Deck 10: Spontaneity, Entropy, and Free Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/143
Play
Full screen (f)
Deck 10: Spontaneity, Entropy, and Free Energy
1
A gas expands isothermally and irreversibly.
ΔS is
A) less than zero.
B) More information is needed.
C) greater than zero.
D) equal to zero.
ΔS is
A) less than zero.
B) More information is needed.
C) greater than zero.
D) equal to zero.
greater than zero.
2
In an isothermal process, the pressure on 1 mol of an ideal monatomic gas suddenly changes from 4.00 atm to 100.0 atm at 25°C.
Calculate ΔT.
A) 25°C
B) 100°C
C) 0°C
D) 48°C
E) none of these
Calculate ΔT.
A) 25°C
B) 100°C
C) 0°C
D) 48°C
E) none of these
0°C
3
A gas expands isothermally and irreversibly.
ΔE is
A) greater than zero.
B) less than zero.
C) equal to zero.
D) More information is needed.
ΔE is
A) greater than zero.
B) less than zero.
C) equal to zero.
D) More information is needed.
equal to zero.
4
One mole of an ideal gas is compressed isothermally and reversibly at 607.4 K from 5.60 atm to 8.90 atm.
Calculate ΔH.
A) 2.34 kJ
B) -2.34 kJ
C) 0 kJ
D) 3.85 kJ
E) none of these
Calculate ΔH.
A) 2.34 kJ
B) -2.34 kJ
C) 0 kJ
D) 3.85 kJ
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
5
A gas expands isothermally and irreversibly.
q is
A) More information is needed.
B) less than zero.
C) greater than zero.
D) equal to zero.
q is
A) More information is needed.
B) less than zero.
C) greater than zero.
D) equal to zero.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the dissociation reaction of the acid HF.  Why is ΔS negative?
Why is ΔS negative?
A) The reaction is expected to be exothermic, and ΔS thus should be negative.
B) The reaction is expected to be endothermic, and thus ΔS should be negative.
C) Each HF molecule produces two ions when it dissociates.
D) The ions are hydrated.
E) none of these
 Why is ΔS negative?
Why is ΔS negative?A) The reaction is expected to be exothermic, and ΔS thus should be negative.
B) The reaction is expected to be endothermic, and thus ΔS should be negative.
C) Each HF molecule produces two ions when it dissociates.
D) The ions are hydrated.
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the process A(l)  A(s). Which direction favors positional randomness?
A(s). Which direction favors positional randomness?
A) to the left
B) neither
C) It depends on the temperature.
D) to the right
 A(s). Which direction favors positional randomness?
A(s). Which direction favors positional randomness?A) to the left
B) neither
C) It depends on the temperature.
D) to the right

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
8
A gas expands isothermally and irreversibly.
ΔH is
A) less than zero.
B) More information is needed.
C) equal to zero.
D) greater than zero.
ΔH is
A) less than zero.
B) More information is needed.
C) equal to zero.
D) greater than zero.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
9
One mole of an ideal gas expands isothermally and reversibly at 0°C. The pressure on 1 mol of an ideal monatomic gas changes from 100.0 atm to 1.00 atm.
Calculate q.
A) -225 kJ
B) 0
C) -10.5 kJ
D) 10.5 kJ
E) 225 kJ
Calculate q.
A) -225 kJ
B) 0
C) -10.5 kJ
D) 10.5 kJ
E) 225 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
10
A gas expands isothermally and irreversibly.
ΔG is
A) More information is needed.
B) less than zero.
C) greater than zero.
D) equal to zero.
ΔG is
A) More information is needed.
B) less than zero.
C) greater than zero.
D) equal to zero.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
11
One mole of an ideal gas is compressed isothermally and reversibly at 607.4 K from 5.60 atm to 8.90 atm.
Calculate w.
A) -298 kJ
B) 2.34 kJ
C) 298 kJ
D) -2.34 kJ
E) none of these
Calculate w.
A) -298 kJ
B) 2.34 kJ
C) 298 kJ
D) -2.34 kJ
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the process A(l)  A(s). An increase in temperature favors which direction?
A(s). An increase in temperature favors which direction?
A) to the right
B) to the left
C) More information is needed.
D) neither
 A(s). An increase in temperature favors which direction?
A(s). An increase in temperature favors which direction?A) to the right
B) to the left
C) More information is needed.
D) neither

