Deck 13: Bonding: General Concepts
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/140
Play
Full screen (f)
Deck 13: Bonding: General Concepts
1
For the elements Cs, F, and Cl, the order of increasing electronegativity is
A) Cl < Cs < F.
B) Cs < Cl < F.
C) F < Cs < Cl.
D) F < Cl < Cs.
E) None of these
A) Cl < Cs < F.
B) Cs < Cl < F.
C) F < Cs < Cl.
D) F < Cl < Cs.
E) None of these
Cs < Cl < F.
2
Based on electronegativities, which of the following would you expect to be most ionic?
A) N2
B) CO2
C) CF4
D) CH4
E) CaF2
A) N2
B) CO2
C) CF4
D) CH4
E) CaF2
CaF2
3
Which of the following is nonpolar?
A) Cl2O
B) CS2
C) SF4
D) IF3
E) NCl3
A) Cl2O
B) CS2
C) SF4
D) IF3
E) NCl3
CS2
4
The electron pair in a C-F bond could be considered
A) an inadequate model because the bond is ionic.
B) closer to C because carbon has a lower electronegativity than fluorine.
C) closer to C because carbon has a larger radius and thus exerts greater control over the shared electron pair.
D) closer to F because fluorine has a higher electronegativity than carbon.
E) centrally located directly between the C and F.
A) an inadequate model because the bond is ionic.
B) closer to C because carbon has a lower electronegativity than fluorine.
C) closer to C because carbon has a larger radius and thus exerts greater control over the shared electron pair.
D) closer to F because fluorine has a higher electronegativity than carbon.
E) centrally located directly between the C and F.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following bonds would be the most polar without being considered ionic?
A) C-O
B) Mg-O
C) O-O
D) Si-O
E) N-O
A) C-O
B) Mg-O
C) O-O
D) Si-O
E) N-O

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following elements forms the most ionic bond with chlorine?
A) Kr
B) Ga
C) N
D) I
E) K
A) Kr
B) Ga
C) N
D) I
E) K

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following groups contains no ionic compounds?
A) KH, SrF2, NaNH2
B) KOH, CBr4, SF4
C) HCN, NO2, Sr(NO3)2
D) CH2O, H2O, NBr3
E) PCl5, LiBr, Zn(OH)2
A) KH, SrF2, NaNH2
B) KOH, CBr4, SF4
C) HCN, NO2, Sr(NO3)2
D) CH2O, H2O, NBr3
E) PCl5, LiBr, Zn(OH)2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
8
In the gaseous phase, which of the following diatomic molecules would be the most polar?
A) NaCl
B) CsF
C) NaF
D) LiF
E) CsCl
A) NaCl
B) CsF
C) NaF
D) LiF
E) CsCl

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following shows these molecules in order from most polar to least polar?
A) CF2Cl2 > CF2H2 > CCl2H2 > CH4 = CCl4
B) CH4 > CF2Cl2 > CF2H2 > CCl4 > CCl2H2
C) CF2H2 > CCl2H2 > CF2Cl2 > CH4 = CCl4
D) CF2Cl2 > CF2H2 > CCl4 > CCl2H2 > CH4
E) CH4 > CF2H2 > CF2Cl2 > CCl4 > CCl2H2
A) CF2Cl2 > CF2H2 > CCl2H2 > CH4 = CCl4
B) CH4 > CF2Cl2 > CF2H2 > CCl4 > CCl2H2
C) CF2H2 > CCl2H2 > CF2Cl2 > CH4 = CCl4
D) CF2Cl2 > CF2H2 > CCl4 > CCl2H2 > CH4
E) CH4 > CF2H2 > CF2Cl2 > CCl4 > CCl2H2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
10
Choose the compound with the most ionic bond.
A) LiCl
B) RbI
C) KCl
D) KF
E) NaCl
A) LiCl
B) RbI
C) KCl
D) KF
E) NaCl

