Deck 9: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 9: Acids and Bases
1
An Arrhenius acid is
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.
D
2
Which species is the conjugate base of NH3?
A)NH4+
B)NH2
C)NH2-
D)H2O
E)NH4OH
A)NH4+
B)NH2
C)NH2-
D)H2O
E)NH4OH
C
3
In the acid-base reaction: HCN(aq)+ F-(aq) 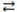 CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
A)products are favored.
B)reactants are favored.
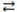 CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, the
CN-(aq)+ HF(aq), where Ka (HCN)= 4.9 × 10-10 and Ka (HF)= 7.2 × 10-4, theA)products are favored.
B)reactants are favored.
B
4
Which species can act as a Brønsted-Lowry acid?
A)CO32-
B)HBr
C)Br2
D)LiOH
A)CO32-
B)HBr
C)Br2
D)LiOH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
Which ion is the strongest base?
A)Br-
B)F-
C)I-
D)NO3-
A)Br-
B)F-
C)I-
D)NO3-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
A Brønsted-Lowry acid is
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.
A)a compound that contains hydroxide and dissolves in water to form OH-.
B)a compound that is a proton donor.
C)a compound that is a proton acceptor.
D)a compound that contains a hydrogen atom and dissolves in water to form a hydrogen ion, H+.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
Which acid is the weakest?
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Which species is the conjugate acid of HCO3-?
A)CO32-
B)H2CO3
C)CO2
D)H2O
A)CO32-
B)H2CO3
C)CO2
D)H2O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
Which compound is a weak acid?
A)HNO3
B)HBr
C)CH3COOH
D)H2SO4
A)HNO3
B)HBr
C)CH3COOH
D)H2SO4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
Which species is a diprotic acid?
A)Mg(OH)2
B)CH3COOH
C)H2
D)H2CO3
A)Mg(OH)2
B)CH3COOH
C)H2
D)H2CO3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
Which solution has the highest pH?
A)4.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M -OH
E)1.0 × 10-2 M H3O+
A)4.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M -OH
E)1.0 × 10-2 M H3O+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
Which acid has the strongest conjugate base?
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
Which species can act as a Brønsted-Lowry base?
A)CO32-
B)HBr
C)H2CO3
D)NH4+
A)CO32-
B)HBr
C)H2CO3
D)NH4+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
Which acid is the strongest?
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)
A)Hydrogen sulfate ion HSO4- (Ka = 1.2 × 10-2)
B)Hydrocyanic acid HCN (Ka = 4.9 × 10-10)
C)Hydrofluoric acid HF (Ka = 7.2 × 10-4)
D)Ammonium ion NH4+ (Ka = 5.6 × 10-10)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
The [H3O+] in a cabernet sauvignon wine is 5.9 × 10-4 M. What is the [-OH] in this wine?
A)5.9 × 10-4 M -OH
B)1.0 × 10-7 M -OH
C)5.9 × 10-18 M -OH
D)1.7 × 10-11 M -OH
E)1.0 × 10-14 M -OH
A)5.9 × 10-4 M -OH
B)1.0 × 10-7 M -OH
C)5.9 × 10-18 M -OH
D)1.7 × 10-11 M -OH
E)1.0 × 10-14 M -OH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
In the acid-base reaction: F-(aq)+ HNO3(aq) 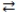 HF(aq)+ NO3-(aq)
HF(aq)+ NO3-(aq)
A)F- is the acid and its conjugate base is HF, and HNO3 is the base and its conjugate acid is NO3-(aq).
B)HNO3 is the acid and its conjugate base is NO3-(aq), and F- is the base and its conjugate acid is HF.
C)F- is the acid and its conjugate base is NO3-(aq), and HNO3 is the base and its conjugate acid is HF.
D)HNO3 is the acid and its conjugate base is HF, and F- is the base and its conjugate acid is NO3-(aq).
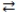 HF(aq)+ NO3-(aq)
HF(aq)+ NO3-(aq)A)F- is the acid and its conjugate base is HF, and HNO3 is the base and its conjugate acid is NO3-(aq).
B)HNO3 is the acid and its conjugate base is NO3-(aq), and F- is the base and its conjugate acid is HF.
C)F- is the acid and its conjugate base is NO3-(aq), and HNO3 is the base and its conjugate acid is HF.
D)HNO3 is the acid and its conjugate base is HF, and F- is the base and its conjugate acid is NO3-(aq).