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
13
A gas expands isothermally and irreversibly.
w is
A) less than zero.
B) greater than zero.
C) More information is needed.
D) equal to zero.
w is
A) less than zero.
B) greater than zero.
C) More information is needed.
D) equal to zero.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
14
One mole of an ideal gas expands isothermally and reversibly at 0°C. The pressure on 1 mol of an ideal monatomic gas changes from 100.0 atm to 1.00 atm.
Calculate w.
A) 10.5 kJ
B) 225 kJ
C) -10.5 kJ
D) 0
E) -225 kJ
Calculate w.
A) 10.5 kJ
B) 225 kJ
C) -10.5 kJ
D) 0
E) -225 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following result(s) in an increase in the entropy of the system?  I. (See diagram.)
I. (See diagram.)
II. Br2(g) → Br2(l)
III. NaBr(s) → Na+(aq) + Br-(aq)
IV. O2(298 K) → O2(373 K)
V. NH3(1 atm, 298 K) → NH3(3 atm, 298 K)
A) I, III, IV
B) I, II, III, IV
C) I
D) II, V
E) I, II, III, V
 I. (See diagram.)
I. (See diagram.)II. Br2(g) → Br2(l)
III. NaBr(s) → Na+(aq) + Br-(aq)
IV. O2(298 K) → O2(373 K)
V. NH3(1 atm, 298 K) → NH3(3 atm, 298 K)
A) I, III, IV
B) I, II, III, IV
C) I
D) II, V
E) I, II, III, V

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
16
One mole of an ideal gas is compressed isothermally and reversibly at 607.4 K from 5.60 atm to 8.90 atm.
Calculate q.
A) 298 kJ
B) -298 kJ
C) 2.34 kJ
D) -2.34 kJ
E) none of these
Calculate q.
A) 298 kJ
B) -298 kJ
C) 2.34 kJ
D) -2.34 kJ
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
17
In an isothermal process, the pressure on 1 mol of an ideal monatomic gas suddenly changes from 4.00 atm to 100.0 atm at 25°C.
Calculate ΔV.
A) -5.87 L
B) 6.11 L
C) -6.11 L
D) 5.87 L
E) none of these
Calculate ΔV.
A) -5.87 L
B) 6.11 L
C) -6.11 L
D) 5.87 L
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
18
A mixture of hydrogen and chlorine remains unreacted until it is exposed to ultraviolet light from a burning magnesium strip. Then the following reaction occurs very rapidly.  Select the statement below that best explains this behavior.
Select the statement below that best explains this behavior.
A) The reactants are thermodynamically more stable than the products.
B) The reaction is spontaneous, but the reactants are kinetically stable.
C) The reaction has a small equilibrium constant.
D) The ultraviolet light raises the temperature of the system and makes the reaction more favorable.
E) The negative value for ΔS slows down the reaction.
 Select the statement below that best explains this behavior.
Select the statement below that best explains this behavior.A) The reactants are thermodynamically more stable than the products.
B) The reaction is spontaneous, but the reactants are kinetically stable.
C) The reaction has a small equilibrium constant.
D) The ultraviolet light raises the temperature of the system and makes the reaction more favorable.
E) The negative value for ΔS slows down the reaction.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
19
A gas expands isothermally and irreversibly.
ΔSsurr is
A) More information is needed.
B) equal to zero.
C) less than zero.
D) greater than zero.
ΔSsurr is
A) More information is needed.
B) equal to zero.
C) less than zero.
D) greater than zero.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
20
One mole of an ideal gas is compressed isothermally at 607.4 K from 5.60 atm to 8.90 atm. If the process is carried out in two irreversible steps (intermediate step at P = 7.00 atm), calculate the following.ΔS
A) 0 J/K
B) -3.85 J/K
C) 3.85 J/K
D) -2.34 J/K
E) 2.34 J/K
A) 0 J/K
B) -3.85 J/K
C) 3.85 J/K
D) -2.34 J/K
E) 2.34 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
21
1.8 mol of an ideal gas at 303 K is compressed isothermally and reversibly from 2.2 L to 0.20 L. What is ΔS?
A) -10.9 kJ/K
B) 36 J/K
C) -36 J/K
D) -18 J/K
E) -20 J/K
A) -10.9 kJ/K
B) 36 J/K
C) -36 J/K
D) -18 J/K
E) -20 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement is true?
A) There is always more heat given off to the surroundings in a reversible process than in an unharnessed one.
B) In a reversible process, the state functions of the system are always much greater than those of the surroundings.
C) A thermodynamically reversible process takes place infinitely fast.
D) All real processes are irreversible.
E) All these statements are true.
A) There is always more heat given off to the surroundings in a reversible process than in an unharnessed one.
B) In a reversible process, the state functions of the system are always much greater than those of the surroundings.
C) A thermodynamically reversible process takes place infinitely fast.
D) All real processes are irreversible.
E) All these statements are true.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
23
In a certain reversible expansion, a system at 300. K absorbs exactly 6.00 × 102 J of heat. In the irreversible recompression to the original state of the system, twice as much work is done on the system as is performed on the surroundings in the expansion. What is the entropy change of the system in the recompression step?
A) 0.00 J/K
B) 2.00 J/K
C) -4.00 J/K
D) -2.00 J/K
E) 4.00 J/K
A) 0.00 J/K
B) 2.00 J/K
C) -4.00 J/K
D) -2.00 J/K
E) 4.00 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
24
At 1 atm, a liquid is heated above its normal boiling point.
ΔSuniv for this process is
A) equal to zero.
B) less than zero.
C) greater than zero.
D) cannot be determined
ΔSuniv for this process is
A) equal to zero.
B) less than zero.
C) greater than zero.
D) cannot be determined

