Deck 5: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 5: Gases
1
The volume of a helium balloon is 1.85 L at 24.0°C and 1.00 atm at sea level. The balloon is released and floats upward. At a certain altitude, the balloon has a volume of 2.14 L and the temperature is 15.2°C. What is the atmospheric pressure at this altitude?
A) 0.538 atm
B) 1.36 atm
C) 0.839 atm
D) 0.891 atm
E) none of these
A) 0.538 atm
B) 1.36 atm
C) 0.839 atm
D) 0.891 atm
E) none of these
0.839 atm
2
Air is 79% N2 and 21% O2 by volume. Calculate the density of air at 1.0 atm, 25°C.
A) 1.18 g/L
B) 2.46 g/L
C) 0.590 g/L
D) 14.1 g/L
E) none of these
A) 1.18 g/L
B) 2.46 g/L
C) 0.590 g/L
D) 14.1 g/L
E) none of these
1.18 g/L
3
Consider three 1-L flasks at the same temperature and pressure. Flask A contains CO gas, flask B contains N2 gas, and flask C contains O2 gas.Which contains the lowest density?
A) flask A
B) flask B
C) flask C
D) All are the same.
E) Two of the flasks contain gases at the same density.
A) flask A
B) flask B
C) flask C
D) All are the same.
E) Two of the flasks contain gases at the same density.
Two of the flasks contain gases at the same density.
4
Consider a sample of neon gas in a container fitted with a movable piston (assume the piston is massless and frictionless). The temperature of the gas is increased from 20.0°C to 40.0°C. The density of neon
A) decreases less than 10%.
B) decreases more than 10%.
C) does not change.
D) increases more than 10%.
E) increases less than 10%.
A) decreases less than 10%.
B) decreases more than 10%.
C) does not change.
D) increases more than 10%.
E) increases less than 10%.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
A balloon contains 10.0 g of neon gas. With the temperature kept constant, 10.0 g of argon gas is added. What happens?
A) The volume of the balloon expands by more than 2 times.
B) The volume of the balloon expands by less than 2 times.
C) The balloon stays the same size, but the pressure increases.
D) The balloon doubles in volume.
E) none of these
A) The volume of the balloon expands by more than 2 times.
B) The volume of the balloon expands by less than 2 times.
C) The balloon stays the same size, but the pressure increases.
D) The balloon doubles in volume.
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following relationships is not true?
A) PV = constant when temperature and moles of gas are held constant.
B) V/T = constant when pressure and moles of gas are held constant.
C) nT = constant when pressure and volume are held constant.
D) P/n = constant when volume and temperature are held constant.
E) All of these are true.
A) PV = constant when temperature and moles of gas are held constant.
B) V/T = constant when pressure and moles of gas are held constant.
C) nT = constant when pressure and volume are held constant.
D) P/n = constant when volume and temperature are held constant.
E) All of these are true.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
A sample of oxygen gas has a volume of 4.50 L at 27°C and 800.0 torr. How many oxygen molecules does it contain?
A) 1.16 × 1022
B) 1.16 × 1023
C) 2.32 × 1024
D) 5.8 × 1022
E) none of these
A) 1.16 × 1022
B) 1.16 × 1023
C) 2.32 × 1024
D) 5.8 × 1022
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
A cylinder is fitted with a movable piston. The pressure inside the cylinder is Pi and the volume is Vi. What is the new pressure in the system when the piston decreases the volume of the cylinder by half?
A) 2ViPi
B) 2Pi
C) (1/4)Pi
D) (1/2)Pi
E) Pi2
A) 2ViPi
B) 2Pi
C) (1/4)Pi
D) (1/2)Pi
E) Pi2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
Mercury vapor contains Hg atoms. What is the volume of 200. g of mercury vapor at a temperature of 822 K and 0.500 atm?
A) 135 L
B) 329 L
C) 82.2 L
D) 67.2 L
E) none of these
A) 135 L
B) 329 L
C) 82.2 L
D) 67.2 L
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
The ratio of volumes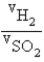
A) 32
B) 0.18
C) 180
D) 5.6
E) 1.0
The ratio of volumes
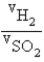
A) 32
B) 0.18
C) 180
D) 5.6
E) 1.0

