Deck 19: Transition Metals and Coordination Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/96
Play
Full screen (f)
Deck 19: Transition Metals and Coordination Chemistry
1
Which of the following coordination compounds will form a precipitate when treated with an aqueous solution of AgNO3?
A) Na3[CrCl6]
B) [Cr(NH3)3Cl3]
C) Na3[Cr(CN)6]
D) [Cr(NH3)6]Cl3
E) all of these
A) Na3[CrCl6]
B) [Cr(NH3)3Cl3]
C) Na3[Cr(CN)6]
D) [Cr(NH3)6]Cl3
E) all of these
[Cr(NH3)6]Cl3
2
Which of the metal ions in the following complex ions has a d5 electron configuration?
A) [FeCl6]4-
B) [Cr(CN)6]3-
C) [Ti(H2O)6]2+
D) [Mo(NH3)6]3+
E) [Fe(CN)6]3-
A) [FeCl6]4-
B) [Cr(CN)6]3-
C) [Ti(H2O)6]2+
D) [Mo(NH3)6]3+
E) [Fe(CN)6]3-
[Fe(CN)6]3-
3
What transition metal is used in magnets, catalysts, and drill bits?
A) platinum
B) nickel
C) copper
D) cobalt
E) titanium
A) platinum
B) nickel
C) copper
D) cobalt
E) titanium
cobalt
4
Which of the following is a d7 ion?
A) Co2+
B) Zn2+
C) Fe2+
D) Cr3+
E) Cu+
A) Co2+
B) Zn2+
C) Fe2+
D) Cr3+
E) Cu+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
5
The corrosion of which transition metal results in a characteristic green patina?
A) lead
B) copper
C) chromium
D) silver
E) iron
A) lead
B) copper
C) chromium
D) silver
E) iron

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is true about coordination complexes?
A) When the ligands approach a transition metal ion in an octahedral field, the dxz, dyz, and dxy atomic orbitals are affected the least by the ligands.
B) The metal is a Lewis base and the ligands are Lewis acids.
C) Only complexes with coordination number 6 are found in nature.
D) None of these is true.
E) All of these are true.
A) When the ligands approach a transition metal ion in an octahedral field, the dxz, dyz, and dxy atomic orbitals are affected the least by the ligands.
B) The metal is a Lewis base and the ligands are Lewis acids.
C) Only complexes with coordination number 6 are found in nature.
D) None of these is true.
E) All of these are true.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
7
Addition of AgNO3 to aqueous solutions of the complex results in a cloudy white precipitate, presumably AgCl. You dissolve 0.1000 g of the complex in H2O and perform a precipitation titration with 0.0500 M AgNO3 as the titrant. Using an electrode that is sensitive to [Ag+], you reach the endpoint after 9.00 mL of titrant is added. How many grams of chloride ion were present in the 0.1000-g sample?
A) 4.50 × 10-4 g
B) 1.77 × 10-3 g
C) 5.00 × 10-3 g
D) 1.60 × 10-2 g
E) 6.38 × 10-2 g
A) 4.50 × 10-4 g
B) 1.77 × 10-3 g
C) 5.00 × 10-3 g
D) 1.60 × 10-2 g
E) 6.38 × 10-2 g

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
8
The phenomenon called the __________ contraction is responsible for the great similarity in atomic size and chemistry between 4d and 5d elements.
A) transition
B) coordination
C) none of these
D) isomeric
E) lanthanide
A) transition
B) coordination
C) none of these
D) isomeric
E) lanthanide

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
9
A coordination compound of Cu2+ can be described as Cu(NH3)xSO4 and is known to contain 29.9% NH3. What is the value of x?
A) 6
B) 3
C) 4
D) 2
E) none of these
A) 6
B) 3
C) 4
D) 2
E) none of these

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
10
What is the electron configuration of the Co(II) ion?
A) [Ar]3d6
B) [Ar]3d7
C) [Ar]4s23d5
D) [Ar]4s24d2
E) [Ar]4s23d7
A) [Ar]3d6
B) [Ar]3d7
C) [Ar]4s23d5
D) [Ar]4s24d2
E) [Ar]4s23d7