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
25
A machine employs the isothermal expansion of 1 mol of an ideal gas from 4.50 L to 15.0 L. At 25°C, the machine performs 3.00 kJ of work. What percent of the maximum possible work is the machine producing?
A) 15.0%
B) 50.3%
C) This amount of work cannot be correct.
D) 0.60%
E) 4.50%
A) 15.0%
B) 50.3%
C) This amount of work cannot be correct.
D) 0.60%
E) 4.50%

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
26
If the change in entropy of the surroundings for a process at 443 K and constant pressure is -347 J/K, what is the heat flow absorbed by the system?
A) 154 kJ
B) 783 kJ
C) -154 kJ
D) -18.5 kJ
E) 18.5 kJ
A) 154 kJ
B) 783 kJ
C) -154 kJ
D) -18.5 kJ
E) 18.5 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is true?
A) For any process, ΔSsurr and ΔSsys have opposite signs.
B) As long as the disorder of the surroundings is increasing, a process will be spontaneous.
C) If ΔSsurr = -ΔSsys , the process is at equilibrium.
D) ΔH° is zero for a chemical reaction at constant temperature.
E) none of these
A) For any process, ΔSsurr and ΔSsys have opposite signs.
B) As long as the disorder of the surroundings is increasing, a process will be spontaneous.
C) If ΔSsurr = -ΔSsys , the process is at equilibrium.
D) ΔH° is zero for a chemical reaction at constant temperature.
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
28
One mole of an ideal gas at 25°C is expanded isothermally and reversibly from 125.0 L to 250.0 L. Which statement is correct?
A) ΔSgas = ΔSsurr
B) ΔSgas = Rln2
C) ΔSgas = 0
D) ΔSuniv = 0
E) ΔSsurr = 0
A) ΔSgas = ΔSsurr
B) ΔSgas = Rln2
C) ΔSgas = 0
D) ΔSuniv = 0
E) ΔSsurr = 0

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the entropy change when 5.00 mol of a monatomic ideal gas is cooled from 135°C to 85°C at 1 atm.
A) -48.9 J/K
B) -13.6 J/K
C) -250.0 J/K
D) -9.62 J/K
E) -2.74 J/K
A) -48.9 J/K
B) -13.6 J/K
C) -250.0 J/K
D) -9.62 J/K
E) -2.74 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
30
ΔSsurr is _______ for exothermic reactions and ______ for endothermic reactions.
A) favorable, unfavorable
B) favorable, favorable
C) unfavorable, favorable
D) unfavorable, unfavorable
E) cannot tell
A) favorable, unfavorable
B) favorable, favorable
C) unfavorable, favorable
D) unfavorable, unfavorable
E) cannot tell

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
31
ΔS is _______ for exothermic reactions and ______ for endothermic reactions.
A) unfavorable, unfavorable
B) favorable, unfavorable
C) favorable, favorable
D) unfavorable, favorable
E) cannot tell
A) unfavorable, unfavorable
B) favorable, unfavorable
C) favorable, favorable
D) unfavorable, favorable
E) cannot tell

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
32
In an isothermal process, the pressure on 1 mol of an ideal monatomic gas suddenly changes from 4.00 atm to 100.0 atm at 25°C.
Calculate w.
A) 0
B) 23.5 kJ
C) 59.5 kJ
D) -59.5 kJ
E) -23.5 kJ
Calculate w.
A) 0
B) 23.5 kJ
C) 59.5 kJ
D) -59.5 kJ
E) -23.5 kJ

