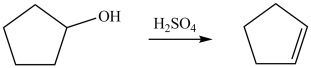
A)An H atom and an OH group have been removed from the reactant.
B)The OH group was removed from the reactant.
C)The OH group was replaced by an H atom.
D)Two H atoms were removed from the reactant.
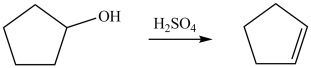
A)An H atom and an OH group have been removed from the reactant.
B)The OH group was removed from the reactant.
C)The OH group was replaced by an H atom.
D)Two H atoms were removed from the reactant.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
52
Which compound is a glycol?
A)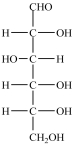
B)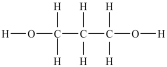
C)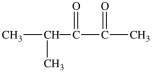
D)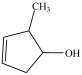
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
53
Which compound can be oxidized to a carboxylic acid?
A)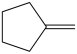
B)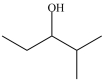
C)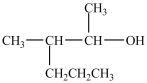
D)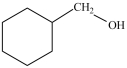
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
54
What dithiol is formed when the cyclic disulfide shown below is reduced? 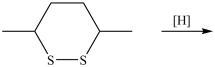
A)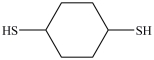
B)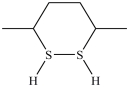
C)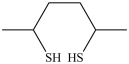
D)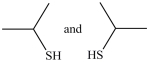

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
55
What product forms when the following disulfide is reduced? 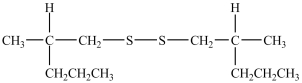

A)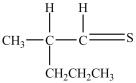
B)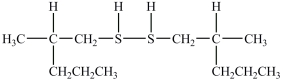
C)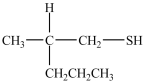
D)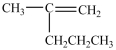
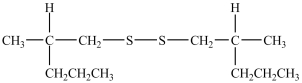

A)

B)
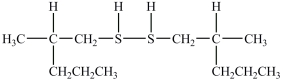
C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
56
Secondary (2°)alcohols are first oxidized to aldehydes (RCHO), which are further oxidized to ketones (RCOR).

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
57
Lactic acid gives milk its sour taste. What product is formed when lactic acid is oxidized? 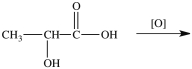
A)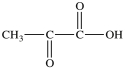
B)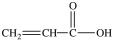
C)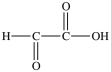
D)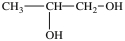

A)

B)
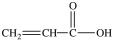
C)

D)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
58
Which statement concerning the compound shown below is incorrect? 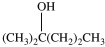
A)It is a tertiary alcohol.
B)Its molecular formula is C6H14O.
C)Its name is 2-methyl-2-pentanol.
D)It can be oxidized to give a ketone.
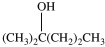
A)It is a tertiary alcohol.
B)Its molecular formula is C6H14O.
C)Its name is 2-methyl-2-pentanol.
D)It can be oxidized to give a ketone.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
59
Which compound is a glycol?
A)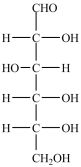
B)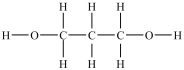
C)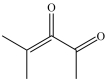
D)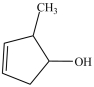
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
60
Alcohol dehydration reactions are important biological reactions. For example, 2-phosphogylcerate (shown below)undergoes dehydration in the glycolysis pathway to produce phosphoenolpyruvate. Which structure represents the product that results when 2-phosphoglycerate is dehydrated? 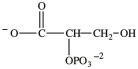
A)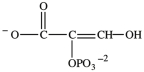
B)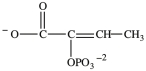
C)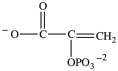
D)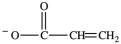

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
61
CHCl2F is classified as a CFC.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
62
The alcohol shown below can be oxidized with K2Cr2O7 to give the indicated product. 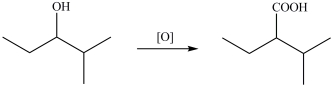


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
63
Propyl bromide is a primary alkyl halide.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
64
Two ether molecules can form hydrogen bonds with each other.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
65
The alcohol shown below can be oxidized with K2Cr2O7 to give the indicated product. 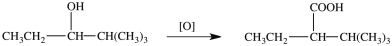


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
66
Thiols have higher boiling points and melting points than alcohols having the same size and shape.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
67
Cyclopentanol has a higher boiling point than methylcyclopentane.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
68
The compound below is expected to be soluble in water. 


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
69
Alkyl halides are soluble in water when the alkyl group has less than five carbons.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
70
CH3CH2CH2CH2-S-S-CH2CH2CH3 is an example of a disulfide compound.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
71
The IUPAC name of the compound below is 2-pentanol. 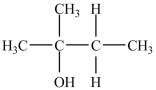


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
72
Ethers have stronger intermolecular forces than alkanes but weaker intermolecular forces than alcohols.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
73
CH3CH2CH2CH2Cl has a higher melting point than CH3CH2CH2CH2Br.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
74
The two alcohols below are dehydrated with H2SO4 to give the same major product. 


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
75
The compound below is expected to be soluble in water. 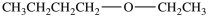
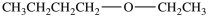

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
76
Antabuse, a drug given to alcoholics to prevent them from consuming alcoholic beverages, acts by interfering with the normal oxidation of ethanol. Antabuse inhibits the oxidation of ethanol to acetaldehyde.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
77
In the simple sugar D-glucose (structure shown), there are two primary -OH groups. 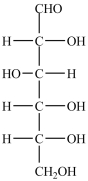


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
78
Thiols are oxidized to sulfates.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
79
Ethers are organic compounds that have two alkyl groups bonded to an oxygen atom.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
80
The compound below is a secondary alkyl halide. 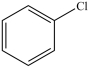


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck


