Deck 1: Matter and Measurement
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/89
Play
Full screen (f)
Deck 1: Matter and Measurement
1
The measurement 78, 005, 760 expressed correctly using scientific notation is
A)7.8005760 × 107
B)7.8005760 × 10-7
C)7.8 × 107
D)7.800576 × 10-7
E)7.800576 × 107
A)7.8005760 × 107
B)7.8005760 × 10-7
C)7.8 × 107
D)7.800576 × 10-7
E)7.800576 × 107
E
2
Which metric relationship is incorrect?
A)1 milligram = 1, 000 grams
B)1 dL = 100 mL
C)1 km = 1, 000 m
D)100 cg = 1 g
E)1 liter = 1, 000, 000 microliters
A)1 milligram = 1, 000 grams
B)1 dL = 100 mL
C)1 km = 1, 000 m
D)100 cg = 1 g
E)1 liter = 1, 000, 000 microliters
A
3
Carry out the following calculation and report the answer using the proper number of significant figures: 549.101 + 8.12 + 95.0076 - 651.9
A)3.286
B)0.3286
C)0.33
D)0.3
E)1268.1
A)3.286
B)0.3286
C)0.33
D)0.3
E)1268.1
D
4
The number 0.0035880 expressed correctly using scientific notation is
A)0.0035889
B)3.5880 × 103
C)3.5880 × 10-3
D)3.5880 × 10-4
E)3.588 × 10-3
A)0.0035889
B)3.5880 × 103
C)3.5880 × 10-3
D)3.5880 × 10-4
E)3.588 × 10-3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
5
Which number contains four significant figures?
A)3.978
B)0.780
C)0.0085
D)1700
E)Two or more of the above numbers contain four significant figures.
A)3.978
B)0.780
C)0.0085
D)1700
E)Two or more of the above numbers contain four significant figures.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
6
Which is an example of a physical change?
A)The rusting of an iron nail
B)The burning of propane in a gas grill
C)Baking cookies
D)Polishing tarnished silver
E)Melting of an ice cube in a glass of soda
A)The rusting of an iron nail
B)The burning of propane in a gas grill
C)Baking cookies
D)Polishing tarnished silver
E)Melting of an ice cube in a glass of soda

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
7
Which number is the smallest?
A)4.38 × 103
B)4.38 × 102
C)4.38 × 10-3
D)4.38 × 10-2
E)438
A)4.38 × 103
B)4.38 × 102
C)4.38 × 10-3
D)4.38 × 10-2
E)438

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
8
Carry out the following calculation and report the answer using the proper number of significant figures: 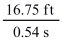
A)31.0185 ft/s
B)31.01 ft/s
C)31.02 ft/s
D)31.0 ft/s
E)31 ft/s
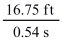
A)31.0185 ft/s
B)31.01 ft/s
C)31.02 ft/s
D)31.0 ft/s
E)31 ft/s

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
9
When 0.022189 is correctly rounded to two significant figures the number becomes
A)0.02
B)0.022
C)22
D)0.023
A)0.02
B)0.022
C)22
D)0.023

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
10
Which is not an example of a pure substance?
A)Sugar
B)Air
C)Aluminum foil
D)Water
E)A block of dry ice
A)Sugar
B)Air
C)Aluminum foil
D)Water
E)A block of dry ice

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
11
What is the correct metric relationship between milliliters and microliters?
A)1 milliliter = 1 microliter
B)1, 000 milliliters = 1 microliter
C)1 milliliter = 1, 000 microliters
D)1, 000, 000 milliliters = 1 microliter
E)1 milliliter = 1, 000, 000 microliters
A)1 milliliter = 1 microliter
B)1, 000 milliliters = 1 microliter
C)1 milliliter = 1, 000 microliters
D)1, 000, 000 milliliters = 1 microliter
E)1 milliliter = 1, 000, 000 microliters

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
12
Which is the proper conversion factor for converting a mass expressed in pounds (lb)to the same mass expressed in grams (g)?
A)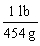
B)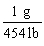
C)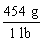
D)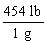
A)
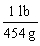
B)
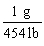
C)
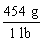
D)
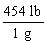

