Deck 8: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 8: Solutions
1
The attraction of an ion with a dipole in a molecule is called
A)a dipole-dipole interaction.
B)an ion-dipole interaction.
C)hydrogen bonding.
D)London dispersion forces.
E)van der Waals forces.
A)a dipole-dipole interaction.
B)an ion-dipole interaction.
C)hydrogen bonding.
D)London dispersion forces.
E)van der Waals forces.
B
2
A particular wine contains 11.2% (v/v)ethanol. What volume of ethanol is in a 750.-mL bottle of this wine?
A)84.0 mL ethanol
B)0.840 mL ethanol
C)6.70 mL ethanol
D)14.9 mL ethanol
A)84.0 mL ethanol
B)0.840 mL ethanol
C)6.70 mL ethanol
D)14.9 mL ethanol
A
3
Which pair of compounds will form a solution?
A)Benzene (C6H6)and hexane (C6H14)
B)Na2SO4 and benzene (C6H6)
C)NaCl and hexane (C6H14)
D)H2O and CCl4
E)More than one of the combinations above will form solutions.
A)Benzene (C6H6)and hexane (C6H14)
B)Na2SO4 and benzene (C6H6)
C)NaCl and hexane (C6H14)
D)H2O and CCl4
E)More than one of the combinations above will form solutions.
A
4
Henry's law states that the solubility of a gas in a liquid is proportional to the
A)partial pressure of the gas above the liquid.
B)temperature of the liquid.
C)temperature of the gas above the liquid.
D)molecular weight of the gas above the liquid.
A)partial pressure of the gas above the liquid.
B)temperature of the liquid.
C)temperature of the gas above the liquid.
D)molecular weight of the gas above the liquid.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
A saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92% (w/v)NaCl in water. How many grams of NaCl are contained in 575 mL of this solution?
A)53 g NaCl
B)529 g NaCl
C)5.3 g NaCl
D)0.016 g NaCl
E)1.6 g NaCl
A)53 g NaCl
B)529 g NaCl
C)5.3 g NaCl
D)0.016 g NaCl
E)1.6 g NaCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Which ionic compound is soluble in water?
A)PbBr2
B)Fe(OH)3
C)BaSO4
D)Ca(NO3)2
E)More than one of the ionic compounds above is soluble in water.
A)PbBr2
B)Fe(OH)3
C)BaSO4
D)Ca(NO3)2
E)More than one of the ionic compounds above is soluble in water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
A sample of seawater contains 1.3 g of calcium ions in 3, 100 kg of solution. What is the calcium ion concentration of this solution in units of ppm?
A)4.2 × 10-4 ppm Ca2+ ions
B)0.42 ppm Ca2+ ions
C)420 ppm Ca2+ ions
D)4.0 ppm Ca2+ ions
E)4.0 × 103 ppm Ca2+ ions
A)4.2 × 10-4 ppm Ca2+ ions
B)0.42 ppm Ca2+ ions
C)420 ppm Ca2+ ions
D)4.0 ppm Ca2+ ions
E)4.0 × 103 ppm Ca2+ ions

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Nonpolar compounds are soluble in
A)ionic compounds.
B)electrolytes.
C)polar solvents.
D)nonpolar solvents.
A)ionic compounds.
B)electrolytes.
C)polar solvents.
D)nonpolar solvents.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
What is the molarity of a solution made by dissolving 36.29 g of NaCl in 2.30 L of solution?
A)15.78 M NaCl
B)0.0634 M NaCl
C)0.270 M NaCl
D)2.70 M NaCl
A)15.78 M NaCl
B)0.0634 M NaCl
C)0.270 M NaCl
D)2.70 M NaCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
What is the molarity of a solution made by dissolving 3.09 moles of NaCl in 1.50 L of solution?
A)4.64 M NaCl
B)4.85 M NaCl
C)2.06 M NaCl
D)0.673 M NaCl
A)4.64 M NaCl
B)4.85 M NaCl
C)2.06 M NaCl
D)0.673 M NaCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
A saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92% (w/v)NaCl in water. What volume of the saline solution must be administered to the patient in order to deliver 7.7 g of NaCl?
A)8.4 mL of saline solution
B)840 mL of saline solution
C)7.1 mL of saline solution
D)140 mL of saline solution
A)8.4 mL of saline solution
B)840 mL of saline solution
C)7.1 mL of saline solution
D)140 mL of saline solution