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following bonds would be the least polar and yet still be considered polar covalent?
A) Mg-O
B) Si-O
C) B-O
D) N-O
E) O-O
A) Mg-O
B) Si-O
C) B-O
D) N-O
E) O-O

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
12
What of the following shows the bonds in order of decreasing polarity?
A) N-Cl, P-Cl, As-Cl
B) As-Cl, P-Cl, N-Cl
C) P-Cl, As-Cl, N-Cl
D) P-Cl, N-Cl, As-Cl
E) As-Cl, N-Cl, P-Cl
A) N-Cl, P-Cl, As-Cl
B) As-Cl, P-Cl, N-Cl
C) P-Cl, As-Cl, N-Cl
D) P-Cl, N-Cl, As-Cl
E) As-Cl, N-Cl, P-Cl

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
13
Which element listed below has the highest electronegativity?
A) K
B) Te
C) Br
D) I
E) Rb
A) K
B) Te
C) Br
D) I
E) Rb

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
14
For the elements Rb, F, and O, the order of increasing electronegativity is
A) Rb < O < F.
B) Rb < F < O.
C) O < F < Rb.
D) F < Rb < O.
E) none of these.
A) Rb < O < F.
B) Rb < F < O.
C) O < F < Rb.
D) F < Rb < O.
E) none of these.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is incorrect?
A) A molecule with very polar bonds can be nonpolar.
B) Ionic bonding results from the transfer of electrons from one atom to another.
C) Dipole moments result from the unequal distribution of electrons in a molecule.
D) Linear molecules cannot have a net dipole moment.
E) The electrons in a polar bond are found nearer to the more electronegative element.
A) A molecule with very polar bonds can be nonpolar.
B) Ionic bonding results from the transfer of electrons from one atom to another.
C) Dipole moments result from the unequal distribution of electrons in a molecule.
D) Linear molecules cannot have a net dipole moment.
E) The electrons in a polar bond are found nearer to the more electronegative element.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
16
In which case is the bond polarity incorrect?
A) δ+ Cl-Br δ-
B) δ+ Na-O δ-
C) δ+ H-Br δ-
D) δ+ Ca-H δ-
E) δ+ C-O δ-
A) δ+ Cl-Br δ-
B) δ+ Na-O δ-
C) δ+ H-Br δ-
D) δ+ Ca-H δ-
E) δ+ C-O δ-

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
17
Atoms having greatly differing electronegativities are expected to form
A) polar covalent bonds.
B) nonpolar covalent bonds.
C) no bonds.
D) ionic bonds.
E) covalent bonds.
A) polar covalent bonds.
B) nonpolar covalent bonds.
C) no bonds.
D) ionic bonds.
E) covalent bonds.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
18
Which compound does not contain both polar covalent and ionic bonds?
A) Ca(CN)2
B) NH4ClO3
C) CH3OH
D) KOH
E) CsC2H3O2
A) Ca(CN)2
B) NH4ClO3
C) CH3OH
D) KOH
E) CsC2H3O2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
19
As a general pattern, electronegativity is inversely related to
A) atomic size
B) polarity of the atom.
C) the number of neutrons in the nucleus.
D) ionization energy.
E) two of these.
A) atomic size
B) polarity of the atom.
C) the number of neutrons in the nucleus.
D) ionization energy.
E) two of these.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following bonds is the least polar?
A) H-N
B) H-C
C) H-O
D) H-F
E) All are the same.
A) H-N
B) H-C
C) H-O
D) H-F
E) All are the same.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
21
Choose the statement that best describes the PbCl4 molecule in the gas phase.
A) The molecule is polar.
B) The bonds are nonpolar.
C) The molecule is polar with bond angles of about 109°.
D) The bond angles are all about 109°.
E) The molecule has a dipole moment.
A) The molecule is polar.
B) The bonds are nonpolar.
C) The molecule is polar with bond angles of about 109°.
D) The bond angles are all about 109°.
E) The molecule has a dipole moment.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement is correct?
A) H2O is linear.
B) The molecule ClO2 cannot be accurately described by a Lewis structure consistent with the octet rule.
C) The diatomic molecule Cl2 is an example of a polar molecule.
D) The bonds in LiF have a more covalent character than those in F2.
E) none of these
A) H2O is linear.
B) The molecule ClO2 cannot be accurately described by a Lewis structure consistent with the octet rule.
C) The diatomic molecule Cl2 is an example of a polar molecule.
D) The bonds in LiF have a more covalent character than those in F2.
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following ionic compounds has the largest lattice energy; that is, which has the lattice energy most favorable to a stable lattice?
A) LiF
B) LiI
C) CsF
D) MgO
E) CsI
A) LiF
B) LiI
C) CsF
D) MgO
E) CsI