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
13
Which number is the largest?
A)4.38 × 103
B)4.38 × 102
C)4.38 × 10-3
D)4.38 × 10-2
E)438
A)4.38 × 103
B)4.38 × 102
C)4.38 × 10-3
D)4.38 × 10-2
E)438

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
14
Carry out the following calculation and report the answer using the proper number of significant figures: 38.251 + 73.1
A)111
B)111.3
C)111.4
D)111.35
E)111.351
A)111
B)111.3
C)111.4
D)111.35
E)111.351

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
15
When 4.870 × 10-3 is correctly converted to its standard form the number becomes
A)4870
B)4870.
C)0.00487
D)0.004870
E)0.0004870
A)4870
B)4870.
C)0.00487
D)0.004870
E)0.0004870

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
16
Which quantity is an exact number?
A)3 cars
B)1, 000 m
C)2 L
D)453.6 g
A)3 cars
B)1, 000 m
C)2 L
D)453.6 g

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
17
Which length is the longest?
A)12 m
B)12, 000 mm
C)12, 000 m
D)12, 000 cm
E)0.0012 km
A)12 m
B)12, 000 mm
C)12, 000 m
D)12, 000 cm
E)0.0012 km

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
18
When 5.5490× 108 is correctly rounded to three significant figures the number becomes
A)5.55
B)5.55 × 108
C)555
D)554
E)5.54 × 108
A)5.55
B)5.55 × 108
C)555
D)554
E)5.54 × 108

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
19
Which measurement has the fewest number of significant figures?
A)12.80 m
B)0.1280 m
C)0.001280 m
D)1280 m
E)All of the measurements have the same number of significant figures.
A)12.80 m
B)0.1280 m
C)0.001280 m
D)1280 m
E)All of the measurements have the same number of significant figures.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
20
Carry out the following calculation and report the answer using the proper number of significant figures: 38.251 × 73.1
A)2796.1481
B)2796.15
C)2796.1
D)2796
E)2.80 × 103
A)2796.1481
B)2796.15
C)2796.1
D)2796
E)2.80 × 103

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
21
If honey has a density of 1.36 g/mL, what is the mass of 1.25 qt, reported in kilograms?
A)1.60 kg
B)1.6 × 103 kg
C)0.974 kg
D)974 kg
E)1.80 kg
A)1.60 kg
B)1.6 × 103 kg
C)0.974 kg
D)974 kg
E)1.80 kg

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
22
If a piece of rock has a volume of 0.73 L and a mass of 1524 g, what is the density of the rock in g/mL?
A)2.1 × 103 g/mL
B)0.48 g/mL
C)4.8 × 10-4 g/mL
D)2.1 g/mL
E)2.088 g/mL
A)2.1 × 103 g/mL
B)0.48 g/mL
C)4.8 × 10-4 g/mL
D)2.1 g/mL
E)2.088 g/mL

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
23
The recommended dietary allowance for calcium for teenage children is 1, 300 mg per day. If a typical 8.0-fl oz glass of reduced-fat milk contains 298 mg of calcium, how many fluid ounces of milk does a teenager need to drink to get the entire recommended amount of calcium from this milk?
A)4.4 fl oz
B)1.8 fl oz
C)3.5 fl oz
D)35 fl oz
E)32 fl oz
A)4.4 fl oz
B)1.8 fl oz
C)3.5 fl oz
D)35 fl oz
E)32 fl oz

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
24
For a person between the ages of 10 and 29, the normal range of blood triglycerides is 53 × 104 mg/dL. What is the correct interpretation of the units in this measurement?
A)milligrams times deciliter
B)micrograms per deciliter
C)megagrams per deciliter
D)milligrams per deciliter
A)milligrams times deciliter
B)micrograms per deciliter
C)megagrams per deciliter
D)milligrams per deciliter

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following temperatures is the hottest?
A)100 °C
B)100 °F
C)100 K
D)All would feel equally warm.
A)100 °C
B)100 °F
C)100 K
D)All would feel equally warm.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
26
The estimated average daily requirement of folic acid for pregnant females is 520 micrograms. Which accurately expresses this value?
A)520 mg
B)520 Mg
C)520 mG
D)520 0g
A)520 mg
B)520 Mg
C)520 mG
D)520 0g