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
Body temperature is about 308 K. On a cold day, what volume of air at 273 K must a person with a lung capacity of 2.00 L breathe in to fill the lungs?
A) 1.13 L
B) 2.26 L
C) 1.77 L
D) 3.54 L
E) none of these
A) 1.13 L
B) 2.26 L
C) 1.77 L
D) 3.54 L
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Three 1.00-L flasks at 25°C and 725 torr contain the gases CH4 (flask A), CO2 (flask B), and C2H6 (flask C).In which flask is there 0.039 mol of gas?
A) flask A
B) flask B
C) flask C
D) all
E) none
A) flask A
B) flask B
C) flask C
D) all
E) none

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
Given a cylinder of fixed volume filled with 1 mol of argon gas, which of the following is correct? (Assume all gases obey the ideal gas law.)
A) If a second mole of argon is added to the cylinder, the ratio T/P will remain constant.
B) If the temperature of the cylinder is changed from 25°C to 50°C, the pressure inside the cylinder will double.
C) A cylinder of identical volume filled with the same pressure of helium must contain more atoms of gas because He has a smaller atomic radius than argon.
D) Two of these are correct.
E) None of these is correct.
A) If a second mole of argon is added to the cylinder, the ratio T/P will remain constant.
B) If the temperature of the cylinder is changed from 25°C to 50°C, the pressure inside the cylinder will double.
C) A cylinder of identical volume filled with the same pressure of helium must contain more atoms of gas because He has a smaller atomic radius than argon.
D) Two of these are correct.
E) None of these is correct.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Consider two samples of helium in separate containers of the same volume. Sample 1 has an absolute temperature four times that of Sample 2. Both samples are at the same pressure.Calculate the ratio n1/n2.
A) 2:1
B) 1:1
C) 1:2
D) 4:1
E) 1:4
A) 2:1
B) 1:1
C) 1:2
D) 4:1
E) 1:4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
The valve between a 3.00-L tank containing O2(g) at 7.36 atm and a 2.10-L tank containing Ne(g) at 5.08 atm is opened. Calculate the ratio of partial pressures (O2:Ne) in the container.
A) 0.592
B) 1.45
C) 1.43
D) 2.07
E) 0.483
A) 0.592
B) 1.45
C) 1.43
D) 2.07
E) 0.483

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
A glass column is filled with mercury and inverted in a pool of mercury. The mercury column stabilizes at a height of 735 mm above the pool of mercury. What is the pressure of the atmosphere?
A) 1.03 atm
B) 194 atm
C) 0.697 atm
D) 0.967 atm
E) 0.735 atm
A) 1.03 atm
B) 194 atm
C) 0.697 atm
D) 0.967 atm
E) 0.735 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
A 8.80-g piece of solid CO2 (dry ice) is allowed to sublime in a balloon. The final volume of the balloon is 1.30 L at 290. K. What is the pressure of the gas?
A) 7.36 × 10-5 atm
B) 0.273 atm
C) 3.66 atm
D) 1.61 × 102 atm
E) 44.6 atm
A) 7.36 × 10-5 atm
B) 0.273 atm
C) 3.66 atm
D) 1.61 × 102 atm
E) 44.6 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
A sample of nitrogen gas has a volume of 180.0 mL at STP. What volume does the gas occupy if the absolute temperature and pressure are each doubled?
A) 90.00 mL
B) 360.0 mL
C) 720.0 mL
D) 44.8 L
E) 180.0 mL
A) 90.00 mL
B) 360.0 mL
C) 720.0 mL
D) 44.8 L
E) 180.0 mL

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
The volume of a balloon is 3.02 L at 22.7°C. The balloon is heated to 43.6°C. Calculate the new volume of the balloon.
A) 1.57 L
B) 3.02 L
C) 2.82 L
D) 3.23 L
E) 5.80 L
A) 1.57 L
B) 3.02 L
C) 2.82 L
D) 3.23 L
E) 5.80 L

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
A cylinder of oxygen gas contains 26.4 g of O2. Another cylinder, twice the volume of the cylinder containing oxygen (and at the same conditions of pressure and temperature), contains CO2 gas. Assuming ideal behavior, what is the mass of the carbon dioxide?
A) 13.2 g
B) 72.6 g
C) 36.3 g
D) 52.8 g
E) none of these
A) 13.2 g
B) 72.6 g
C) 36.3 g
D) 52.8 g
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
The density of a certain diatomic gas is 1.696 g/L at STP. Identify the gas.
A) Cl2
B) O2
C) F2
D) H2
E) N2
A) Cl2
B) O2
C) F2
D) H2
E) N2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
Magnesium metal reacts with hydrochloric acid to form aqueous magnesium chloride and hydrogen gas. An excess of magnesium is reacted with 20.0 mL of 3.00 M hydrochloric acid, and all of the hydrogen is collected in a balloon at 25°C and 1.00 atm. What is the expected volume of the balloon?
A) 0.734 L
B) 1.47 L
C) 0.672 L
D) 22.4 L
E) 1.34 L
A) 0.734 L
B) 1.47 L
C) 0.672 L
D) 22.4 L
E) 1.34 L