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
24
Use the following electronegativity values to answer the question:

This molecule is the most polar.
A) CH3Cl
B) C2H6
C) CO2
D) CH3CHO
E) none of these

This molecule is the most polar.
A) CH3Cl
B) C2H6
C) CO2
D) CH3CHO
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following molecules has a dipole moment?
A) PCl3
B) BCl3
C) Cl2
D) SiCl4
E) none of these
A) PCl3
B) BCl3
C) Cl2
D) SiCl4
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following has a zero dipole moment?
A) SO2
B) NO2
C) PF5
D) NH3
E) HCN
A) SO2
B) NO2
C) PF5
D) NH3
E) HCN

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following has the smallest radius?
A) Se2-
B) Sr2+
C) Br+
D) Kr
E) Rb+
A) Se2-
B) Sr2+
C) Br+
D) Kr
E) Rb+

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following molecules does not have a dipole moment?
A) H2O
B) H2S
C) H2Xe
D) All of these have a dipole moment.
E) None of these has a dipole moment.
A) H2O
B) H2S
C) H2Xe
D) All of these have a dipole moment.
E) None of these has a dipole moment.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the following molecules.
I. BF3
II. CHBr3 (C is the central atom.)
III. Br2
IV. XeCl2
V. CO
VI. SF4
Select the molecule(s) that fit the given statement.These molecules have a zero net dipole moment.
A) III, V
B) III, IV, V
C) I, III, IV
D) I, III, IV, VI
E) none of them
I. BF3
II. CHBr3 (C is the central atom.)
III. Br2
IV. XeCl2
V. CO
VI. SF4
Select the molecule(s) that fit the given statement.These molecules have a zero net dipole moment.
A) III, V
B) III, IV, V
C) I, III, IV
D) I, III, IV, VI
E) none of them

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following molecules has a nonzero dipole moment?
A) CO2
B) CBr4
C) SiH4
D) SO3
E) PCl3
A) CO2
B) CBr4
C) SiH4
D) SO3
E) PCl3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
31
The first electron affinity value for oxygen is _______ and the second electron affinity value is ________.
A) favorable (exothermic), favorable (exothermic)
B) More information is needed.
C) favorable (exothermic), unfavorable (endothermic)
D) unfavorable (endothermic), favorable (exothermic)
E) unfavorable (endothermic), unfavorable (endothermic)
A) favorable (exothermic), favorable (exothermic)
B) More information is needed.
C) favorable (exothermic), unfavorable (endothermic)
D) unfavorable (endothermic), favorable (exothermic)
E) unfavorable (endothermic), unfavorable (endothermic)

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following molecules has a zero dipole moment?
A) NCl3
B) XeF2
C) SCl4
D) H2S
E) ICl3
A) NCl3
B) XeF2
C) SCl4
D) H2S
E) ICl3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the lattice energy for LiF(s) given the following: 
A) -650. kJ/mol
B) -1047 kJ/mol
C)800. kJ/mol
D) 285 kJ/mol
E) none of these