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
A solution is made by mixing 569 mL of water and 238 mL ethanol. What is the concentration of ethanol in units of volume/volume percent?
A)41.8% (v/v)ethanol
B)0.418% (v/v)ethanol
C)29.5% (v/v)ethanol
D)0.295% (v/v)ethanol
E)70.5% (v/v)ethanol
A)41.8% (v/v)ethanol
B)0.418% (v/v)ethanol
C)29.5% (v/v)ethanol
D)0.295% (v/v)ethanol
E)70.5% (v/v)ethanol

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
Which substance is a nonelectrolyte?
A)NaCl
B)(NH4)2SO4
C)H2O2
D)KOH
A)NaCl
B)(NH4)2SO4
C)H2O2
D)KOH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
A solution is made by dissolving 3.88 g of NaCl in enough water to make 67.8 mL of solution. What is the concentration of sodium chloride in units of weight/volume percent?
A)5.41% (w/v)NaCl
B)94.3% (w/v)NaCl
C)5.72% (w/v)NaCl
D)0.0572% (w/v)NaCl
A)5.41% (w/v)NaCl
B)94.3% (w/v)NaCl
C)5.72% (w/v)NaCl
D)0.0572% (w/v)NaCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
Which ionic compound is not soluble in water?
A)NaCl
B)AgCl
C)(NH4)2SO4
D)Ca(CH3CO2)2
A)NaCl
B)AgCl
C)(NH4)2SO4
D)Ca(CH3CO2)2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
How many grams of glucose (C6H12O6)are contained in 555 mL of a 1.77 M glucose solution?
A)0.982 g C6H12O6
B)177 g C6H12O6
C)0.555 g C6H12O6
D)0.177 g C6H12O6
A)0.982 g C6H12O6
B)177 g C6H12O6
C)0.555 g C6H12O6
D)0.177 g C6H12O6

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
What is the molarity of a solution made by dissolving 4.88 g of KCl in 423 mL of solution?
A)0.0115 M KCl
B)11.5 M KCl
C)1.55 × 10-4 M KCl
D)0.155 M KCl
A)0.0115 M KCl
B)11.5 M KCl
C)1.55 × 10-4 M KCl
D)0.155 M KCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
Which solution contains the smallest number of moles of sucrose (C12H22O11, molar mass = 342.30 g/mol)?
A)2, 000 mL of a 5.0 × 10-5% (w/v)sucrose solution
B)2, 000 mL of a 5.0 ppm sucrose solution
C)20 mL of a 5.0 M sucrose solution
D)All of the solutions above contain the same number of moles of sucrose.
A)2, 000 mL of a 5.0 × 10-5% (w/v)sucrose solution
B)2, 000 mL of a 5.0 ppm sucrose solution
C)20 mL of a 5.0 M sucrose solution
D)All of the solutions above contain the same number of moles of sucrose.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Which is not an example of a solution?
A)a dental filling
B)chicken noodle soup
C)gasoline
D)tap water
A)a dental filling
B)chicken noodle soup
C)gasoline
D)tap water

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
Which substance is a colloid?
A)mayonnaise
B)a dental filling
C)mint chocolate chip ice cream
D)gasoline
A)mayonnaise
B)a dental filling
C)mint chocolate chip ice cream
D)gasoline

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
Which compound will be the most soluble in water?
A)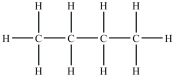
B)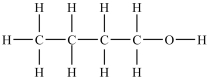
C)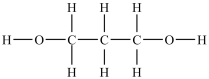
D)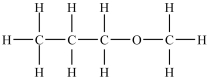
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
What is the concentration of a solution formed by adding 65.0 mL of water to 25.0 mL of a 3.2 M NaCl solution?
A)0.89 M NaCl
B)1.2 M NaCl
C)2.3 M NaCl
D)12 M NaCl
A)0.89 M NaCl
B)1.2 M NaCl
C)2.3 M NaCl
D)12 M NaCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
A flask contains two compartments (A and
A)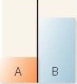
B)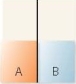
B)with equal volumes of solution separated by a semipermeable membrane. Which diagram represents the final level of the liquids if A is initially a 10% (w/v)glucose solution and B is initially a 20% (w/v)glucose solution?
C)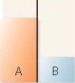
A)

B)