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
11
You analyze for pyridine (Kb is approximately 10-9) by dissolving 0.1000 g of the complex in 100 mL of H2O and titrating with a 0.01 M HCl solution. Which of the following indicators should be used to detect the endpoint? (Assume that the initial concentration of pyridine is approximately 0.01 M.)
A) thymol blue, pH range of color change = 8.0-9.6
B) methyl red, pH range of color change = 4.8-6.0
C) bromophenol blue, pH range of color change = 3.0-4.6
D) bromothymol blue, pH range of color change = 6.0-7.6
E) alizarin yellow, pH range of color change = 10.1-12.0
A) thymol blue, pH range of color change = 8.0-9.6
B) methyl red, pH range of color change = 4.8-6.0
C) bromophenol blue, pH range of color change = 3.0-4.6
D) bromothymol blue, pH range of color change = 6.0-7.6
E) alizarin yellow, pH range of color change = 10.1-12.0

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
12
Transition metals show great similarities both within a given period and within a given vertical group.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
13
What is the maximum oxidation state of chromium?
A) +4
B) +6
C) +3
D) +5
E) none of these
A) +4
B) +6
C) +3
D) +5
E) none of these

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
14
In which of the following complexes does the transition metal have a d8 configuration?
A) [Zn(NH3)4]2+
B) [NiBr4]2-
C) [Mn(CN)6]3-
D) [Ni(CO)4]
E) [Ti(H2O)6]2+
A) [Zn(NH3)4]2+
B) [NiBr4]2-
C) [Mn(CN)6]3-
D) [Ni(CO)4]
E) [Ti(H2O)6]2+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
15
You discover that the complex decomposes in water. You dissolve 0.1000 g of the complex in H2O and add excess NaHg(SCN)4, which precipitates Co(II) as CoHg(SCN)4(s). After the precipitate is washed and dried, its mass is 0.1102 g. How many grams of cobalt are contained in 0.100 g of the complex?
A) 0.0396 g
B) 0.0132 g
C) 0.1102 g
D) 0.437 g
E) 0.0548 g
A) 0.0396 g
B) 0.0132 g
C) 0.1102 g
D) 0.437 g
E) 0.0548 g

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
16
How many d electrons are present on the metal ion in the complex ion PtCl62-?
A) 3
B) 8
C) 2
D) 4
E) 6
A) 3
B) 8
C) 2
D) 4
E) 6

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
17
Which transition metal can exist in all oxidation states from +2 to +7?
A) iron
B) vanadium
C) copper
D) manganese
E) chromium
A) iron
B) vanadium
C) copper
D) manganese
E) chromium

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a d2 ion?
A) Mn3+
B) Ti+
C) Fe3+
D) Cr2+
E) Ti2+
A) Mn3+
B) Ti+
C) Fe3+
D) Cr2+
E) Ti2+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
19
A compound of which transition metal is responsible for the white color of most paper?
A) chromium
B) titanium
C) zinc
D) nickel
E) copper
A) chromium
B) titanium
C) zinc
D) nickel
E) copper

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
20
Which metal ion has a d4 electron configuration?
A) Ti2+
B) Mn2+
C) Ni2+
D) Fe3+
E) Mn3+
A) Ti2+
B) Mn2+
C) Ni2+
D) Fe3+
E) Mn3+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
21
How many of the following compounds exhibit geometric isomers?
I.Pd(NH3)2Br2 (square planar)
II.[Co(H2O)2]Br3
III.[Ni(H2O)4(NO2)2]
IV.K2[CoCl4]
A) 2
B) 3
C) 1
D) 4
E) 0
I.Pd(NH3)2Br2 (square planar)
II.[Co(H2O)2]Br3
III.[Ni(H2O)4(NO2)2]
IV.K2[CoCl4]
A) 2
B) 3
C) 1
D) 4
E) 0

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
22
_____ isomers and _______ isomers are classes of structural isomers.
A) Geometric, optical
B) Geometric, linkage
C) Linkage, geometric
D) Coordination, linkage
E) Coordination, geometric
A) Geometric, optical
B) Geometric, linkage
C) Linkage, geometric
D) Coordination, linkage
E) Coordination, geometric