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
Equimolar amounts of the gases N2 and H2 are mixed in a closed container fitted with a piston (allowing the volume of the container to change, thus keeping the pressure constant). Calculate the ratio of the final volume of the container to the initial volume of the container when the reaction N2 + 3H2 → 2NH3 goes to completion.
A) 1
B) 2/3
C) 3/2
D) 1/3
E) none of these
A) 1
B) 2/3
C) 3/2
D) 1/3
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and
1 atm pressure.
Which gas has the highest density?
A) NH3
B) CH4
C) Cl2
D) He
E) All the gases have the same density.
1 atm pressure.
Which gas has the highest density?
A) NH3
B) CH4
C) Cl2
D) He
E) All the gases have the same density.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
Into a 3.50-liter container at 25°C are placed 1.10 mol of O2 gas and 4.68 mol of solid C (graphite). If the carbon and oxygen react completely to form CO(g), what will be the final pressure in the container at 25°C?
A) 0.645 atm
B) 7.69 atm
C) 32.7 atm
D) 40.4 atm
E) 15.4 atm
A) 0.645 atm
B) 7.69 atm
C) 32.7 atm
D) 40.4 atm
E) 15.4 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
Air has an average molar mass of 29.0 g/mol. The density of air at 1.00 atm and 30°C is
A) 40.0 g/mL.
B) 1.17 g/L.
C) 1.29 g/L.
D) 12 g/L.
E) 29.0 g/L.
A) 40.0 g/mL.
B) 1.17 g/L.
C) 1.29 g/L.
D) 12 g/L.
E) 29.0 g/L.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
A volatile liquid produced 0.379 g of vapor in a 262.0-mL flask at 100.0°C and 0.960 atm. What is the molar mass of the liquid?
A) 11.9 g/mol
B) 35.4 g/mol
C) 46.1 g/mol
D) 8.22 g/mol
E) 30.7 g/mol
A) 11.9 g/mol
B) 35.4 g/mol
C) 46.1 g/mol
D) 8.22 g/mol
E) 30.7 g/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
An excess of potassium hydroxide is treated with 1.6 L of dry hydrogen bromide gas measured at STP. What is the mass of potassium bromide formed?
A) 8.2 × 102 g
B) 8.5 g
C) 1.0 × 103 g
D) 1.7 × 103 g
E) 1.4 × 103 g
A) 8.2 × 102 g
B) 8.5 g
C) 1.0 × 103 g
D) 1.7 × 103 g
E) 1.4 × 103 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
At STP the mass of 680.0 mL of a certain gas is 0.850
A) CO
B) Ar
C) O2
D) CO2
E) H2
A) CO
B) Ar
C) O2
D) CO2
E) H2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
What volume of carbon dioxide measured at STP will be formed by the reaction of 1.30 mol of oxygen with 9.00 × 10-1 mol of ethyl alcohol (CH3CH2OH)?
A) 91.9 L
B) 8.70 L
C) 28.0 L
D) 19.4 L
E) 40.3 L
A) 91.9 L
B) 8.70 L
C) 28.0 L
D) 19.4 L
E) 40.3 L

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
Air is 79% N2 and 21% O2 by volume. Calculate the mass of oxygen in a 2.0-L bottle of air at 1.0 atm, 25°C.
A) 0.550 g
B) 2.36 g
C) 1.81 g
D) 2.62 g
E) none of these
A) 0.550 g
B) 2.36 g
C) 1.81 g
D) 2.62 g
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the density of nitrogen at STP.
A) 1.25 g/L
B) 0.625 g/L
C) 0.312 g/L
D) 1.60 g/L
E) 0.800 g/L
A) 1.25 g/L
B) 0.625 g/L
C) 0.312 g/L
D) 1.60 g/L
E) 0.800 g/L