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
27
An oven is set for a temperature of 298 °F. What is the oven temperature in K?
A)166 K
B)421 K
C)148 K
D)571 K
E)439 K
A)166 K
B)421 K
C)148 K
D)571 K
E)439 K

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
28
What is the mass in kilograms of an individual who weighs 197 lb?
A)197 kg
B)8.95 kg
C)89.5 kg
D)90 kg
E)433 kg
A)197 kg
B)8.95 kg
C)89.5 kg
D)90 kg
E)433 kg

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
29
What is the mass in grams of 85.32 mL of blood plasma with a density of 1.03 g/mL?
A)85.32 g
B)82.83 g
C)82.8 g
D)87.88 g
E)87.9 g
A)85.32 g
B)82.83 g
C)82.8 g
D)87.88 g
E)87.9 g

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
30
What is the density of a sample of rubbing alcohol if it has a specific gravity of 0.789?
A)1.27 g/mL
B)0.789 g/mL
C)1.00 g/mL
D)0.895 g/mL
A)1.27 g/mL
B)0.789 g/mL
C)1.00 g/mL
D)0.895 g/mL

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
31
Which volume is equivalent to 225 mL?
A)2.25 × 105 L
B)2.25 × 102 L
C)2.25 L
D)2.25 × 10-5 L
E)0.225 L
A)2.25 × 105 L
B)2.25 × 102 L
C)2.25 L
D)2.25 × 10-5 L
E)0.225 L

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
32
The density of human urine is normally between 1.003 and 1.030g/mL, and is often used as a diagnostic tool. If a 25.00 mL sample of urine from a patient has a mass of 26.875 g, how does the density of the urine sample compare to the normal range?
A)the density of the sample is lower than the normal range
B)the density of the sample is greater than the normal range
C)the density of the sample is within the normal range
D)there is insufficient information to make a comparison
A)the density of the sample is lower than the normal range
B)the density of the sample is greater than the normal range
C)the density of the sample is within the normal range
D)there is insufficient information to make a comparison

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
33
A patient's urine sample has a density of 1.02 g/mL. If 1250 mL of urine was excreted by the patient in one day, what mass of urine was eliminated?
A)1.28 kg
B)1225 g
C)1275 g
D)128 g
A)1.28 kg
B)1225 g
C)1275 g
D)128 g

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following conversions is correct and expresses the answer using the proper number of significant figures?
A)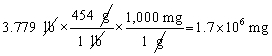
B)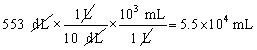
C)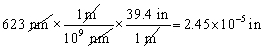
D)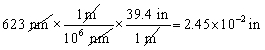
A)
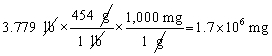
B)
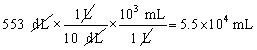
C)
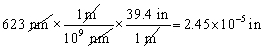
D)
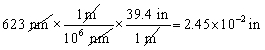

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
35
If a package of nuts weighs 41.3 oz, what is the mass of the package expressed in milligrams?
A)1.17 mg
B)1.17 × 103 mg
C)1.17 × 106 mg
D)117 mg
E)3.00 × 105 mg
A)1.17 mg
B)1.17 × 103 mg
C)1.17 × 106 mg
D)117 mg
E)3.00 × 105 mg

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
36
On an autumn day in Washington, DC the outdoor temperature was 21 °C. What was this outdoor temperature in °F?
A)44 °F
B)57 °F
C)69 °F
D)70 °F
A)44 °F
B)57 °F
C)69 °F
D)70 °F

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
37
If a balloon has a volume of 21.6 cups, what is the volume of this balloon expressed in L?
A)86.4 L
B)81.51 L
C)5.72 L
D)5.094 L
E)5.09 L
A)86.4 L
B)81.51 L
C)5.72 L
D)5.094 L
E)5.09 L

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
38
If a 185-lb patient is prescribed 145 mg of the cholesterol lowering drug Tricor daily, what dosage is the patient receiving in mg/kg of his body weight?
A)0.784 mg/kg
B)1.28 mg/kg
C)0.356 mg/kg
D)1.72 mg/kg
E)0.580 mg/kg
A)0.784 mg/kg
B)1.28 mg/kg
C)0.356 mg/kg
D)1.72 mg/kg
E)0.580 mg/kg