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
23
A metal ion in a high-spin octahedral complex has two more unpaired electrons than the same ion does in a low-spin octahedral complex. Which of the following could the metal ion be?
A) Cr3+
B) Mn2+
C) Co2+
D) Ti2+
E) Cu2+
A) Cr3+
B) Mn2+
C) Co2+
D) Ti2+
E) Cu2+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following complexes shows geometric isomerism?
A) K[Co(H2O)2Cl4]
B) [Co(H2O)5Cl]Cl2
C) [Co(H2O)5Cl]SO4
D) Na3[CoCl6]
E) [Co(H2O)6]Cl3
A) K[Co(H2O)2Cl4]
B) [Co(H2O)5Cl]Cl2
C) [Co(H2O)5Cl]SO4
D) Na3[CoCl6]
E) [Co(H2O)6]Cl3

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
25
For which of the following metal ions would there be no distinction between low spin and high spin in octahedral complexes?
A) Cr2+
B) Mn2+
C) Ni3+
D) V2+
E) Co3+
A) Cr2+
B) Mn2+
C) Ni3+
D) V2+
E) Co3+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
26
Give the number of geometric isomers for the octahedral compound [Ma2B2C2], where A, B, and C represent ligands.
A) 2
B) 1
C) 3
D) 5
E) none of these
A) 2
B) 1
C) 3
D) 5
E) none of these

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
27
For the process Co(NH3)5Cl2+ + Cl- → Co(NH3)4Cl2+ + NH3, what would be the ratio of cis to trans isomers in the product?
A) 4:1
B) 1:1
C) 2:1
D) 1:2
E) 1:4
A) 4:1
B) 1:1
C) 2:1
D) 1:2
E) 1:4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
28
A complex ion is a square planar complex. It has a d8 electron configuration. What is the most reasonable d orbital scheme for this complex?
A)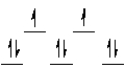
B)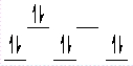
C)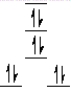
D)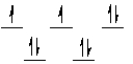
E)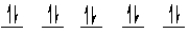
A)
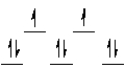
B)
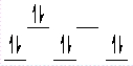
C)
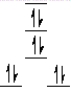
D)
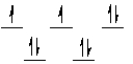
E)

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
29
The complex FeL62+, where L is a neutral ligand, is known to be diamagnetic. How many d electrons are there in this complex ion?
A) 7
B) 8
C) 6
D) 4
E) 5
A) 7
B) 8
C) 6
D) 4
E) 5

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements about the complex ion Co(en)2Cl2+ is true?
(en = ethylenediamine, NH2CH2CH2NH2)
A) The geometric isomers of the complex ion have identical chemical properties.
B) The complex ion exhibits two geometric isomers (cis and trans) and two optical isomers.
C) Because en is a strong field ligand (large Δ), the complex ion is paramagnetic.
D) The complex ion exhibits cis and trans geometric isomers, but no optical isomers.
E) The complex ion contains Co(I).
(en = ethylenediamine, NH2CH2CH2NH2)
A) The geometric isomers of the complex ion have identical chemical properties.
B) The complex ion exhibits two geometric isomers (cis and trans) and two optical isomers.
C) Because en is a strong field ligand (large Δ), the complex ion is paramagnetic.
D) The complex ion exhibits cis and trans geometric isomers, but no optical isomers.
E) The complex ion contains Co(I).

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements is true of the crystal field model?
A) The electrons are assumed to be localized.
B) The metal ion-ligand bonds are considered completely ionic.
C) The ligands are treated as negative point charges.
D) The interaction between metal ion and ligand is treated as a Lewis acid-base interaction.
E) None of these statements is true.
A) The electrons are assumed to be localized.
B) The metal ion-ligand bonds are considered completely ionic.
C) The ligands are treated as negative point charges.
D) The interaction between metal ion and ligand is treated as a Lewis acid-base interaction.
E) None of these statements is true.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
32
How many unpaired electrons are there in a complex ion having a d5 electron configuration and an octahedral geometry in the weak-field case?
A) 2
B) 1
C) 3
D) 5
E) 4
A) 2
B) 1
C) 3
D) 5
E) 4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
33
Which model(s) account(s) for the magnetism and color of coordination compounds?
I.the localized electron model
II.the crystal field model
A) both I and II
B) neither I nor II
C) II only
D) I only
I.the localized electron model
II.the crystal field model
A) both I and II
B) neither I nor II
C) II only
D) I only