B)with equal volumes of solution separated by a semipermeable membrane. Which diagram represents the final level of the liquids if A is initially a 10% (w/v)glucose solution and B is initially a 20% (w/v)glucose solution?
C)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
An unknown amount of water is added to 75 mL of a 3.5 M aqueous glucose solution. What can be said about the concentration of the resulting solution?
A)The concentration of the resultant glucose solution will be less than 3.5 M.
B)The concentration of the resultant glucose solution will be greater than 3.5 M.
C)The concentration of the resultant glucose solution will remain the same because the amount of glucose has not changed.
D)It is impossible to say anything about the concentration of the resultant glucose solution because the amount of added water has not been provided.
A)The concentration of the resultant glucose solution will be less than 3.5 M.
B)The concentration of the resultant glucose solution will be greater than 3.5 M.
C)The concentration of the resultant glucose solution will remain the same because the amount of glucose has not changed.
D)It is impossible to say anything about the concentration of the resultant glucose solution because the amount of added water has not been provided.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
A hypotonic solution has _____ osmotic pressure than/as body fluids.
A)a higher
B)a lower
C)the same
A)a higher
B)a lower
C)the same

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
If cells are placed in a hypertonic solution,
A)water diffuses out of the cells and the cells shrink in a process called hemolysis.
B)water diffuses out of the cells and the cells shrink in a process called crenation.
C)water diffuses into the cells and the cells swell and eventually burst in a process called hemolysis.
D)water diffuses into the cells and the cells swell and eventually burst in a process called crenation.
A)water diffuses out of the cells and the cells shrink in a process called hemolysis.
B)water diffuses out of the cells and the cells shrink in a process called crenation.
C)water diffuses into the cells and the cells swell and eventually burst in a process called hemolysis.
D)water diffuses into the cells and the cells swell and eventually burst in a process called crenation.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
Which compound will be the least soluble in water?
A)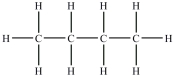
B)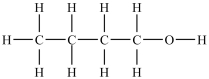
C)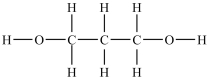
D)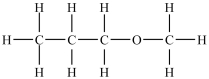
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
Which solution has the greatest mass of solute?
A)2.00 L of a 0.75 M NaCl solution
B)2.00 L of a 0.75 M CaCl2 solution
C)2.00 L of a 0.75 M Ca(NO3)2 solution
D)2.00 L of a 0.75 M Na2CO3 solution
E)All of the solutions above have the same mass of solute.
A)2.00 L of a 0.75 M NaCl solution
B)2.00 L of a 0.75 M CaCl2 solution
C)2.00 L of a 0.75 M Ca(NO3)2 solution
D)2.00 L of a 0.75 M Na2CO3 solution
E)All of the solutions above have the same mass of solute.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
Which solution has the highest freezing point?
A)A solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water.
B)A solution formed by dissolving 0.25 mol of KCl in 1.00 kg of water.
C)A solution formed by dissolving 0.75 mol of KCl in 4.00 kg of water.
D)A solution formed by dissolving 0.25 mol of KCl in 0.50 kg of water.
E)All of the solutions described above have the same freezing point.
A)A solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water.
B)A solution formed by dissolving 0.25 mol of KCl in 1.00 kg of water.
C)A solution formed by dissolving 0.75 mol of KCl in 4.00 kg of water.
D)A solution formed by dissolving 0.25 mol of KCl in 0.50 kg of water.
E)All of the solutions described above have the same freezing point.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
What is the molarity of a 11.5% (w/v)glucose (C6H12O6, molar mass 180.16 g/mol)solution?
A)0.0638 M
B)0.638 M
C)1.15 M
D)1.76 M
A)0.0638 M
B)0.638 M
C)1.15 M
D)1.76 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
Which solution has the lowest boiling point?
A)A solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water.
B)A solution formed by dissolving 0.75 mol of glucose (C6H12O6)in 1.00 kg of water.
C)A solution formed by dissolving 0.75 mol of Ca(NO3)2 in 1.00 kg of water.
D)A solution formed by dissolving 0.75 mol of Na3PO4 in 1.00 kg of water.
E)All of the solutions described above have the same boiling point.
A)A solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water.
B)A solution formed by dissolving 0.75 mol of glucose (C6H12O6)in 1.00 kg of water.
C)A solution formed by dissolving 0.75 mol of Ca(NO3)2 in 1.00 kg of water.
D)A solution formed by dissolving 0.75 mol of Na3PO4 in 1.00 kg of water.
E)All of the solutions described above have the same boiling point.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
What is the concentration of a solution formed by diluting 25.0 mL of a 3.2 M NaCl solution to 135.0 mL?
A)17 M NaCl
B)0.59 M NaCl
C)0.50 M NaCl
D)2.7 M NaCl
A)17 M NaCl
B)0.59 M NaCl
C)0.50 M NaCl
D)2.7 M NaCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
What is the molarity of a 25.0% (v/v)aqueous isopropyl alcohol solution? The density of isopropyl alcohol (C3H8O, molar mass 60.09 g/mol)is 0.786 g/mL.
A)0.529 M
B)1.18 M
C)0.327 M
D)3.27 M
A)0.529 M
B)1.18 M
C)0.327 M
D)3.27 M