A) -650. kJ/mol
B) -1047 kJ/mol
C)800. kJ/mol
D) 285 kJ/mol
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
34
Given the following information: 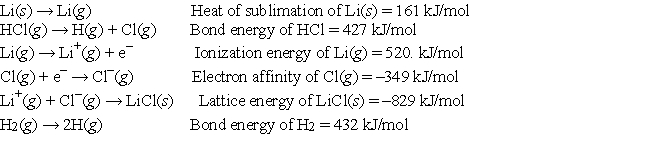 calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)
calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)
A) -179 kJ
B) 362 kJ
C) -70. kJ
D) -572 kJ
E) None of these
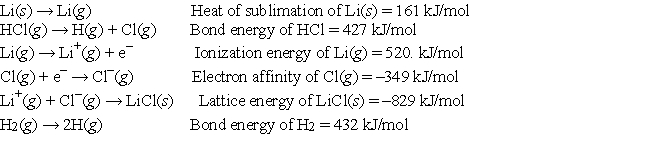 calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)
calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)A) -179 kJ
B) 362 kJ
C) -70. kJ
D) -572 kJ
E) None of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
35
In the reaction between magnesium and sulfur, the magnesium atoms
A) share electrons with sulfur.
B) become part of polyatomic ions.
C) become anions.
D) become cations.
A) share electrons with sulfur.
B) become part of polyatomic ions.
C) become anions.
D) become cations.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is polar?
A) PBr3
B) SiF4
C) SBr6
D) KrF2
E) BF3
A) PBr3
B) SiF4
C) SBr6
D) KrF2
E) BF3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following series is isoelectronic?
A) B, C, N, O
B) Sn, As, S, F
C) Na, K, Rb, Cs
D) S2-, Cl-, K+, Ca2+
E) F-, Cl-, K+, Rb+
A) B, C, N, O
B) Sn, As, S, F
C) Na, K, Rb, Cs
D) S2-, Cl-, K+, Ca2+
E) F-, Cl-, K+, Rb+

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules has a dipole moment?
A) SCl6
B) CO2
C) OF2
D) BH3
E) None of these has a dipole moment.
A) SCl6
B) CO2
C) OF2
D) BH3
E) None of these has a dipole moment.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following has the largest radius?
A) K+
B) Ar
C) S2-
D) Ca2+
E) Cl-
A) K+
B) Ar
C) S2-
D) Ca2+
E) Cl-

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following molecules has a dipole moment?
A) SF4
B) CF4
C) XeF4
D) All of these have a dipole moment.
E) None of these has a dipole moment.
A) SF4
B) CF4
C) XeF4
D) All of these have a dipole moment.
E) None of these has a dipole moment.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds contains only one unshared pair of valence electrons?
A) NaCl
B) NH3
C) BeF3
D) CH4
E) H2O
A) NaCl
B) NH3
C) BeF3
D) CH4
E) H2O

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the compound crotonaldehyde, whose skeleton is 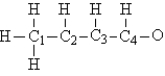
How many electrons must be shown in the Lewis structure of this molecule?
A) 32
B) 28
C) 18
D) 24
E) 12
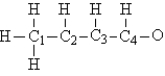
How many electrons must be shown in the Lewis structure of this molecule?
A) 32
B) 28
C) 18
D) 24
E) 12

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
43
Given the following bond energies:  estimate ΔH for the reaction H2O2 + CH3OH → H2CO + 2H2O.
estimate ΔH for the reaction H2O2 + CH3OH → H2CO + 2H2O.
A) -145 kJ
B) +291 kJ
C) -291 kJ
D) +145 kJ
E) -287 kJ
 estimate ΔH for the reaction H2O2 + CH3OH → H2CO + 2H2O.
estimate ΔH for the reaction H2O2 + CH3OH → H2CO + 2H2O.A) -145 kJ
B) +291 kJ
C) -291 kJ
D) +145 kJ
E) -287 kJ