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
34
The spectrochemical series is
I- < Br- < Cl- < F- < OH- < H2O < NH3 < en < NO2- < CN-
Which of the following complexes will absorb visible radiation of the highest energy (shortest wavelength)?
A) [Co(OH)6]3-
B) [Co(NH3)6]3+
C) [Co(I)6]3-
D) [Co(H2O)6]3+
E) [Co(en)3]3+
I- < Br- < Cl- < F- < OH- < H2O < NH3 < en < NO2- < CN-
Which of the following complexes will absorb visible radiation of the highest energy (shortest wavelength)?
A) [Co(OH)6]3-
B) [Co(NH3)6]3+
C) [Co(I)6]3-
D) [Co(H2O)6]3+
E) [Co(en)3]3+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
35
Analysis of the data from a titration indicates that a 0.1000-g sample of the complex contains 0.0708 g of py. Further analysis shows that 0.1000 g of the complex contains 0.0132 g of cobalt and 0.0160 g of chloride. What is the empirical formula of the complex?
A) Co(py)6(Cl) (NO3)
B) Co(py)4(NO3)2
C) Co2(py)5(Cl)2(NO3)2
D) Co3(py)8(Cl)2(NO3)4
E) Co(py)4Cl2
A) Co(py)6(Cl) (NO3)
B) Co(py)4(NO3)2
C) Co2(py)5(Cl)2(NO3)2
D) Co3(py)8(Cl)2(NO3)4
E) Co(py)4Cl2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
36
The complex ion [NiCl4]2- is tetrahedral. How many unpaired electrons are there in the complex?
A) 4
B) 1
C) 2
D) 3
E) 0
A) 4
B) 1
C) 2
D) 3
E) 0

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following are structural isomers?
I.coordination isomers
II.linkage isomers
III.geometric isomers
IV.optical isomers
A) II, IV
B) II, III
C) I, II
D) I, III, IV
E) I, III
I.coordination isomers
II.linkage isomers
III.geometric isomers
IV.optical isomers
A) II, IV
B) II, III
C) I, II
D) I, III, IV
E) I, III

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
38
The color of a transition metal complex results from
A) nuclear magnetic resonance.
B) transition of an electron between d orbitals.
C) bending vibrations.
D) stretching vibrations.
E) transition of an electron between an s orbital and a p orbital.
A) nuclear magnetic resonance.
B) transition of an electron between d orbitals.
C) bending vibrations.
D) stretching vibrations.
E) transition of an electron between an s orbital and a p orbital.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
39
The empirical formula of a compound with a mass percent composition of 6.78% H, 31.43% N, 39.76% Cl, and 22.03% Co is consistent with which of the following complexes?
A) [Co(NH3)4Cl2]Cl
B) [Co(NH3)3Cl3]
C) [Co(NH3)6]Cl3
D) [Co(NH3)5Cl]Cl2
E) none of these
A) [Co(NH3)4Cl2]Cl
B) [Co(NH3)3Cl3]
C) [Co(NH3)6]Cl3
D) [Co(NH3)5Cl]Cl2
E) none of these

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
40
Fluoride ion ranks low in the spectrochemical series and produces a weak crystal field in complex ions. Based on this information, predict the number of unpaired electrons in [CoF6]3-.
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
41
Specify the number of unpaired electrons in Co(en) 33+ (strong field).
A) 0
B) 1
C) 4
D) 5
E) 2
A) 0
B) 1
C) 4
D) 5
E) 2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
42
Specify the number of unpaired electrons in NiCl42- (tetrahedral).
A) 0
B) 2
C) 4
D) 1
E) 5
A) 0
B) 2
C) 4
D) 1
E) 5

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
43
Specify the number of unpaired electrons in [CuCl2]-.
A) 3
B) 5
C) 4
D) 2
E) 0
A) 3
B) 5
C) 4
D) 2
E) 0