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
33
One mole of an ideal gas is compressed isothermally and reversibly at 607.4 K from 5.60 atm to 8.90 atm.Calculate ΔS.
A) 0
B) 3.85 J/K
C) 2.34 J/K
D) -2.34 J/K
E) -3.85 J/K
A) 0
B) 3.85 J/K
C) 2.34 J/K
D) -2.34 J/K
E) -3.85 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
34
In an isothermal process, the pressure on 1 mol of an ideal monatomic gas suddenly changes from 4.00 atm to 100.0 atm at 25°C.Calculate ΔE.
A) 0 kJ
B) 119 kJ
C) 59.5 kJ
D) -59.5 kJ
E) none of these
A) 0 kJ
B) 119 kJ
C) 59.5 kJ
D) -59.5 kJ
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
35
One mole of an ideal gas at 25°C is expanded isothermally from 5.0 L to 10.0 L under such conditions that no work is produced in the surroundings. Which statement is correct?
A) ΔSgas = 0
B) ΔSgas = Rln2/298
C) ΔSsurr = 0
D) ΔSuniv = 0
E) ΔSgas = ΔSsurr
A) ΔSgas = 0
B) ΔSgas = Rln2/298
C) ΔSsurr = 0
D) ΔSuniv = 0
E) ΔSgas = ΔSsurr

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate ΔG for the isothermal compression of 1 mol of an ideal monatomic gas from 1.4 atm to 5.0 atm at 25°C.
A) 1.5 × 103 J
B) 3.2 × 103 J
C) 4.7 × 103 J
D) -3.2 × 103 J
E) -1.5 × 103 J
A) 1.5 × 103 J
B) 3.2 × 103 J
C) 4.7 × 103 J
D) -3.2 × 103 J
E) -1.5 × 103 J

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
37
At 1 atm, a liquid is heated above its normal boiling point.
ΔS for this process is
A) greater than zero.
B) less than zero.
C) equal to zero.
D) cannot be determined
ΔS for this process is
A) greater than zero.
B) less than zero.
C) equal to zero.
D) cannot be determined

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
38
One mole of an ideal gas expands isothermally and reversibly at 0°C. The pressure on 1 mol of an ideal monatomic gas changes from 100.0 atm to 1.00 atm.Calculate ΔE.
A) 450 kJ
B) -21.0 kJ
C) -450 kJ
D) 21.0 kJ
E) none of these
A) 450 kJ
B) -21.0 kJ
C) -450 kJ
D) 21.0 kJ
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
39
At 1 atm, a liquid is heated above its normal boiling point.
ΔSsurr for this process is
A) less than zero.
B) cannot be determined
C) greater than zero.
D) equal to zero.
ΔSsurr for this process is
A) less than zero.
B) cannot be determined
C) greater than zero.
D) equal to zero.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
40
A rigid insulated box contains 20.0 g of He(g) at 25.0° C and 1.00 atm in one compartment and 20.0 g of N2(g) at 115° C and 2.00 atm in the other compartment. These compartments are connected by a partition that transmits heat. What is the final temperature in the box at thermal equilibrium? (Cv(He) = 12.5 J/K•mol; Cv(N2) = 20.7 J/K•mol)
A) 81.0°C
B) 70.0°C
C) 58.9°C
D) 42.3°C
E) none of these
A) 81.0°C
B) 70.0°C
C) 58.9°C
D) 42.3°C
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
41
In an isothermal process, the pressure on 1 mol of an ideal monatomic gas suddenly changes from 4.00 atm to 100.0 atm at 25°C.
Calculate ΔS.
A) -20.5 J/K
B) -26.8 J/K
C) 26.8 J/K
D) 20.5 J/K
E) none of these
Calculate ΔS.
A) -20.5 J/K
B) -26.8 J/K
C) 26.8 J/K
D) 20.5 J/K
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
42
Substance X has a heat of vaporization of 55.4 kJ/mol at its normal boiling point (423°C). For the process X(l) → X(g) at 1 atm and 423°C, calculate the value of:
ΔG
A) 79.6 J/K•mol
B) -79.6 J/K•mol
C) 0
D) 103 J/K•mol
E) -103 J/K•mol
ΔG
A) 79.6 J/K•mol
B) -79.6 J/K•mol
C) 0
D) 103 J/K•mol
E) -103 J/K•mol

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
43
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
What is ΔS?
A) More information is needed.
B) less than zero
C) equal to zero
D) greater than zero
What is ΔS?
A) More information is needed.
B) less than zero
C) equal to zero
D) greater than zero