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
Which solution has the highest pH?
A)4.3 × 10-8 M H3O+
B)1.0 × 10-7 M H3O+
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M H3O+
E)1.0 × 10-2 M H3O+
A)4.3 × 10-8 M H3O+
B)1.0 × 10-7 M H3O+
C)5.1 × 10-2 M H3O+
D)1.9 × 10-8 M H3O+
E)1.0 × 10-2 M H3O+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
The [-OH] in a sample of egg whites is 6.3 × 10-7 M. What is the [H3O+] in these egg whites?
A)6.3 × 10-7 M H3O+
B)1.0 × 10-7 M H3O+
C)6.3 × 10-21 M H3O+
D)1.6 × 10-8 M H3O+
E)1.0 × 10-14 M H3O+
A)6.3 × 10-7 M H3O+
B)1.0 × 10-7 M H3O+
C)6.3 × 10-21 M H3O+
D)1.6 × 10-8 M H3O+
E)1.0 × 10-14 M H3O+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Ammonia, NH3, is an example of a
A)strong acid
B)strong base
C)weak acid
D)weak base
A)strong acid
B)strong base
C)weak acid
D)weak base

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
What is the expression for Kw, the ion-product constant for water?
A)Kw = [H2O]2
B)Kw =![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://storage.examlex.com/TB5866/11eaaeeb_de2f_9896_9547_45ea193798ec_TB5866_11.jpg)
C)Kw =![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://storage.examlex.com/TB5866/11eaaeeb_de2f_9897_9547_2da9c4e98288_TB5866_11.jpg)
D)Kw =![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://storage.examlex.com/TB5866/11eaaeeb_de2f_bfa8_9547_ebf851b48095_TB5866_11.jpg)
A)Kw = [H2O]2
B)Kw =
![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://storage.examlex.com/TB5866/11eaaeeb_de2f_9896_9547_45ea193798ec_TB5866_11.jpg)
C)Kw =
![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://storage.examlex.com/TB5866/11eaaeeb_de2f_9897_9547_2da9c4e98288_TB5866_11.jpg)
D)Kw =
![<strong>What is the expression for K<sub>w</sub>, the ion-product constant for water?</strong> A)K<sub>w</sub> = [H<sub>2</sub>O]<sup>2</sup> <sup> </sup> B)K<sub>w</sub> = C)K<sub>w</sub> = D)K<sub>w</sub> =](https://storage.examlex.com/TB5866/11eaaeeb_de2f_bfa8_9547_ebf851b48095_TB5866_11.jpg)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
What is the pH of a cleaning solution with a [H3O+] = 7.4 × 10-9 M H3O+?
A)5.9
B)7.13
C)8.13
D)5.87
A)5.9
B)7.13
C)8.13
D)5.87

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
Which buffer solution has the lowest pH (HF has Ka = 7.2 × 10-4)?
A)0.10 M HF and 0.10 M NaF
B)0.20 M HF and 0.20 M NaF
C)0.20 M HF and 0.10 M NaF
D)0.10 M HF and 0.20 M NaF
E)All of the buffer solutions described above have the same pH.
A)0.10 M HF and 0.10 M NaF
B)0.20 M HF and 0.20 M NaF
C)0.20 M HF and 0.10 M NaF
D)0.10 M HF and 0.20 M NaF
E)All of the buffer solutions described above have the same pH.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
Which salt forms a solution with a pH < 7 when dissolved in water?
A)RbI
B)NaCH3COO
C)LiNO2
D)(NH4)2SO4
A)RbI
B)NaCH3COO
C)LiNO2
D)(NH4)2SO4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
How many milliliters of 0.653 M NaOH are needed to neutralize 25.0 mL of a 1.02 M HBr solution? The neutralization reaction is: NaOH(aq)+ HBr(aq) H2O(l)+ NaBr(aq)
A)16.0 mL NaOH
B)25.5 mL NaOH
C)39.1 mL NaOH
D)16.3 mL NaOH
A)16.0 mL NaOH
B)25.5 mL NaOH
C)39.1 mL NaOH
D)16.3 mL NaOH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
What is the molarity of an HNO3 solution if 24.1 mL of a 0.250 M Ba(OH)2 solution are needed to titrate a 15.0 mL sample of the acid according to the equation below? Ba(OH)2(aq)+ 2 HNO3(aq) 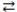 2 H2O(l)+ Ba(NO3)2(aq)
2 H2O(l)+ Ba(NO3)2(aq)
A)0.402 M HNO3
B)0.156 M HNO3
C)0.311 M HNO3
D)0.201 M HNO3
E)0.803 M HNO3
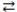 2 H2O(l)+ Ba(NO3)2(aq)
2 H2O(l)+ Ba(NO3)2(aq)A)0.402 M HNO3
B)0.156 M HNO3
C)0.311 M HNO3
D)0.201 M HNO3
E)0.803 M HNO3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
Which solution has the lowest pH?
A)1.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M -OH
D)3.9 × 10-8 M -OH
E)2.3 × 10-3 M -OH
A)1.3 × 10-8 M -OH
B)1.0 × 10-7 M -OH
C)5.1 × 10-2 M -OH
D)3.9 × 10-8 M -OH
E)2.3 × 10-3 M -OH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
What is the conjugate base of the hydronium ion, H3O+?
A)OH-
B)H2O-
C)H2O
D)H3O+ has no conjugate base
A)OH-
B)H2O-
C)H2O
D)H3O+ has no conjugate base