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
44
How many unpaired electrons are found in MnCl42- (tetrahedral)?
A) 5
B) 0
C) 2
D) 1
E) 4
A) 5
B) 0
C) 2
D) 1
E) 4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following crystal field diagrams is correct for Mn(CN)63- , where CN- is a strong-field ligand?
A)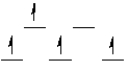
B)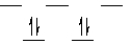
C)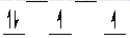
D)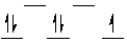
E) none of these
A)
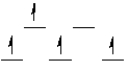
B)
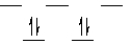
C)
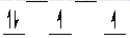
D)
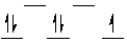
E) none of these

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following complexes would be diamagnetic (all electrons paired)?
A) Fe(CN)6]4-
B) Mn(CN)6]4-
C) V(CN)6]3-
D) [Cr(CN)6]3-
A) Fe(CN)6]4-
B) Mn(CN)6]4-
C) V(CN)6]3-
D) [Cr(CN)6]3-

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
47
How many unpaired electrons are found in NiBr42- (tetrahedral)?
A) 0
B) 4
C) 1
D) 5
E) 2
A) 0
B) 4
C) 1
D) 5
E) 2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
48
How many unpaired electrons are found in Mn(NH3)43+ (tetrahedral)?
A) 4
B) 5
C) 1
D) 0
E) 2
A) 4
B) 5
C) 1
D) 0
E) 2

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
49
How many unpaired electrons are there in the complex ion [Co(NO3)6]4-? For this ion, the nitrate ligands produce a very strong crystal field.
A) 4
B) 5
C) 3
D) 2
E) 1
A) 4
B) 5
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
50
How many unpaired electrons are found in [Co(CN)4]3- (square planar)?
A) 2
B) 0
C) 1
D) 4
E) 5
A) 2
B) 0
C) 1
D) 4
E) 5

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
51
How many unpaired electrons are found in Mn(CN)63- (strong field)?
A) 2
B) 0
C) 4
D) 5
E) 1
A) 2
B) 0
C) 4
D) 5
E) 1

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
52
How many unpaired electrons are found in Fe(en)32+ (strong field)?
A) 5
B) 1
C) 2
D) 0
E) 4
A) 5
B) 1
C) 2
D) 0
E) 4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
53
How many unpaired electrons are found in Zn(H2O)62+?
A) 4
B) 2
C) 5
D) 0
E) 1
A) 4
B) 2
C) 5
D) 0
E) 1

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
54
How many unpaired electrons are found in [CoCl6]3- (weak field)?
A) 2
B) 0
C) 1
D) 5
E) 4
A) 2
B) 0
C) 1
D) 5
E) 4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
55
A certain complex ion has a distorted octahedral structure in which the ligands along the plus and minus z axes are compressed (pushed in closer to the central metal ion). The d orbital splitting diagram for this complex ion would be
A)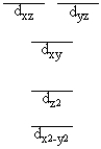
B)
C)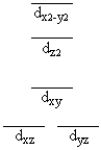
D)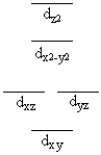
E)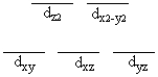
A)

B)

C)
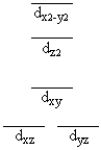
D)
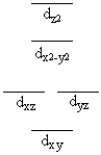
E)


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following crystal field diagrams is correct for Co(CN)64-, where CN- is a strong-field ligand?
A)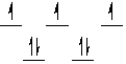
B)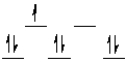
C)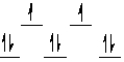
D)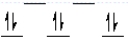
E) none of these
A)

B)
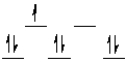
C)
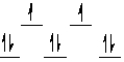
D)
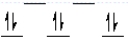
E) none of these

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
57
Specify the number of unpaired electrons in CoF63- (weak field).
A) 4
B) 5
C) 1
D) 2
E) 0
A) 4
B) 5
C) 1
D) 2
E) 0