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the correct statement.
A) A reaction that exhibits a negative value of ΔS cannot be spontaneous.
B) Exothermic reactions are always spontaneous.
C) Free energy is independent of temperature.
D) At constant pressure and temperature, a decrease in free energy ensures an increase in the entropy of the system.
E) none of these
A) A reaction that exhibits a negative value of ΔS cannot be spontaneous.
B) Exothermic reactions are always spontaneous.
C) Free energy is independent of temperature.
D) At constant pressure and temperature, a decrease in free energy ensures an increase in the entropy of the system.
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
45
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
Calculate the minimum work required to compress 1.9 mol of an ideal monatomic gas from 48.0 L to 17.8 L at 29°C.
A) 7.1 × 103 J
B) 4.5 × 102 J
C) 4.7 × 103 J
D) -7.1 × 103 J
E) 2.5 × 102 J
Calculate the minimum work required to compress 1.9 mol of an ideal monatomic gas from 48.0 L to 17.8 L at 29°C.
A) 7.1 × 103 J
B) 4.5 × 102 J
C) 4.7 × 103 J
D) -7.1 × 103 J
E) 2.5 × 102 J

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
46
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
6.00 mol of a monatomic ideal gas is cooled from 254°C to 24°C at constant volume. Calculate ΔS.
A) -31.1 J/K
B) -42.9 J/K
C) -1.77 × 102 J/K
D) -18.6 J/K
E) -71.5 J/K
6.00 mol of a monatomic ideal gas is cooled from 254°C to 24°C at constant volume. Calculate ΔS.
A) -31.1 J/K
B) -42.9 J/K
C) -1.77 × 102 J/K
D) -18.6 J/K
E) -71.5 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
47
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
Calculate ΔS for cooling 2.60 mol of an ideal monatomic gas from 88°C to 39°C at constant volume.
A) -1.82 J/K
B) -17.6 J/K
C) -3.15 J/K
D) -4.73 J/K
E) -26.4 J/K
Calculate ΔS for cooling 2.60 mol of an ideal monatomic gas from 88°C to 39°C at constant volume.
A) -1.82 J/K
B) -17.6 J/K
C) -3.15 J/K
D) -4.73 J/K
E) -26.4 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
48
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
What is q?
A) greater than zero
B) More information is needed.
C) equal to zero
D) less than zero
What is q?
A) greater than zero
B) More information is needed.
C) equal to zero
D) less than zero

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
49
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
Calculate ΔS for an isothermal (95°C) expansion of 2 mol of a monatomic ideal gas from 100.0 atm to 1.00 atm.
A) -38.3 J/K
B) 38.3 J/K
C) -76.6 J/K
D) 76.6 J/K
E) none
Calculate ΔS for an isothermal (95°C) expansion of 2 mol of a monatomic ideal gas from 100.0 atm to 1.00 atm.
A) -38.3 J/K
B) 38.3 J/K
C) -76.6 J/K
D) 76.6 J/K
E) none

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
50
Substance X has a heat of vaporization of 55.4 kJ/mol at its normal boiling point (423°C). For the process X(l) → X(g) at 1 atm and 423°C, calculate the value of:
ΔSsurr
A) -79.6 J/K•mol
B) 0
C) -103 J/K•mol
D) 103 J/K•mol
E) 79.6 J/K•mol
ΔSsurr
A) -79.6 J/K•mol
B) 0
C) -103 J/K•mol
D) 103 J/K•mol
E) 79.6 J/K•mol

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
51
In an isothermal process, the pressure on 1 mol of an ideal monatomic gas suddenly changes from 4.00 atm to 100.0 atm at 25°C.
Calculate ΔG.
A) -7.98 kJ
B) 7.98 kJ
C) 0 kJ
D) 59.5 kJ
E) none of these
Calculate ΔG.
A) -7.98 kJ
B) 7.98 kJ
C) 0 kJ
D) 59.5 kJ
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
52
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
What is ΔH?
A) less than zero
B) More information is needed.
C) greater than zero
D) equal to zero
What is ΔH?
A) less than zero
B) More information is needed.
C) greater than zero
D) equal to zero