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
What are the products of the acid-base reaction of sodium carbonate with acetic acid?
A)CO2(g)+ H2O(l)+ 2 NaCH3COO(aq)
B)5 CO2(g)+ 2 H2O(l)+ 2 NaOH(aq)
C)H2CO3(aq)+ H2O(l)+ 2 NaOH(aq)
D)CO(g)+ H2O(l)+ 2 NaCH3COO(aq)
A)CO2(g)+ H2O(l)+ 2 NaCH3COO(aq)
B)5 CO2(g)+ 2 H2O(l)+ 2 NaOH(aq)
C)H2CO3(aq)+ H2O(l)+ 2 NaOH(aq)
D)CO(g)+ H2O(l)+ 2 NaCH3COO(aq)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
What is the pH of a peach with a [-OH] = 3.2 × 10-11 M -OH?
A)11.32
B)10.49
C)3.51
D)3.2
A)11.32
B)10.49
C)3.51
D)3.2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
The pH of a lime is 1.90. What is the [H3O+]?
A)1.3 × 10-2 M H3O+
B)1.3 × 1012 M H3O+
C)7.9 × 101 M H3O+
D)7.9 × 10-13 M H3O+
E)1.9 M H3O+
A)1.3 × 10-2 M H3O+
B)1.3 × 1012 M H3O+
C)7.9 × 101 M H3O+
D)7.9 × 10-13 M H3O+
E)1.9 M H3O+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
The pH of normal blood is 7.4. The pH of a diabetic's blood was determined to be 6.4. Which comparison of these two blood samples is accurate?
A)The diabetic's blood has a lower [H3O+] than normal blood.
B)The diabetic's blood is more acidic than normal blood.
C)Normal blood has a lower [OH-] than the diabetic's blood.
D)Normal blood has a higher [H3O+] than the diabetic's blood.
A)The diabetic's blood has a lower [H3O+] than normal blood.
B)The diabetic's blood is more acidic than normal blood.
C)Normal blood has a lower [OH-] than the diabetic's blood.
D)Normal blood has a higher [H3O+] than the diabetic's blood.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
What is the pH of a buffer that contains 0.15 M CH3COOH and 0.10 M NaCH3COO (Ka = 1.8 × 10-5)?
A)4.74
B)7.00
C)4.57
D)4.92
A)4.74
B)7.00
C)4.57
D)4.92

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
Which term correctly describes the medical condition in which the pH of blood is greater than 7.45, and therefore the blood is more basic than normal?
A)respiratory alkalosis
B)alkalosis
C)respiratory acidosis
D)acidosis
A)respiratory alkalosis
B)alkalosis
C)respiratory acidosis
D)acidosis