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
44
Estimate the bond energy of the N2 molecule. 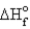 for NH3 = -46.0 kJ/mol
for NH3 = -46.0 kJ/mol
N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
A) 1140 kJ/mol
B) 560 kJ/mol
C) 87 kJ/mol
D) 479 kJ/mol
E) 958 kJ/mol
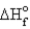 for NH3 = -46.0 kJ/mol
for NH3 = -46.0 kJ/molN-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
A) 1140 kJ/mol
B) 560 kJ/mol
C) 87 kJ/mol
D) 479 kJ/mol
E) 958 kJ/mol

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
45
Choose the molecule with the strongest bond.
A) HBr
B) HCl
C) HF
D) HI
A) HBr
B) HCl
C) HF
D) HI

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following molecules contains a double bond?
A) CO2
B) H2O
C) NH3
D) all
E) none
A) CO2
B) H2O
C) NH3
D) all
E) none

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
47
What does X represent in the Lewis structure X=X?
A) N
B) C
C) B
D) F
E) O
A) N
B) C
C) B
D) F
E) O

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
48
In which of the following compounds does the bond between the central atom and fluorine have the greatest ionic character?
A) SF2
B) SeF2
C) OF2
D) SbF3
E) AsF3
A) SF2
B) SeF2
C) OF2
D) SbF3
E) AsF3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
49
Choose the molecule with the strongest bond.
A) I2
B) F2
C) Br2
D) Cl2
A) I2
B) F2
C) Br2
D) Cl2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
50
In the Lewis structure for elemental nitrogen, there is(are)
A) a triple bond between the nitrogens.
B) three unpaired electrons.
C) a double bond between the nitrogens.
D) a single bond between the nitrogens.
E) none of these
A) a triple bond between the nitrogens.
B) three unpaired electrons.
C) a double bond between the nitrogens.
D) a single bond between the nitrogens.
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
51
Choose the molecule with the strongest bond.
A) HF
B) NH3
C) H2O
D) CH4
A) HF
B) NH3
C) H2O
D) CH4

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following molecules exhibits the greatest bond energy?
A) Cl2
B) Br2
C) F2
D) I2
E) all the same
A) Cl2
B) Br2
C) F2
D) I2
E) all the same

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
53
Using the following data reactions:  calculate the energy of an H-Cl bond.
calculate the energy of an H-Cl bond.
A) 518 kJ
B) 326 kJ
C) 856 kJ
D) 770 kJ
E) 428 kJ
 calculate the energy of an H-Cl bond.
calculate the energy of an H-Cl bond.A) 518 kJ
B) 326 kJ
C) 856 kJ
D) 770 kJ
E) 428 kJ

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
54
Use the following electronegativity values to answer the question:
C 2.5 Cl 3.2
H 2.2 N 3.0
O 3.4
This molecule shows the smallest number of lone pairs in its Lewis structure.
A) CH3CHO
B) CO2
C) CH3Cl
D) C2H6
E) none of these
C 2.5 Cl 3.2
H 2.2 N 3.0
O 3.4
This molecule shows the smallest number of lone pairs in its Lewis structure.
A) CH3CHO
B) CO2
C) CH3Cl
D) C2H6
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the compound crotonaldehyde, whose skeleton is 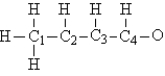
How many nonbonding electrons appear in the Lewis structure of this molecule?
A) 2
B) 4
C) 8
D) 10
E) 6
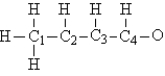
How many nonbonding electrons appear in the Lewis structure of this molecule?
A) 2
B) 4
C) 8
D) 10
E) 6

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following molecules and ions has a lone pair of electrons on the central atom?
A) PCl5
B) XeO4
C) BeCl2
D) CH3+
E) CH3-
A) PCl5
B) XeO4
C) BeCl2
D) CH3+
E) CH3-