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
39
If a tree is 89.5 cm tall, what is the tree's height expressed in yards?
A)0.979 yd
B)6.31 yd
C)18.9 yd
D)35.2 yd
E)227 yd
A)0.979 yd
B)6.31 yd
C)18.9 yd
D)35.2 yd
E)227 yd

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
40
A hiker with hypothermia has a body temperature of 82 °F. What is his body temperature in °C?
A)14 °C
B)28 °C
C)31 °C
D)50 °C
A)14 °C
B)28 °C
C)31 °C
D)50 °C

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
41
The number 900, 027, 300 has nine significant figures.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
42
The number 900, 027, 300 has four significant figures.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
43
A compound cannot be broken down into simpler substances.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
44
The two conversion factors for the equality 1 in = 2.54 cm are properly shown below. 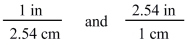
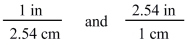

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
45
When the liquid carbon tetrachloride (density = 1.59 g/mL)is added to water, the top layer will be the water layer.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
46
Air has a density of 0.001226 g/mL. What volume of air would have a mass of 1.0 lb?
A)2.7 mL
B)815.6 mL
C)37 mL
D)3.7 x 102 L
A)2.7 mL
B)815.6 mL
C)37 mL
D)3.7 x 102 L

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
47
Changes in state such as melting and boiling are physical changes.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
48
The base unit for volume in the metric system is liter (L).

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
49
Nitrogen gas (N2)would properly be classified as a compound.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
50
An inexact number results from a measurement or observation and contains some uncertainty.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
51
The base unit for mass in the metric system is kilograms (kg).

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
52
The water molecules in this image are best described as being in the liquid state. 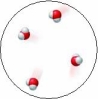


Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
53
Specific gravity is a quantity that compares the density of a substance with the density of water.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
54
In reading a number with a decimal point from left to right, all digits starting with the first nonzero number are significant figures.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
55
PVC plastic, which is used in pipes, is an example of a synthetic material.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
56
A beaker contains 145.675 mL of a saline solution. If 24.2 mL of the saline solution are removed from the beaker, what volume of solution remains?
A)121.475 mL
B)121.4 mL
C)121.5 mL
D)121 mL
A)121.475 mL
B)121.4 mL
C)121.5 mL
D)121 mL

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
57
Which volume has the most uncertainty associated with the measurement?
A)10 mL
B)10.0 mL
C)10.00 mL
D)all have the same degree of uncertainty
A)10 mL
B)10.0 mL
C)10.00 mL
D)all have the same degree of uncertainty

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
58
When a piece of magnesium (density = 1.738 g/mL)is placed in a container of liquid carbon tetrachloride (density = 1.59 g/mL), the piece of magnesium will float on top of the carbon tetrachloride.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
59
The specific gravity of a substance has units of g/mL.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
60
A zero counts as a significant figure when it occurs at the end of a number that contains a decimal point.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
61
One-thousand (1, 000)ms is the same length of time as one (1) s.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
62
For a number written in scientific notation, a negative exponent indicates the value of the number is less than 1.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
63
The density of olive oil is greater at 200 °C than at 25 °C.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
64
The temperature -60 °C is higher than -60 °F.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
65
When subtracting 15 from 762.85 the answer should be reported with two significant figures.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
66
Dissolving sugar in water involves a chemical change.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
67
If the density of a substance is greater than 1 g/mL, the mass of a sample of this substance will be greater than the volume of the sample.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
68
A mixture can be separated into its components by physical changes.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
69
Elements and compounds are both classified as pure substances.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
70
Dividing a number by 105 is the same as multiplying a number by 10-5.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
71
One Kelvin is the same size as one degree Celsius.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
72
The temperature 60 °C is higher than 60 K.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
73
The temperature 60 °C is higher than 60 °F.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
74
The number 0.0007270 is larger than the number 5.7 × 10-3.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
75
The number 87, 927, 000 is larger than the number 9.7 × 106.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
76
Assuming the numbers are measured values, when multiplying 762.85 by 15 the answer should be reported with two significant figures.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
77
The meaning of the metric prefix milli- is 1000.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
78
In scientific notation, a number is written as y × 10x, where x can be any positive or negative number or fraction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
79
The terms used in conversion factors must always be exact numbers.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
80
The measurement 10.3 cm has more significant figures than the measurement 10.3 m.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck