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
The solubility of a substance is best described by which of the following?
A)the ability of the substance to dissolve in water
B)the ability of the substance to dissociate into ions when dissolved in water
C)the maximum amount of a substance that can dissolve in a specific amount of solvent
D)the mass of solvent necessary to completely dissolve 100 g of the substance
A)the ability of the substance to dissolve in water
B)the ability of the substance to dissociate into ions when dissolved in water
C)the maximum amount of a substance that can dissolve in a specific amount of solvent
D)the mass of solvent necessary to completely dissolve 100 g of the substance

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
Which solution has the highest boiling point?
A)A solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water.
B)A solution formed by dissolving 0.75 mol of glucose (C6H12O6)in 1.00 kg of water.
C)A solution formed by dissolving 0.75 mol of Ca(NO3)2 in 1.00 kg of water.
D)A solution formed by dissolving 0.75 mol of Na3PO4 in 1.00 kg of water.
E)All of the solutions described above have the same boiling point.
A)A solution formed by dissolving 0.75 mol of KCl in 1.00 kg of water.
B)A solution formed by dissolving 0.75 mol of glucose (C6H12O6)in 1.00 kg of water.
C)A solution formed by dissolving 0.75 mol of Ca(NO3)2 in 1.00 kg of water.
D)A solution formed by dissolving 0.75 mol of Na3PO4 in 1.00 kg of water.
E)All of the solutions described above have the same boiling point.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements concerning a solution is NOT true?
A)A solution is a heterogeneous mixture of two or more pure substances.
B)A solution is composed of a solvent and one or more solutes; the solute(s)dissolve in the solvent.
C)A solution has its components uniformly distributed.
D)A solution is likely to form when the solute(s)and the solvent have similar polarities.
A)A solution is a heterogeneous mixture of two or more pure substances.
B)A solution is composed of a solvent and one or more solutes; the solute(s)dissolve in the solvent.
C)A solution has its components uniformly distributed.
D)A solution is likely to form when the solute(s)and the solvent have similar polarities.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
What is the maximum volume of a 0.788 M CaCl2 solution that can be prepared using 85.3 g CaCl2?
A)1.00 L
B)0.769 L
C)0.975 L
D)67.2 L
A)1.00 L
B)0.769 L
C)0.975 L
D)67.2 L

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
How many milliliters of a 5.25% (w/v)HCl solution must be used to prepare 250 mL of a 0.175% (w/v)HCl solution?
A)8.3 mL HCl solution
B)7, 500 mL HCl solution
C)230 mL HCl solution
D)240 mL HCl solution
E)8.6 mL HCl solution
A)8.3 mL HCl solution
B)7, 500 mL HCl solution
C)230 mL HCl solution
D)240 mL HCl solution
E)8.6 mL HCl solution

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements concerning solution concentration is NOT true?
A)An unsaturated solution contains less than the maximum amount of solute that can be dissolved in the solvent.
B)A saturated solution contains more than 100 g of dissolved solute.
C)A solution can be made less concentrated by adding additional solvent.
D)The number of moles of solute present in exactly one liter of solution is referred to as the solution's molarity.
A)An unsaturated solution contains less than the maximum amount of solute that can be dissolved in the solvent.
B)A saturated solution contains more than 100 g of dissolved solute.
C)A solution can be made less concentrated by adding additional solvent.
D)The number of moles of solute present in exactly one liter of solution is referred to as the solution's molarity.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
The maximum level of lead allowed in drinking water is 15 g/kg. What is this concentration in units of parts per million?
A)15 ppm
B)1.5 × 10-2 ppm
C)1.5 × 104 ppm
D)3.1 ppm
A)15 ppm
B)1.5 × 10-2 ppm
C)1.5 × 104 ppm
D)3.1 ppm

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
A nonvolatile solute makes it harder for solvent molecules to form a crystalline solid, thus decreasing its melting point.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
For most ionic and molecular solids, solubility generally increases as temperature increases.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
The greater the number of dissolved particles in a solution, the lower the solution's osmotic pressure.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
Ethylene glycol is more soluble in water than propane. 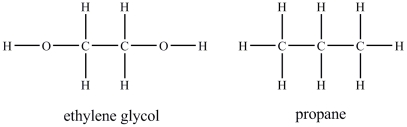