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
57
Using the following bond energies:  estimate the heat of combustion for 1 mol of acetylene:
estimate the heat of combustion for 1 mol of acetylene: 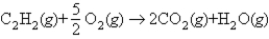
A) +365 kJ
B) 1228 kJ
C) -447 kJ
D) -1228 kJ
E) +447 kJ
 estimate the heat of combustion for 1 mol of acetylene:
estimate the heat of combustion for 1 mol of acetylene: 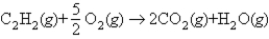
A) +365 kJ
B) 1228 kJ
C) -447 kJ
D) -1228 kJ
E) +447 kJ

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
58
As the number of bonds between two carbon atoms increases, which one of the following decreases?
A) the number of electrons between the carbon atoms
B) the bond length
C) the bond energy
D) all of these
E) none of these
A) the number of electrons between the carbon atoms
B) the bond length
C) the bond energy
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
59
Given the following information:
N2 bond energy = 941 kJ/mol
F2 bond energy = 154 kJ/mol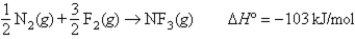
Calculate the N-F bond energy.
A) 113 kJ/mol
B) 268 kJ/mol
C) 66 kJ/mol
D) 317 kJ/mol
E) none of these
N2 bond energy = 941 kJ/mol
F2 bond energy = 154 kJ/mol
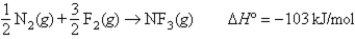
Calculate the N-F bond energy.
A) 113 kJ/mol
B) 268 kJ/mol
C) 66 kJ/mol
D) 317 kJ/mol
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
60
In which pair do both compounds exhibit predominantly ionic bonding?
A) KF and O3
B) PBr5 and HF
C) NaF and H2S
D) NaCl and SrO
E) Na2SO3 and BCl3
A) KF and O3
B) PBr5 and HF
C) NaF and H2S
D) NaCl and SrO
E) Na2SO3 and BCl3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
61
As indicated by Lewis structures, which of the following species could probably not exist as a stable molecule?
A) N2H6
B) N2O4
C) N2H2
D) N2H4
E) NH3
A) N2H6
B) N2O4
C) N2H2
D) N2H4
E) NH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
62
How many acceptable and equivalent resonance structures can be drawn for NO3-?
A) 2
B) 3
C) 4
D) 1
E) 0
A) 2
B) 3
C) 4
D) 1
E) 0

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
63
For which compound is resonance required to describe the structure adequately?
A) PCl3
B) CO32-
C) HCN
D) NH4+
E) none of these
A) PCl3
B) CO32-
C) HCN
D) NH4+
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
64
Draw the Lewis structures of the molecules below, and use them to answer the following questions.
I. BH3
II. NO2
III. SF6
IV. O3
V. PCl5
Which of the molecules obeys the octet rule?
A) II
B) IV
C) III
D) V
E) I
I. BH3
II. NO2
III. SF6
IV. O3
V. PCl5
Which of the molecules obeys the octet rule?
A) II
B) IV
C) III
D) V
E) I

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
65
Which molecule or ion violates the octet rule?
A) I3-
B) PF3
C) H2O
D) NO3-
E) none of these
A) I3-
B) PF3
C) H2O
D) NO3-
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
66
How many of the following molecules and ions contain double or triple bonds?
N2 H2CO C2H4 C2H6 SCN-
A) 5
B) 1
C) 4
D) 2
E) 3
N2 H2CO C2H4 C2H6 SCN-
A) 5
B) 1
C) 4
D) 2
E) 3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
67
Select the best Lewis structure for acetone, CH3COCH3.
A)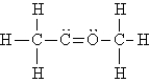
B)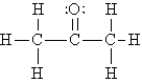
C)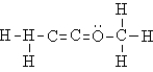
D)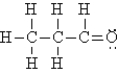
E)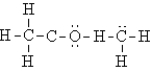
A)
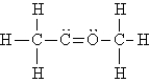
B)

C)
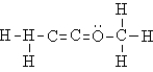
D)
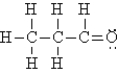
E)
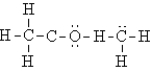