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
An aqueous solution with a low pH is necessary for a certain industrial process. Which of the following solutions would have the lowest pH?
A)2.0 M NaOH
B)0.5 M HCl
C)1.0 M acetic acid, CH3COOH
D)1.0 M HCl
E)0.15 M NaOH
A)2.0 M NaOH
B)0.5 M HCl
C)1.0 M acetic acid, CH3COOH
D)1.0 M HCl
E)0.15 M NaOH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
What is the net ionic equation for the acid-base reaction of hydrobromic acid with sodium hydroxide?
A)NaOH(aq)+ HBr(aq) H2O(l)+ NaBr(aq)
B)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H2O(l)+ Na+(aq)+ Br-(aq)
C)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H+(aq)+ -OH(aq)+ Na+(aq)+ Br-(aq)
D)-OH(aq)+ H+(aq) H2O(l)
A)NaOH(aq)+ HBr(aq) H2O(l)+ NaBr(aq)
B)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H2O(l)+ Na+(aq)+ Br-(aq)
C)Na+(aq)+ -OH(aq)+ H+(aq)+ Br-(aq) H+(aq)+ -OH(aq)+ Na+(aq)+ Br-(aq)
D)-OH(aq)+ H+(aq) H2O(l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
Which solution containing an equal number of moles of each of the substances is a buffer?
A)HCl and NaCl
B)HNO2 and HNO3
C)CH3COOH and NaCH3COO
D)H2CO3 and CO32-
E)More than one of the solutions above is a buffer.
A)HCl and NaCl
B)HNO2 and HNO3
C)CH3COOH and NaCH3COO
D)H2CO3 and CO32-
E)More than one of the solutions above is a buffer.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
What is the molarity of an HCl solution if 43.6 mL of a 0.125 M KOH solution are needed to titrate a 25.0 mL sample of the acid according to the equation below? KOH(aq)+ HCl(aq) H2O(l)+ KCl(aq)
A)0.218 M HCl
B)0.573 M HCl
C)0.0717 M HCl
D)4.58 M HCl
E)1.74 M HCl
A)0.218 M HCl
B)0.573 M HCl
C)0.0717 M HCl
D)4.58 M HCl
E)1.74 M HCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
Which diagram properly depicts the dissolved species present in an aqueous solution of KOH? (water molecules are not shown)
A)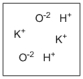
B)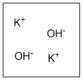
C)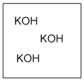
D)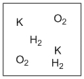
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
Which salt forms a basic solution when dissolved in water?
A)KCl
B)NH4Br
C)LiNO3
D)Na3PO4
A)KCl
B)NH4Br
C)LiNO3
D)Na3PO4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
What is the balanced equation for the acid-base reaction of nitrous acid with lithium hydroxide?
A)LiOH(aq)+ HNO3(aq)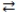 H2O(l)+ LiNO3(aq)
H2O(l)+ LiNO3(aq)
B)Li(OH)2(aq)+ 2 HNO3(aq)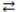 2 H2O(l)+ Li(NO3)2(aq)
2 H2O(l)+ Li(NO3)2(aq)
C)LiOH(aq)+ HNO2(aq)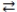 H2O(l)+ LiNO2(aq)
H2O(l)+ LiNO2(aq)
D)2 LiOH(aq)+ H2NO2(aq)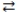 2 H2O(l)+ Li2NO2(aq)
2 H2O(l)+ Li2NO2(aq)
A)LiOH(aq)+ HNO3(aq)
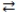 H2O(l)+ LiNO3(aq)
H2O(l)+ LiNO3(aq)B)Li(OH)2(aq)+ 2 HNO3(aq)
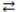 2 H2O(l)+ Li(NO3)2(aq)
2 H2O(l)+ Li(NO3)2(aq)C)LiOH(aq)+ HNO2(aq)
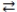 H2O(l)+ LiNO2(aq)
H2O(l)+ LiNO2(aq)D)2 LiOH(aq)+ H2NO2(aq)
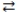 2 H2O(l)+ Li2NO2(aq)
2 H2O(l)+ Li2NO2(aq)
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
All compounds can be classified as either an acid or a base.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
The pH of a 1.0 × 10-5 M H3O+ solution is 5.00.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
A solution in which [-OH] = 6.3 × 10-7 M has a higher pH than a solution with a [-OH] = 4.3 × 10-2 M.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
An aqueous solution of NaHCO3 is basic.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
A sample of water from the Chesapeake Bay has [H3O+]=3.1 × 10-9 M. Which statement below accurately describes this water sample?
A)The pH of the water sample is 7.5.
B)The water sample is a basic solution.
C)The water sample doesn't contain any hydroxide ions.
D)The water sample has a higher concentration of hydronium ions than pure water does.
A)The pH of the water sample is 7.5.
B)The water sample is a basic solution.
C)The water sample doesn't contain any hydroxide ions.
D)The water sample has a higher concentration of hydronium ions than pure water does.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
When phosphoric acid (H3PO4)dissolves in water, the equilibrium shown below is established. If Ka = 7.5 × 10-3 for H3PO4, which statement concerning an aqueous solution of phosphoric acid is correct?
H3PO4 + H2O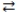 H3O+ + H2PO4-
H3O+ + H2PO4-
A)An aqueous solution of phosphoric acid contains mainly H3PO4 molecules.
B)An aqueous solution of phosphoric acid contains predominantly H3O+ and H2PO4- ions.
C)An aqueous solution of phosphoric acid contains equal amounts of H3O+ and H3PO4.
D)An aqueous solution of phosphoric acid contains a greater concentration of dissolved ions than it does neutral phosphoric acid molecules.
H3PO4 + H2O
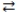 H3O+ + H2PO4-
H3O+ + H2PO4-A)An aqueous solution of phosphoric acid contains mainly H3PO4 molecules.
B)An aqueous solution of phosphoric acid contains predominantly H3O+ and H2PO4- ions.
C)An aqueous solution of phosphoric acid contains equal amounts of H3O+ and H3PO4.
D)An aqueous solution of phosphoric acid contains a greater concentration of dissolved ions than it does neutral phosphoric acid molecules.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
If two solutions differ in their [H3O+] by a factor of 2.0, the difference in their pH will be 2.0.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
The pH of a 1.0 × 10-6 M -OH solution is 6.00.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
The value of Kw = 1.0 × 10-14 at all temperatures below 100 °C.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
An aqueous solution of KCl has a lower pH than an aqueous solution of Li2SO3.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
A solution in which [H3O+] = 7.4 × 10-5 M has a higher pH than a solution with a [-OH] = 4.8 × 10-8 M.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
The products are favored in the acid-base reaction: HI(aq)+ NH3(aq) 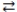 NH4+(aq)+ I-(aq).
NH4+(aq)+ I-(aq).
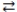 NH4+(aq)+ I-(aq).
NH4+(aq)+ I-(aq).
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
A salt derived from a strong base and a weak acid forms an acidic solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
In an acidic solution, [H3O+] > [-OH].