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
It is found that 250. mL of gas at STP has a mass of 1.00
A) 14.0 g/mol
B) 89.6 g/mol
C) 22.4 g/mol
D) 28.0 g/mol
E) none of these
A) 14.0 g/mol
B) 89.6 g/mol
C) 22.4 g/mol
D) 28.0 g/mol
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
The mass of 1.12 L of gas Y is found to be 6.23
A) 0.180 g/L.
B) 0.200 g/L.
C) 10.6 g/L.
D) 15.6 g/L.
E) 5.56 g/L.
A) 0.180 g/L.
B) 0.200 g/L.
C) 10.6 g/L.
D) 15.6 g/L.
E) 5.56 g/L.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
Potassium chlorate decomposes upon heating as follows:
2KClO3(s) → 2KCl(s) + 3O2(g)
A 2.72-g sample of KClO3 decomposes, and the oxygen at 23.4°C and 0.935 atm is collected. What volume of oxygen gas will be collected, assuming 100% yield?
A) 2.21 mL
B) 0.577 mL
C) 0.0456 mL
D) 0.0684 mL
E) 0.866 mL
2KClO3(s) → 2KCl(s) + 3O2(g)
A 2.72-g sample of KClO3 decomposes, and the oxygen at 23.4°C and 0.935 atm is collected. What volume of oxygen gas will be collected, assuming 100% yield?
A) 2.21 mL
B) 0.577 mL
C) 0.0456 mL
D) 0.0684 mL
E) 0.866 mL

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
When a mixture is prepared from 15.0 L of ammonia and 15.0 L of chlorine measured at the same conditions, these compounds react according to the following equation:
2NH3(g) + 3Cl2(g) → N2(g) + 6HCl(g)
When the reaction is completed, what are the volumes of the gases (NH3, Cl2, N2, and HCl, respectively)? Assume the final volumes are measured under identical conditions.
A) 0.00 L, 0.00 L, 5.00 L, and 30.0 L
B) 0.00 L, 0.00 L, 7.50 L, and 45.0 L
C) 0.00 L, 5.00 L, 7.50 L, and 45.0 L
D) 5.00 L, 0.00 L, 5.00 L, and 30.0 L
E) 0.00 L, 10.0 L, 15.0 L, and 90.0 L
2NH3(g) + 3Cl2(g) → N2(g) + 6HCl(g)
When the reaction is completed, what are the volumes of the gases (NH3, Cl2, N2, and HCl, respectively)? Assume the final volumes are measured under identical conditions.
A) 0.00 L, 0.00 L, 5.00 L, and 30.0 L
B) 0.00 L, 0.00 L, 7.50 L, and 45.0 L
C) 0.00 L, 5.00 L, 7.50 L, and 45.0 L
D) 5.00 L, 0.00 L, 5.00 L, and 30.0 L
E) 0.00 L, 10.0 L, 15.0 L, and 90.0 L

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
The molar mass of gas Y is
A) 89.0 g/mol.
B) 56.0 g/mol.
C) 157 g/mol.
D)140. g/mol.
E) 125 g/mol.
A) 89.0 g/mol.
B) 56.0 g/mol.
C) 157 g/mol.
D)140. g/mol.
E) 125 g/mol.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
If a 2.15-g sample of a gas occupies 750. mL at STP, what is the molar mass of the gas at 125°C?
A) 75.0
B) 3.07 × 10-2
C) 64.2
D) 70.1
E) Not enough information is given.
A) 75.0
B) 3.07 × 10-2
C) 64.2
D) 70.1
E) Not enough information is given.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and
1 atm pressure.
Which gas sample has the greatest number of molecules?
A) He
B) NH3
C) Cl2
D) CH4
E) All the gases have the same number of molecules.
1 atm pressure.
Which gas sample has the greatest number of molecules?
A) He
B) NH3
C) Cl2
D) CH4
E) All the gases have the same number of molecules.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
A mixture of KCl and KClO3 weighing 1.80 g was heated; the dry O2 generated occupied 1.40 × 102 mL at STP. What percent of the original mixture was KClO3? KClO3 decomposes as follows:
2KClO3(s) → 2KCl(s) + 3O2(g)
A) 37.2%
B) 72.6%
C) 42.6%
D) 63.8%
E) 28.4%
2KClO3(s) → 2KCl(s) + 3O2(g)
A) 37.2%
B) 72.6%
C) 42.6%
D) 63.8%
E) 28.4%