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
58
The complex ions of Zn2+ are all colorless. The most likely explanation for this is that
A) Zn2+ exhibits "d orbital" splittings in its complexes such that they absorb all wavelengths in the visible region.
B) because Zn2+ is a d10 ion, it does not absorb visible light even though the "d orbital" splittings are correct for absorbing visible wavelengths.
C) Zn2+ is paramagnetic.
D) Zn2+ is not a transition metal ion.
E) None of these explanations could be correct.
A) Zn2+ exhibits "d orbital" splittings in its complexes such that they absorb all wavelengths in the visible region.
B) because Zn2+ is a d10 ion, it does not absorb visible light even though the "d orbital" splittings are correct for absorbing visible wavelengths.
C) Zn2+ is paramagnetic.
D) Zn2+ is not a transition metal ion.
E) None of these explanations could be correct.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
59
Specify the number of unpaired electrons in [Co(NH3)6]3+ (strong field).
A) 2
B) 5
C) 0
D) 4
E) 7
A) 2
B) 5
C) 0
D) 4
E) 7

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is paramagnetic?
A) [Co(NH3)6]3+ (strong field)
B) [Cu(en)3]+
C) [Mn(CN)6]4- (strong field)
D) [Fe(CN)6]4- (strong field)
E) [Zn(H2O)6]2+
A) [Co(NH3)6]3+ (strong field)
B) [Cu(en)3]+
C) [Mn(CN)6]4- (strong field)
D) [Fe(CN)6]4- (strong field)
E) [Zn(H2O)6]2+

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate the total number of unpaired electrons in the following complex ions: [Ti(H2O)6]2+, [NiCl4]2- (tetrahedral), [Co(H2O)6]3+ (weak field).
A) 8
B) 6
C) 3
D) 4
E) 1
A) 8
B) 6
C) 3
D) 4
E) 1

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
62
The complex ion Co(NH3)62+ (three unpaired electrons) is classified as
A) There is no way to tell.
B) strong field.
C) weak field.
A) There is no way to tell.
B) strong field.
C) weak field.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
63
Carboxyhemoglobin is formed when __________ prevents the normal uptake of oxygen in the blood.
A) CN-
B) CH4
C) CO2
D) NH3
E) CO
A) CN-
B) CH4
C) CO2
D) NH3
E) CO

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
64
In _________, all bonds are the same, but the spatial arrangements of atoms are different.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
65
Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
Fe(OH2)63+ (assume weak field)
A)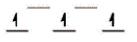
B)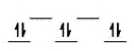
C)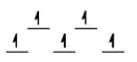
D)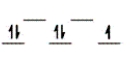
E)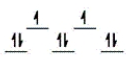
Fe(OH2)63+ (assume weak field)
A)
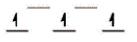
B)
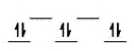
C)
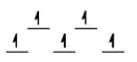
D)
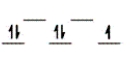
E)
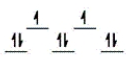

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
66
How many unpaired electrons are there in Ir(Br)64-? (Br- is a weak-field ligand.)
A) 2
B) 4
C) 3
D) 0
E) 1
A) 2
B) 4
C) 3
D) 0
E) 1

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
67
In _________, the isomers contain the same atoms, but one or more bonds differ.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
68
A d6 ion (Fe2+) is complexed with six strong-field ligands (for example, SCN-). What is the number of unpaired electrons in this complex?
A) 3
B) 2
C) 0
D) 1
E) 4
A) 3
B) 2
C) 0
D) 1
E) 4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
69
Which has the greater number of unpaired electrons?
I. square planar Ni(CN)42-
II. tetrahedral FeCl4-
A) I
B) II
C) Neither I nor II has any unpaired electrons.
D) More information is needed.
E) Both I and II have the same (nonzero) number of unpaired electrons.
I. square planar Ni(CN)42-
II. tetrahedral FeCl4-
A) I
B) II
C) Neither I nor II has any unpaired electrons.
D) More information is needed.
E) Both I and II have the same (nonzero) number of unpaired electrons.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
70
According to crystal field theory, how many unpaired electrons are present in the complex ion [Zn(H2O)6]2+?
A) 2
B) 3
C) 4
D) 1
E) 0
A) 2
B) 3
C) 4
D) 1
E) 0

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
71
Oxygen is stored in mammalian tissue in which type of molecule?
A) chlorophyll
B) cytochrome
C) hemoglobin
D) prophyrin
E) myoglobin
A) chlorophyll
B) cytochrome
C) hemoglobin
D) prophyrin
E) myoglobin

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following transition metals is a component of vitamin B12?
A) chromium
B) zinc
C) copper
D) manganese
E) cobalt
A) chromium
B) zinc
C) copper
D) manganese
E) cobalt