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
Normal gastric juice has a pH of about 2. Assuming that normal gastric juice is primarily aqueous HCl, what is the concentration of HCl in the stomach?
A)2 M HCl
B)1.0 × 102 M HCl
C)0.01 M HCl
D)0.14 M HCl
A)2 M HCl
B)1.0 × 102 M HCl
C)0.01 M HCl
D)0.14 M HCl

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
Although a Brønsted-Lowry acid must contain a hydrogen atom, it may be a neutral molecule or contain a net positive or negative charge.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
A sample of urine has a pH of 8.2. Which statement describing the urine sample is NOT true?
A)The urine sample is basic.
B)The urine sample has a hydronium ion concentration greater than that of a neutral solution.
C)The urine sample has a hydroxide ion concentration greater than its hydronium ion concentration.
D)The urine sample has a hydroxide ion concentration greater than 1.0 × 10-7.
A)The urine sample is basic.
B)The urine sample has a hydronium ion concentration greater than that of a neutral solution.
C)The urine sample has a hydroxide ion concentration greater than its hydronium ion concentration.
D)The urine sample has a hydroxide ion concentration greater than 1.0 × 10-7.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
A compound can be an acid or a base, but not both.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
The acid-base indicator phenolphthalein is colorless in acidic solutions and bright pink in basic solutions.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
The value of Kw applies to any aqueous solution at 25 °C , not just pure water.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
The addition of a base to water decreases the pH of the solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
Two species that differ by the presence of a proton are called a conjugate acid-base pair.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
An aqueous solution whose pH is greater than 7 has a higher [H3O+] than pure water itself.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
The principal buffer in the blood is carbonic acid/bicarbonate (H2CO3/HCO3-), keeping the normal blood pH of a healthy individual in the range of 7.35 to 7.45.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
OH- and NH4+ are both examples of Brønsted-Lowry bases.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
If the pH of blood is lower than 7.35, the blood is more acidic than normal, and the condition is called alkalosis.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
In an acid-base reaction, a proton is transferred from the acid (HA)to the base (B:).

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
All salts where the anion is derived from a strong acid form basic solutions.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
In the reaction: SO32-(aq)+ H2O(l) 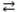 HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
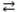 HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
HSO3-(aq)+ -OH(aq), H2O acts as a Brønsted-Lowry acid.
Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
A Brønsted-Lowry base must contain a lone pair of electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
HOBr and CH3CH2CH2COOH are both examples of Brønsted-Lowry acids.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
An aqueous solution containing an equal number of moles of Na2HPO4 and H3PO4 is an example of a buffer solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
Consider two acids, A and B. If acid A is weaker than acid B, then acid B dissociates in water to a greater extent than acid A.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
Consider two acids, A and B. If acid A is stronger than acid B, then the conjugate base of B is stronger than the conjugate base of A.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
An aqueous solution containing an equal number of moles of NaF and HF is an example of a buffer solution.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
For a solution labeled 0.15 M CH3COOH, the [H3O+] is 0.15 M.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
Water is amphoteric; it can act as an acid or a base.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
Most acids are weak acids.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
The addition of an acid to water increases the [H3O+] of the solution and increases the solution pH.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
A solution containing a low concentration HCl is a weak acid.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