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
How many of the following gases at STP are less dense than air at STP?
NH3, He, Kr, and F2
A) 4
B) 0
C) 2
D) 1
E) 3
NH3, He, Kr, and F2
A) 4
B) 0
C) 2
D) 1
E) 3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
A 0.234-g sample of a gas in a 275-mL container at 23.5°C exerts a pressure of 0.292 atm What is the molar mass of the gas?
A) 70.9 g/mol
B) 0.0141 g/mol
C) 5.62 g/mol
D) 32.0 g/mol
E) none of these
A) 70.9 g/mol
B) 0.0141 g/mol
C) 5.62 g/mol
D) 32.0 g/mol
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
The purity of a sample containing zinc and weighing 0.198 g is determined by measuring the amount of hydrogen formed when the sample reacts with an excess of hydrochloric acid. The determination shows the sample to be 84.0% zinc. What amount of hydrogen (measured at STP) was obtained?
A) 1.53 × 1021 atoms
B) 3.42 × 10-3 mol
C) 0.152 L
D) 0.0330 g
E) 1.53 × 1021 molecules
A) 1.53 × 1021 atoms
B) 3.42 × 10-3 mol
C) 0.152 L
D) 0.0330 g
E) 1.53 × 1021 molecules

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
A 3.82-g sample of lead(II) nitrate, Pb(NO3)2, molar mass = 331 g/mol, is heated in an evacuated cylinder with a volume of 1.70 L. The salt decomposes when heated, according to the equation
2Pb(NO3)2(s) → 2PbO(s) + 4NO2(g) + O2(g)
Assuming complete decomposition, what is the pressure in the cylinder after decomposition and cooling to a temperature of 290. K? Assume the PbO(s) takes up negligible volume.
A) 0.565 atm
B) 0.162 atm
C) 0.784 atm
D) 0.808 atm
E) 0.404 atm
2Pb(NO3)2(s) → 2PbO(s) + 4NO2(g) + O2(g)
Assuming complete decomposition, what is the pressure in the cylinder after decomposition and cooling to a temperature of 290. K? Assume the PbO(s) takes up negligible volume.
A) 0.565 atm
B) 0.162 atm
C) 0.784 atm
D) 0.808 atm
E) 0.404 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
A 226.4-L cylinder contains 65.5% He(g) and 34.5% Kr(g) by mass at 27.0°C and 1.40 atm total pressure. What is the mass of He in this container?
A) 26.5 g
B) 50.3 g
C) 76.8 g
D) 559 g
E) 33.8 g
A) 26.5 g
B) 50.3 g
C) 76.8 g
D) 559 g
E) 33.8 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Oxygen gas, generated by the reaction 2KClO3(s) → 2KCl(s) + 3O2(g), is collected over water at 27°C in a 3.72-L vessel at a total pressure of 730. torr. (The vapor pressure of H2O at 27°C is 26.0 torr.) How many moles of KClO3 were consumed in the reaction?
A) 0.100 mol
B) 0.210 mol
C) 0.0933 mol
D) 70.9 mol
E) 1.04 mol
A) 0.100 mol
B) 0.210 mol
C) 0.0933 mol
D) 70.9 mol
E) 1.04 mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
A balloon contains an anesthetic mixture of cyclopropane (C3H6) and oxygen (O2) at 160 torr and 550 torr, respectively. What is the ratio of the number of moles of cyclopropane to the number of moles of oxygen?
Ncp/no2 = ?
A) 0.29
B) 0.77
C) 0.23
D) 3.4
E) 0.26
Ncp/no2 = ?
A) 0.29
B) 0.77
C) 0.23
D) 3.4
E) 0.26