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
73
Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH3)2(CN)4]2-, where CN- produces a much stronger crystal field than does NH3.
A)![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_9387_892c_57a0d90e0804_TB6422_00.jpg)
B)![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_9388_892c_b1a740c53c57_TB6422_00.jpg)
C)![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_ba99_892c_6ba15e0bc10d_TB6422_00.jpg)
D)![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_ba9a_892c_e156caf38dd5_TB6422_00.jpg)
E)![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_ba9b_892c_3ba497e06c31_TB6422_00.jpg)
A)
![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_9387_892c_57a0d90e0804_TB6422_00.jpg)
B)
![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_9388_892c_b1a740c53c57_TB6422_00.jpg)
C)
![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_ba99_892c_6ba15e0bc10d_TB6422_00.jpg)
D)
![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_ba9a_892c_e156caf38dd5_TB6422_00.jpg)
E)
![<strong>Choose the most likely pattern for the crystal field diagram for the complex trans-[Ni(NH<sub>3</sub>)<sub>2</sub>(CN)<sub>4</sub>]<sup>2-</sup>, where CN<sup>-</sup> produces a much stronger crystal field than does NH<sub>3</sub>.</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dd5_ba9b_892c_3ba497e06c31_TB6422_00.jpg)

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
74
Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species.
[Co(NH3)4Br2]+
A)![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7a8_0eb5_a7f5_07bca148637b_TB34225555_11.jpg)
B)![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7a9_9556_a7f5_2fdbbe6b1f70_TB34225555_11.jpg)
C)![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7aa_cdd7_a7f5_6b26001b42ea_TB34225555_11.jpg)
D)![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7ac_0658_a7f5_29bedac5d693_TB34225555_11.jpg)
E)![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7ad_3ed9_a7f5_e360b43e684d_TB34225555_11.jpg)
[Co(NH3)4Br2]+
A)
![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7a8_0eb5_a7f5_07bca148637b_TB34225555_11.jpg)
B)
![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7a9_9556_a7f5_2fdbbe6b1f70_TB34225555_11.jpg)
C)
![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7aa_cdd7_a7f5_6b26001b42ea_TB34225555_11.jpg)
D)
![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7ac_0658_a7f5_29bedac5d693_TB34225555_11.jpg)
E)
![<strong>Here are some crystal field representations of d electrons in an octahedral field: Choose the representation that fits the transition metal atom in the following species. [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup></strong> A) B) C) D) E)](https://storage.examlex.com/TB34225555/11ec7dc6_e7ad_3ed9_a7f5_e360b43e684d_TB34225555_11.jpg)

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
75
The complex ion Ni(NH3)62+ (two unpaired electrons) is classified as
A) There is no way to tell.
B) strong field.
C) weak field.
A) There is no way to tell.
B) strong field.
C) weak field.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
76
Specify the number of unpaired electrons in [MnBr4]- (tetrahedral).
A) 2
B) 1
C) 5
D) 4
E) 0
A) 2
B) 1
C) 5
D) 4
E) 0

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following ligands are capable of linkage isomerism? 


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
78
This molecule is toxic because it has about 200 times the affinity for the Fe2+ in hemoglobin that oxygen does, causing asphyxiation if enough of it is present in the air.
A) NH3
B) CO2
C) CO
D) CN-
E) CH4
A) NH3
B) CO2
C) CO
D) CN-
E) CH4

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
79
The complex ion Fe(CN)64- (no unpaired electrons) is classified as
A) There is no way to tell.
B) strong field.
C) weak field.
A) There is no way to tell.
B) strong field.
C) weak field.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements is true about the octahedral complexes of Ni2+?
A) Both strong- and weak-field complexes are paramagnetic.
B) The strong-field complex is paramagnetic and the weak-field complex is diamagnetic.
C) Both strong- and weak-field complexes are diamagnetic.
D) The strong-field complex is diamagnetic and the weak-field complex is paramagnetic.
A) Both strong- and weak-field complexes are paramagnetic.
B) The strong-field complex is paramagnetic and the weak-field complex is diamagnetic.
C) Both strong- and weak-field complexes are diamagnetic.
D) The strong-field complex is diamagnetic and the weak-field complex is paramagnetic.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck