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
53
When a gas is expanded isothermally and reversibly at 39°C, the work done is -43.4 kJ. Calculate ΔS.
A) 1.11 kJ/K
B) -1113 J/K
C) -1156 J/K
D) -1.11 kJ/K
E) 1.39 × 102 J/K
A) 1.11 kJ/K
B) -1113 J/K
C) -1156 J/K
D) -1.11 kJ/K
E) 1.39 × 102 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
54
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
Calculate ΔS for cooling 2.1 mol of an ideal monatomic gas from 81°C to 44°C at constant pressure.
A) -26.6 J/K
B) -2.89 J/K
C) -4.82 J/K
D) -2.29 J/K
E) -1.93 J/K
Calculate ΔS for cooling 2.1 mol of an ideal monatomic gas from 81°C to 44°C at constant pressure.
A) -26.6 J/K
B) -2.89 J/K
C) -4.82 J/K
D) -2.29 J/K
E) -1.93 J/K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
55
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
What is ΔG?
A) less than zero
B) equal to zero
C) greater than zero
D) More information is needed.
What is ΔG?
A) less than zero
B) equal to zero
C) greater than zero
D) More information is needed.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
56
Substance X has a heat of vaporization of 55.4 kJ/mol at its normal boiling point (423°C). For the process X(l) → X(g) at 1 atm and 423°C, calculate the value of:
ΔSuniv
A) 0
B) -79.6 J/K•mol
C) 79.6 J/K•mol
D) -103 J/K•mol
E) 103 J/K•mol
ΔSuniv
A) 0
B) -79.6 J/K•mol
C) 79.6 J/K•mol
D) -103 J/K•mol
E) 103 J/K•mol

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
57
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
What is w?
A) greater than zero
B) More information is needed.
C) equal to zero
D) less than zero
What is w?
A) greater than zero
B) More information is needed.
C) equal to zero
D) less than zero

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
58
A 100-mL sample of water is placed in a coffee cup calorimeter. When 1.0 g of an ionic solid is added, the temperature decreases from 21.5°C to 20.8°C as the solid dissolves. Which of the following is true for the dissolving of the solid?
A) ΔH < 0
B) ΔSsys< 0
C) ΔSuniv > 0
D) ΔSsurr > 0
E) none of these
A) ΔH < 0
B) ΔSsys< 0
C) ΔSuniv > 0
D) ΔSsurr > 0
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
59
For which process is ΔS negative?
A) evaporation of 1 mol of CCl4(l)
B) grinding a large crystal of KCl to powder
C) raising the temperature of 100 g of Cu from 275 K to 295 K
D) mixing 5 mL of ethanol with 25 mL of water
E) compressing 1 mol of Ne at constant temperature from 1.5 atm to 0.5 atm
A) evaporation of 1 mol of CCl4(l)
B) grinding a large crystal of KCl to powder
C) raising the temperature of 100 g of Cu from 275 K to 295 K
D) mixing 5 mL of ethanol with 25 mL of water
E) compressing 1 mol of Ne at constant temperature from 1.5 atm to 0.5 atm

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
60
The process H2O(g) → H2O(l) takes place at 1 atm and 95°C.
Assume that the enthalpy of fusion of ice is 6020 J/mol and does not vary appreciably over the temperature range 270-290 K. If 1.40 mol of ice at 0°C is melted by heat supplied from surroundings at 284 K, what is the entropy change in the surroundings in J/K?
A) +30.9
B) -30.9
C) 0.0
D) -27.9
E) +27.9
Assume that the enthalpy of fusion of ice is 6020 J/mol and does not vary appreciably over the temperature range 270-290 K. If 1.40 mol of ice at 0°C is melted by heat supplied from surroundings at 284 K, what is the entropy change in the surroundings in J/K?
A) +30.9
B) -30.9
C) 0.0
D) -27.9
E) +27.9

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
61
For the process moleene(l) → moleene(g) at 1 atm, ΔH°vap = 26.0 kJ/mol and ΔS°vap = 83.4 J/mol•K. Assuming these values are independent of T, what is the normal boiling point of moleene?
A) 585°C
B) 312°C
C) 39°C
D) -39°C
E) 382°C
A) 585°C
B) 312°C
C) 39°C
D) -39°C
E) 382°C

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
62
The dissociation of hydrogen
H2(g) 2H(g)
2H(g)
A) is spontaneous at high temperature.
B) is spontaneous at low temperature.
C) is independent of temperature.
D) is spontaneous at any temperature.
E) never takes place.
H2(g)
 2H(g)
2H(g)A) is spontaneous at high temperature.
B) is spontaneous at low temperature.
C) is independent of temperature.
D) is spontaneous at any temperature.
E) never takes place.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
63
The vapor pressure of Br2(l) at 25°C is 0.281 atm, and 193 J is required to vaporize 1.00 g of bromine at 1 atm pressure.
Calculate ΔS° for the vaporization of Br2(l) at 25°C and 1 atm.
A) 103 J/K•mol
B) 92.8 J/K•mol
C) 30.8 J/K•mol
D) 0 J/K•mol
E) none of these
Calculate ΔS° for the vaporization of Br2(l) at 25°C and 1 atm.
A) 103 J/K•mol
B) 92.8 J/K•mol
C) 30.8 J/K•mol
D) 0 J/K•mol
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the reaction
2N2O5(g) 4NO2(g) + O2(g)
4NO2(g) + O2(g)
At 25°C, for which the following data are relevant:
Which of the following is true for this reaction?
A) Both ΔH° and ΔS° favor the reaction's spontaneity.
B) ΔH° opposes the reaction, but ΔS° favors it.
C) Both ΔH° and ΔS° oppose the reaction's spontaneity.
D) The reaction cannot occur at room temperature.
E) ΔH° favors the reaction, but ΔS° opposes it.
2N2O5(g)
 4NO2(g) + O2(g)
4NO2(g) + O2(g)At 25°C, for which the following data are relevant:

Which of the following is true for this reaction?
A) Both ΔH° and ΔS° favor the reaction's spontaneity.
B) ΔH° opposes the reaction, but ΔS° favors it.
C) Both ΔH° and ΔS° oppose the reaction's spontaneity.
D) The reaction cannot occur at room temperature.
E) ΔH° favors the reaction, but ΔS° opposes it.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
65
Substance X has a heat of vaporization of 55.4 kJ/mol at its normal boiling point (423°C). For the process X(l) → X(g) at 1 atm and 423°C, calculate the value of:
Consider the freezing of liquid water at -10°C. For this process what are the signs for ΔH, ΔS, and ΔG, respectively?
A) - - -
B) + - -
C) - + -
D) - + 0
E) + - 0
Consider the freezing of liquid water at -10°C. For this process what are the signs for ΔH, ΔS, and ΔG, respectively?
A) - - -
B) + - -
C) - + -
D) - + 0
E) + - 0

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
66
In an isothermal process, the pressure on 1 mol of an ideal monatomic gas suddenly changes from 4.00 atm to 100.0 atm at 25°C.Calculate ΔH.
A) -59.5 kJ
B) 0 kJ
C) 59.5 kJ
D) -119 kJ
E) none of these
A) -59.5 kJ
B) 0 kJ
C) 59.5 kJ
D) -119 kJ
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
67
Assuming ΔH° and ΔS° are temperature independent, calculate the normal boiling point of bromine.
A) 25°C
B) 332°C
C) 0°C
D) 332 K
E) none of these
A) 25°C
B) 332°C
C) 0°C
D) 332 K
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
68
The vapor pressure of Br2(l) at 25°C is 0.281 atm, and 193 J is required to vaporize 1.00 g of bromine at 1 atm pressure.
Calculate ΔH° for the vaporization of Br2(l) at 25°C and 1 atm.
A) 15.4 kJ/mol
B) 0.193 kJ/mol
C) 1.21 kJ/mol
D) 30.8 kJ/mol
E) none of these
Calculate ΔH° for the vaporization of Br2(l) at 25°C and 1 atm.
A) 15.4 kJ/mol
B) 0.193 kJ/mol
C) 1.21 kJ/mol
D) 30.8 kJ/mol
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
69
When a stable diatomic molecule spontaneously forms from its atoms, what are the signs of ΔH°, ΔS°, and ΔG°, respectively?
A) + + +
B) - - +
C) - + +
D) + - -
E) - - -
A) + + +
B) - - +
C) - + +
D) + - -
E) - - -

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
70
In which case must a reaction be spontaneous at all temperatures?
A) ΔS = 0, ΔH is positive
B) ΔH is negative, ΔS is positive
C) ΔH = 0, ΔS is negative
D) ΔH is positive, ΔS is positive
E) none of these
A) ΔS = 0, ΔH is positive
B) ΔH is negative, ΔS is positive
C) ΔH = 0, ΔS is negative
D) ΔH is positive, ΔS is positive
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
71
For the process involving compound A: A(s) → A(l), ΔH° = 8.8 kJ/mol, and ΔS° = 37.4 J/mol•K. What is the melting point of compound A?
A) 235°C
B) 38°C
C) -38°C
D) -235°C
E) -227°C
A) 235°C
B) 38°C
C) -38°C
D) -235°C
E) -227°C

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
72
For a spontaneous endothermic process, which conditions must hold?
1) wmax = ΔG
2) ΔSsurr > 0
3) ΔS cannot be negative.
4) ΔS is positive.
A) all
B) none
C) 1 and 3 only
D) 1, 2, and 4 only
E) 3 and 4 only
1) wmax = ΔG
2) ΔSsurr > 0
3) ΔS cannot be negative.
4) ΔS is positive.
A) all
B) none
C) 1 and 3 only
D) 1, 2, and 4 only
E) 3 and 4 only