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
The oxidation of nitric oxide to nitrogen dioxide is
2NO(g) + O2(g) → 2NO2(g)
If 100.0 mL of NO (at STP) reacts with 400.0 mL of O2 at STP, calculate the partial pressure of NO2 in the final reaction mixture.
A) 0.222 atm
B) 0.286 atm
C) 0.333 atm
D) 0.250 atm
E) 1.00 atm
2NO(g) + O2(g) → 2NO2(g)
If 100.0 mL of NO (at STP) reacts with 400.0 mL of O2 at STP, calculate the partial pressure of NO2 in the final reaction mixture.
A) 0.222 atm
B) 0.286 atm
C) 0.333 atm
D) 0.250 atm
E) 1.00 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
A gaseous mixture containing 1.5 mol Ar and 3.5 mol CO2 has a total pressure of 7.0 atm. What is the partial pressure of CO2?
A) 2.4 atm
B) 3.5 atm
C) 4.9 atm
D) 1.8 atm
E) 2.1 atm
A) 2.4 atm
B) 3.5 atm
C) 4.9 atm
D) 1.8 atm
E) 2.1 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
A 275.0-mL sample of O2 is collected over water at 60.0°C. The total pressure is 755 torr. What is the volume of the O2 at the STP? (The vapor pressure of water at 60°C is 149 torr).
A) 244.0 mL
B) 224.0 mL
C) 180.0 mL
D) 333.0 mL
E) none of these
A) 244.0 mL
B) 224.0 mL
C) 180.0 mL
D) 333.0 mL
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
When 0.72 g of a liquid is vaporized at 110°C and 0.967 atm, the gas occupies a volume of 0.559 L. The empirical formula of the gas is CH2. What is the molecular formula of the gas?
A) C4H8
B) CH2
C) C2H4
D) C3H6
E) none of these
A) C4H8
B) CH2
C) C2H4
D) C3H6
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
At 1000°C and 10. torr, the density of a certain element in the gaseous state is 2.9 × 10-3 g/L. The element is
A) Ne.
B) Ar.
C) He.
D) Na.
E) Hg.
A) Ne.
B) Ar.
C) He.
D) Na.
E) Hg.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure.
For which gas do the molecules have the highest average velocity?
A) He
B) CH4
C) NH3
D) Cl2
E) The molecules of all the gases have the same average velocity.
For which gas do the molecules have the highest average velocity?
A) He
B) CH4
C) NH3
D) Cl2
E) The molecules of all the gases have the same average velocity.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure.
For which gas are the collisions elastic?
A) He
B) Cl2
C) CH4
D) NH3
E) The collisions are elastic for all the gases.
For which gas are the collisions elastic?
A) He
B) Cl2
C) CH4
D) NH3
E) The collisions are elastic for all the gases.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
A vessel with a volume of 10.1 L contains 3.28 g of nitrogen gas, 0.400 g of hydrogen gas, and 85.0 g of argon gas. At 26.0°C, what is the pressure in the vessel?
A) 215 atm
B) 0.516 atm
C) 8.99 × 104 atm
D) 5.94 atm
E) 3.08 atm
A) 215 atm
B) 0.516 atm
C) 8.99 × 104 atm
D) 5.94 atm
E) 3.08 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
A 130.-mL sample of gas is collected over water at 22°C and 753 torr. What is the volume of the dry gas at STP? (The vapor pressure of water at 22°C is 20. torr.)
A) 111 mL
B) 135 mL
C)130. mL
D) 119 mL
E) none of these
A) 111 mL
B) 135 mL
C)130. mL
D) 119 mL
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
A 1.00-g sample of a gaseous compound of boron and hydrogen occupies 0.820 L at 1.00 atm and 3°C. What is the molecular formula for the compound?
A) B2H6
B) B4H10
C) B5H14
D) B3H12
E) BH3
A) B2H6
B) B4H10
C) B5H14
D) B3H12
E) BH3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
A 250.0-L cylinder contains 65.0% He(g) and 35.0% Kr(g) by mass at 25.0°C and 1.35 atm total pressure. What is the partial pressure of He in this container?
A) 0.675 atm
B) 0.878 atm
C) 1.32 atm
D) 1.35 atm
E) 0.473 atm
A) 0.675 atm
B) 0.878 atm
C) 1.32 atm
D) 1.35 atm
E) 0.473 atm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
Consider three 1.0-L flasks at STP. Flask A contains N2 gas, flask B contains Kr gas, and flask C contains H2 gas.In which flask do the gas particles have the lowest average kinetic energy?
A) flask B
B) The gas particles in all of the flasks have the same average kinetic energy.
C) The gas particles in two of the flasks have the same average kinetic energy.
D) flask C
E) flask A
A) flask B
B) The gas particles in all of the flasks have the same average kinetic energy.
C) The gas particles in two of the flasks have the same average kinetic energy.
D) flask C
E) flask A

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
Three 1.00-L flasks at 25°C and 725 torr contain the gases CH4 (flask A), CO2 (flask B), and C2H6 (flask C).In which single flask do the molecules have the greatest mass, the greatest average velocity, and the highest kinetic energy?
A) flask A
B) flask B
C) flask C
D) all
E) none
A) flask A
B) flask B
C) flask C
D) all
E) none