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Only solids dissolve in water to form solutions.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
The solubility of gases increases with increasing temperature.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
Dialysis is a process that involves the selective passage of water, protein molecules, and ions across a semipermeable membrane.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
What interactions are responsible for holding dissolved Cl- ions in an aqueous solution?
A)ion-ion attractions between K+ and Cl- ions
B)ion-dipole attractions between Cl- ions and the hydrogen atoms of water
C)ion-dipole attractions between Cl- ions and the oxygen atom of water
D)hydrogen bonding between the Cl- ions and water
A)ion-ion attractions between K+ and Cl- ions
B)ion-dipole attractions between Cl- ions and the hydrogen atoms of water
C)ion-dipole attractions between Cl- ions and the oxygen atom of water
D)hydrogen bonding between the Cl- ions and water

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
Since living cells are surrounded by biological solutions separated by a semipermeable membrane, the osmotic pressure must be higher in the cell than outside the cell membrane.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
Magnesium hydroxide can be made by the reaction shown below. If a chemist requires 0.725 moles of NaOH for this reaction, what volume of a 1.50 M NaOH solution is needed to provide this amount? MgCl2(aq)+ 2 NaOH(aq) Mg(OH)2(s)+ 2 NaCl(aq)
A)0.483 L of a 1.50 M NaOH solution
B)0.967 L of a 1.50 M NaOH solution
C)1.09 L of a 1.50 M NaOH solution
D)967 mL of a 1.50 M NaOH solution
A)0.483 L of a 1.50 M NaOH solution
B)0.967 L of a 1.50 M NaOH solution
C)1.09 L of a 1.50 M NaOH solution
D)967 mL of a 1.50 M NaOH solution

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
Heptane (C7H16)is soluble in water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
In solution formation, solvation always releases more energy than that required to separate particles, so the overall process is always exothermic.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
The ionic compound (NH4)2SO4 is soluble in water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
Pure water has an osmotic pressure of 1 atm.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
Any mixture of two or more components is a solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Methanol (CH3OH)is soluble in water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
Water solubility for neutral molecules occurs only for small polar molecules or those with many O or N atoms that can hydrogen bond to water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
The ionic compound CaCO3 is soluble in water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
If the attractive forces between the ions and water are stronger than the attraction between the ions in the crystal, an ionic compound dissolves in water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
The solubility of helium gas in water is greater at 25 °C than at 50 °C.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
The presence of a solute reduces the vapor pressure of the solvent above the solution, raising its boiling point.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
Liquid solutions are always transparent and colorless.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
Vitamin D is tested for its solubility in water and benzene (C6H6), and is found to be insoluble in water and soluble in benzene. These solubility results indicate that Vitamin D is most likely a nonpolar compound.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
An aqueous solution with the label 0.25 M sucrose contains 0.25 grams of sucrose in every 1 L of solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
The boiling point of a solution that contains 0.64 mol of Mg(NO3)2 in 1.00 kg of water is 100.98 °C.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
The solubility of Ba(NO3)2 in water is lower at 25 °C than at 50 °C.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
When calculating the concentration of a solution in units of parts per million, the "parts" must be expressed in units of grams.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
All nonvolatile solutes are ionic compounds that do not readily escape into the vapor phase, and thus they have a negligible vapor pressure at a given temperature.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
A liquid solution that contains a nonvolatile solute has a higher boiling point than the solvent alone.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
A solution containing the maximum number of grams of solute that can dissolve in the solvent is said to be supersaturated.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
Osmosis is the passage of water and small molecules across a semipermeable membrane from a solution of high solute concentration to a solution of lower solute concentration.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
A 6.10 M NaCl solution is made by adding 356 g of NaCl to a flask that contains 1.00 L of water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
Compared to the pure solvent, the freezing point depression caused by adding 0.05 mol of sucrose to 1.0 L of water is greater than the boiling point elevation of the same solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
The solubility of KI in water at 20 °C is 140 g KI/100 g H2O. If 160 g of KI is mixed with 150 g of water, all of the KI will dissolve and the solution that results will be unsaturated.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
The concentration unit of weight/volume percent concentration, (w/v)%, is the number of grams of solute dissolved in 100 mL of solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Dilution is the addition of solute to decrease the concentration of solvent.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
A solution can be made less concentrated by adding additional solvent.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
Nonpolar compounds are soluble in nonpolar solvents and insoluble in polar solvents.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
Colligative properties are properties of a solution that depend on the concentration of the solute particles but not their identity.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
Sodium chloride is soluble in nonpolar solvents such as octane (C8H18).

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