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
73
Substance X has a heat of vaporization of 55.4 kJ/mol at its normal boiling point (423°C). For the process X(l) → X(g) at 1 atm and 423°C, calculate the value of:
ΔS
A) 0
B) 103 J/K•mol
C) -79.6 J/K•mol
D) 79.6 J/K•mol
E) -103 J/K•mol
ΔS
A) 0
B) 103 J/K•mol
C) -79.6 J/K•mol
D) 79.6 J/K•mol
E) -103 J/K•mol

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
74
Calculate ΔG for the vaporization of Br2(l) at 25°C and 1 atm.
A) 3.15 kJ/mol
B) 0.263 kJ/mol
C) 0
D) 0.378 kJ/mol
E) none of these
A) 3.15 kJ/mol
B) 0.263 kJ/mol
C) 0
D) 0.378 kJ/mol
E) none of these

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
75
As O2(l) is cooled at 1 atm, it freezes at 54.5 K to form Solid I. At a lower temperature, Solid I rearranges to Solid II, which has a different crystal structure. Thermal measurements show that ΔH for the I → II phase transition is -743.1 J/mol and that ΔS for the same transition is -17.0 J/K mol. At what temperature are Solids I and II in equilibrium?
A) 43.7 K
B) 2.06 K
C) 53.4 K
D) 31.5 K
E) They can never be in equilibrium because they are both solids.
A) 43.7 K
B) 2.06 K
C) 53.4 K
D) 31.5 K
E) They can never be in equilibrium because they are both solids.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
76
Elemental sulfur exists in two crystalline forms, rhombic and monoclinic. From the following data, calculate the temperature at which monoclinic sulfur and rhombic sulfur are in equilibrium. 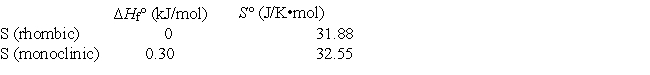
A) 0 K
B) +450 K
C) +210 K
D) -210 K
E) -450 K
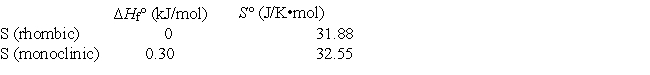
A) 0 K
B) +450 K
C) +210 K
D) -210 K
E) -450 K

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
77
For the vaporization of a liquid at a given pressure,
A) ΔG is positive at all temperatures.
B) ΔG is negative at all temperatures.
C) ΔG is positive at low temperatures but negative at high temperatures (and zero at some temperature).
D) ΔG is negative at low temperatures but positive at high temperatures (and zero at some temperature).
A) ΔG is positive at all temperatures.
B) ΔG is negative at all temperatures.
C) ΔG is positive at low temperatures but negative at high temperatures (and zero at some temperature).
D) ΔG is negative at low temperatures but positive at high temperatures (and zero at some temperature).

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
78
For the reaction A + B → C + D, ΔH° = +40 kJ and ΔS° = +50 J/K. Therefore, the reaction under standard conditions is
A) spontaneous only at temperatures between 10 K and 800 K.
B) spontaneous at temperatures greater than 800 K.
C) spontaneous at all temperatures.
D) nonspontaneous at all temperatures.
E) spontaneous at temperatures less than 10 K.
A) spontaneous only at temperatures between 10 K and 800 K.
B) spontaneous at temperatures greater than 800 K.
C) spontaneous at all temperatures.
D) nonspontaneous at all temperatures.
E) spontaneous at temperatures less than 10 K.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
79
At constant pressure, the reaction
2NO2(g) → N2O4(g)
Is exothermic. The reaction (as written) is
A) spontaneous at low temperatures but not at high temperatures.
B) spontaneous at high temperatures but not at low temperatures.
C) always spontaneous.
D) never spontaneous.
2NO2(g) → N2O4(g)
Is exothermic. The reaction (as written) is
A) spontaneous at low temperatures but not at high temperatures.
B) spontaneous at high temperatures but not at low temperatures.
C) always spontaneous.
D) never spontaneous.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
80
Given the following data, calculate the normal boiling point for formic acid (HCOOH). 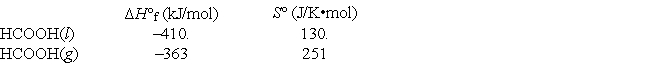
A) 115°C
B) 82°C
C) 2.57 K
D) 1730°C
E) 388°C
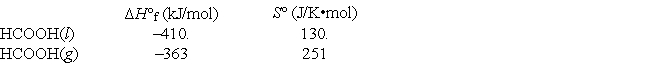
A) 115°C
B) 82°C
C) 2.57 K
D) 1730°C
E) 388°C

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck