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure.
For which gas do the molecules have the smallest average kinetic energy?
A) He
B) CH4
C) NH3
D) Cl2
E) The molecules of all the gases have the same average kinetic energy.
For which gas do the molecules have the smallest average kinetic energy?
A) He
B) CH4
C) NH3
D) Cl2
E) The molecules of all the gases have the same average kinetic energy.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
The kinetic-molecular theory of gases does not assume that
A) gas particles are very small compared to the average distance between the particles.
B) gas particles collide with the walls of their container in elastic collisions.
C) the average velocity of gas particles is directly proportional to the absolute temperature.
D) gases are made up of tiny particles in constant chaotic motion.
E) All of these are correct.
A) gas particles are very small compared to the average distance between the particles.
B) gas particles collide with the walls of their container in elastic collisions.
C) the average velocity of gas particles is directly proportional to the absolute temperature.
D) gases are made up of tiny particles in constant chaotic motion.
E) All of these are correct.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
The ratio of average kinetic energies per molecule (H2:SO2)
A) 0.18
B) 5.6
C) 180
D) 1.0
E) 32
The ratio of average kinetic energies per molecule (H2:SO2)
A) 0.18
B) 5.6
C) 180
D) 1.0
E) 32

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Consider three 1-L flasks at the same temperature and pressure. Flask A contains CO gas, flask B contains N2 gas, and flask C contains O2 gas.In which flask do the molecules have the greatest momentum per impact?
A) The molecules in all the flasks have the same momentum per impact.
B) flask C
C) The molecules in two of the flasks have the same momentum per impact.
D) flask A
E) flask B
A) The molecules in all the flasks have the same momentum per impact.
B) flask C
C) The molecules in two of the flasks have the same momentum per impact.
D) flask A
E) flask B

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
Calculate the temperature at which the average velocity of Ar(g) equals the average velocity of Ne(g) at 25°C.
A) 25°C
B) 151°C
C) 317°C
D) 49.5°C
E) none of these
A) 25°C
B) 151°C
C) 317°C
D) 49.5°C
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
Which statement about kinetic energy (K.E.) is true?
A) The K.E. of a body will double if its velocity doubles.
B) As the velocity of a body increases, its K.E. decreases.
C) The K.E. of a body is independent of its mass.
D) All objects moving with the same velocity have the same K.E.
E) None of these statements is true.
A) The K.E. of a body will double if its velocity doubles.
B) As the velocity of a body increases, its K.E. decreases.
C) The K.E. of a body is independent of its mass.
D) All objects moving with the same velocity have the same K.E.
E) None of these statements is true.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Consider two 1.0-L containers, one containing He(g) at 2.50 atm and 24°C and the other containing Ar(g) at 5.00 atm and 48°C.
Calculate the ratio of average velocities (He:Ar).
A) 0.108
B) 4.98
C) 0.200
D) 9.22
E) none of these
Calculate the ratio of average velocities (He:Ar).
A) 0.108
B) 4.98
C) 0.200
D) 9.22
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
The ratio of the forces per wall impact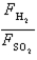 .
.
A) 5.6
B) 0.18
C) 1.0
D) 32
E) 180
The ratio of the forces per wall impact
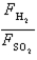 .
.A) 5.6
B) 0.18
C) 1.0
D) 32
E) 180

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
The root-mean-square velocity of a gas in a closed container of fixed volume is increased from 345 m/s to 690 m/s. Which one of the following statements might explain correctly how this change was accomplished?
A) By pumping in more gas at constant temperature, the pressure was quadrupled.
B) By heating the gas, the temperature was doubled.
C) By heating the gas, the pressure was quadrupled.
D) By pumping out 75% of the gas at constant temperature, the pressure was decreased to 25% of its original value.
E) None of these statements correctly explains the change.
A) By pumping in more gas at constant temperature, the pressure was quadrupled.
B) By heating the gas, the temperature was doubled.
C) By heating the gas, the pressure was quadrupled.
D) By pumping out 75% of the gas at constant temperature, the pressure was decreased to 25% of its original value.
E) None of these statements correctly explains the change.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the following gas samples:

Which one of the following statements is false?
A) Assuming identical intermolecular forces in the two samples, sample A should be more nearly ideal than sample B.
B) The root-mean-square velocity of molecules in sample A is twice as large as the root-mean-square velocity of molecules in sample B.
C) The average kinetic energy of the molecules in sample A is twice the average kinetic energy of the molecules in sample B.
D) The fraction of molecules in sample A having a kinetic energies greater than some high fixed value is larger than the fraction of molecules in sample B having kinetic energies greater than that same high fixed value.
E) The volume of sample A is twice the volume of sample B.

Which one of the following statements is false?
A) Assuming identical intermolecular forces in the two samples, sample A should be more nearly ideal than sample B.
B) The root-mean-square velocity of molecules in sample A is twice as large as the root-mean-square velocity of molecules in sample B.
C) The average kinetic energy of the molecules in sample A is twice the average kinetic energy of the molecules in sample B.
D) The fraction of molecules in sample A having a kinetic energies greater than some high fixed value is larger than the fraction of molecules in sample B having kinetic energies greater than that same high fixed value.
E) The volume of sample A is twice the volume of sample B.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
The root-mean-square velocity of N2 gas at 35°C is
A) 52.0 m/s.
B) 177 m/s.
C) 5.58 m/s
D) 524 m/s.
E) 16.6 m/s
A) 52.0 m/s.
B) 177 m/s.
C) 5.58 m/s
D) 524 m/s.
E) 16.6 m/s

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
At 200 K, the molecules or atoms of an unknown gas, X, have an average velocity equal to that of Ar atoms at 400 K. What is X? (Assume ideal behavior.)
A) CO
B) F2
C) He
D) HF
E) HBr
A) CO
B) F2
C) He
D) HF
E) HBr

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following statements is true concerning ideal gases?
A) The temperature of the gas sample is directly related to the average velocity of the gas particles.
B) At STP, 1.0 L of Ar(g) contains about twice the number of atoms as 1.0 L of Ne(g) because the molar mass of Ar is about twice that of Ne.
C) A gas exerts pressure as a result of the collisions of the gas molecules with the walls of the container.
D) The gas particles in a sample exert attraction on one another.
E) All of these statements are false.
A) The temperature of the gas sample is directly related to the average velocity of the gas particles.
B) At STP, 1.0 L of Ar(g) contains about twice the number of atoms as 1.0 L of Ne(g) because the molar mass of Ar is about twice that of Ne.
C) A gas exerts pressure as a result of the collisions of the gas molecules with the walls of the container.
D) The gas particles in a sample exert attraction on one another.
E) All of these statements are false.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Under which of the following conditions does a gas behave most ideally?
A) STP
B) P = 2.0 atm, T = -100.0°C
C) P = 0.50 atm, T = 0.0°C
D) P = 1.0 atm, T = 100.0°C
E) P = 0.50 atm, T = 100.0°C
A) STP
B) P = 2.0 atm, T = -100.0°C
C) P = 0.50 atm, T = 0.0°C
D) P = 1.0 atm, T = 100.0°C
E) P = 0.50 atm, T = 100.0°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Calculate the temperature at which the average kinetic energy of O2 gas is twice that of He gas at 10.0°C.
A) 293°C
B) 20.0°C
C) 160.0°C
D) 2.50°C
E) 10.0°C
A) 293°C
B) 20.0°C
C) 160.0°C
D) 2.50°C
E) 10.0°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
Calculate the following ratios for a gas at Kelvin temperatures T1 and T2 where T2 = 2T1.Average kinetic energy at T1 : Average kinetic energy at T2
A) 2.0
B) 0.50
C) 0.71
D) 1.0
E) 1.4
A) 2.0
B) 0.50
C) 0.71
D) 1.0
E) 1.4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
Consider separate samples of Ar(g) and Ne(g). For what ratio of absolute temperatures (Ne:Ar) are the average kinetic energies equal?
A) 1.41
B) 0.505
C) 1.98
D) 1.00
E) none of these
A) 1.41
B) 0.505
C) 1.98
D) 1.00
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
Calculate the ratio of the change in momentum per wall impact for Ar(g) to that for He(g) if the gases are at the same temperature and pressure.
A) 0.316
B) 3.16
C) 9.98
D) 0.100
E) none of these
A) 0.316
B) 3.16
C) 9.98
D) 0.100
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
The ratio of the root-mean-square velocities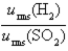 .
.
A) 180
B) 32
C) 0.18
D) 1.0
E) 5.6
The ratio of the root-mean-square velocities
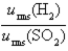 .
.A) 180
B) 32
C) 0.18
D) 1.0
E) 5.6

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
Consider two 1.0-L containers, one containing He(g) at 2.50 atm and 24°C and the other containing Ar(g) at 5.00 atm and 48°C.
Calculate the ratio of impacts with the wall per second (He:Ar).
A) 9.22
B) 1.64
C) 4.98
D) 6.25
E) none of these
Calculate the ratio of impacts with the wall per second (He:Ar).
A) 9.22
B) 1.64
C) 4.98
D) 6.25
E) none of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