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is not a valid resonance structure for N3-?
A)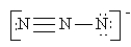
B)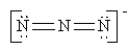
C)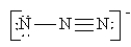
D)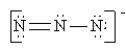
E) All are valid.
A)
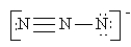
B)
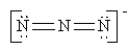
C)
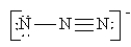
D)
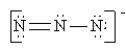
E) All are valid.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
69
How many electrons are in the Lewis structure for SO2?
A) 18
B) 20
C) 32
D) 16
E) 30
A) 18
B) 20
C) 32
D) 16
E) 30

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
70
Given the following Lewis structure: 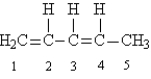
How many electrons are shared between carbons 1 and 2?
A) 6
B) 2
C) 0
D) 8
E) 4
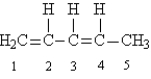
How many electrons are shared between carbons 1 and 2?
A) 6
B) 2
C) 0
D) 8
E) 4

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
71
When molten sulfur reacts with chlorine gas, a vile-smelling orange liquid forms that is found to have the empirical formula SCl. Which of the following could be the correct Lewis structure for this compound?
A)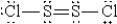
B)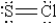
C)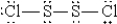
D)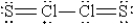
E)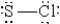
A)
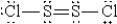
B)
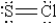
C)
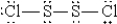
D)
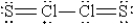
E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
72
In the Lewis structure for I3-, there are _________ electrons around the central iodine atom.
A) 12
B) 4
C) 8
D) 10
E) none of these
A) 12
B) 4
C) 8
D) 10
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
73
Complete the Lewis structure for the molecule 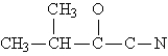 This molecule has __________ single bonds and __________ multiple bonds.
This molecule has __________ single bonds and __________ multiple bonds.
A) 11, 2
B) 6, 3
C) 13, 0
D) 11, 5
E) 4, 2
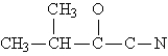 This molecule has __________ single bonds and __________ multiple bonds.
This molecule has __________ single bonds and __________ multiple bonds.A) 11, 2
B) 6, 3
C) 13, 0
D) 11, 5
E) 4, 2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
74
For which of the following can we not draw a stable Lewis structure?
A) PCl5
B) OCl6
C) SCl6
D) All of these have stable Lewis structures.
E) None of these has a stable Lewis structure.
A) PCl5
B) OCl6
C) SCl6
D) All of these have stable Lewis structures.
E) None of these has a stable Lewis structure.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
75
As indicated by Lewis structures, which of the following would probably not exist as a stable molecule?
A) CH2O
B) C2H2
C) CH3O
D) C3H4
E) CH3OH
A) CH2O
B) C2H2
C) CH3O
D) C3H4
E) CH3OH

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
76
The Lewis structure for H3BO3 is
A)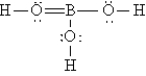
B)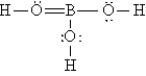
C)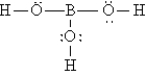
D)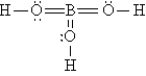
E)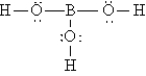
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
77
How many of the following exhibit resonance?
O3 OCl2 NF3 CCl4
A) 2
B) 0
C) 3
D) 1
E) 4
O3 OCl2 NF3 CCl4
A) 2
B) 0
C) 3
D) 1
E) 4

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
78
How many resonance structures does the molecule SO2 have?
A) 2
B) 1
C) 4
D) 0
E) 3
A) 2
B) 1
C) 4
D) 0
E) 3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
79
Given the following Lewis structure: 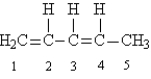
How many unshared pairs of electrons are present in this molecule?
A) 1
B) 4
C) 0
D) 3
E) 2
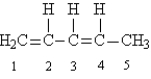
How many unshared pairs of electrons are present in this molecule?
A) 1
B) 4
C) 0
D) 3
E) 2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
80
Which species has an unpaired electron?
A) OH-
B) N2
C) CO
D) NO
E) none of these
A) OH-
B) N2
C) CO
D) NO
E) none of these

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck


