Deck 20: The Nucleus: a Chemists View
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 20: The Nucleus: a Chemists View
1
Which of the following is a product of α decay of 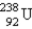 ?
?
A)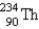
B)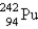
C)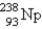
D)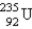
E)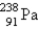
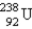 ?
?A)
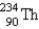
B)
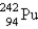
C)
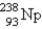
D)
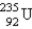
E)
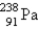
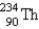
2
The nuclide 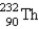 is radioactive. When one of these atoms decays, a series of α- and β−-particle emissions occurs, taking the atom through many transformations to end up as an atom of
is radioactive. When one of these atoms decays, a series of α- and β−-particle emissions occurs, taking the atom through many transformations to end up as an atom of 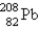
) How many α particles are emitted in converting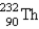
Into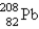
?
A) 8
B) 214
C) 4
D) 6
E) 2
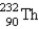 is radioactive. When one of these atoms decays, a series of α- and β−-particle emissions occurs, taking the atom through many transformations to end up as an atom of
is radioactive. When one of these atoms decays, a series of α- and β−-particle emissions occurs, taking the atom through many transformations to end up as an atom of 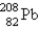
) How many α particles are emitted in converting
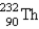
Into
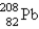
?
A) 8
B) 214
C) 4
D) 6
E) 2
6
3
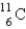 is an unstable isotope. Which radioactive decay would be expected?
is an unstable isotope. Which radioactive decay would be expected?A)
B) α
C) fission
D) β
E)
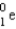
4
When 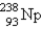 undergoes β emission, what are the products?
undergoes β emission, what are the products?
A)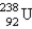 + β
+ β
B)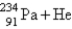
C)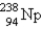 + β
+ β
D)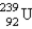 + β
+ β
E)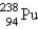 + β
+ β
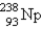 undergoes β emission, what are the products?
undergoes β emission, what are the products?A)
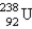 + β
+ βB)
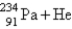
C)
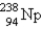 + β
+ βD)
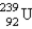 + β
+ βE)
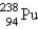 + β
+ β
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
An unstable isotope of Ta-184 is a beta producer. What is the other product of the reaction?
A) Hf-184
B) W-184
C) W-183
D) Pt-192
E) Ta-185
A) Hf-184
B) W-184
C) W-183
D) Pt-192
E) Ta-185

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
The nuclide Ra-222 is the daughter nuclide resulting from the α decay of what parent nuclide?
A) Th-226
B)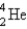
C) Rn-208
D) U-224
E) Hg-297
A) Th-226
B)
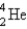
C) Rn-208
D) U-224
E) Hg-297

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
The stable nuclide 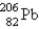 is formed from
is formed from 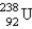
By a long series of α and β decays. Which of the following nuclides could not be involved in this decay series?
A) Po-218
B) Pu-239
C) Tl-210
D) Ra-226
E) Th-234
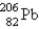 is formed from
is formed from 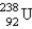
By a long series of α and β decays. Which of the following nuclides could not be involved in this decay series?
A) Po-218
B) Pu-239
C) Tl-210
D) Ra-226
E) Th-234

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
The U-238 nucleus decays to form Pb-206 by α and β decays.
Calculate the number of α decays.
A) 2
B) 4
C) 6
D) 8
E) none of these
Calculate the number of α decays.
A) 2
B) 4
C) 6
D) 8
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
Which types of processes are likely when the neutron-to-proton ratio in a nucleus is too large?
I.α decay
II.β decay
III.positron production
IV.electron capture
A) IV only
B) III, IV
C) II, III
D) I, II
E) II only
I.α decay
II.β decay
III.positron production
IV.electron capture
A) IV only
B) III, IV
C) II, III
D) I, II
E) II only

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
Heavy nuclides with too few neutrons to be in the band of stability are most likely to decay by what mode?
A) α-particle production.
B) fission
C) β production
D) positron production
E) none of these
A) α-particle production.
B) fission
C) β production
D) positron production
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
The U-238 nucleus decays to form Pb-206 by α and β decays.
Calculate the number of β decays.
A) 4
B) 2
C) 8
D) 6
E) none of these
Calculate the number of β decays.
A) 4
B) 2
C) 8
D) 6
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
Electron capture transforms 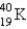 into what nuclide?
into what nuclide?
A)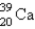
B)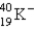
C)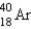
D)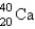
E)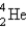
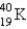 into what nuclide?
into what nuclide?A)
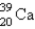
B)
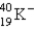
C)
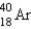
D)
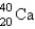
E)
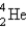

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
It is desired to determine the concentration of arsenic in a lake sediment sample by means of neutron activation analysis. The nuclide 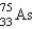 captures a neutron to form
captures a neutron to form 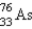
, which in turn undergoes β decay. The daughter nuclide produces the characteristic γ rays used for the analysis. What is the daughter nuclide?
A)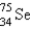
B)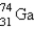
C)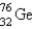
D)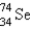
E)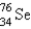
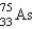 captures a neutron to form
captures a neutron to form 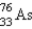
, which in turn undergoes β decay. The daughter nuclide produces the characteristic γ rays used for the analysis. What is the daughter nuclide?
A)
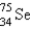
B)
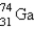
C)
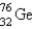
D)
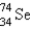
E)
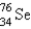

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
A radioactive isotope of vanadium, 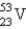 , decays by producing β particles and gamma rays. The nuclide formed has the atomic number
, decays by producing β particles and gamma rays. The nuclide formed has the atomic number
A)23
B)21
C)22
D)24
E) none of these
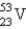 , decays by producing β particles and gamma rays. The nuclide formed has the atomic number
, decays by producing β particles and gamma rays. The nuclide formed has the atomic numberA)23
B)21
C)22
D)24
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
Which reaction will produce an isotope of the parent nuclide?
A)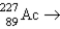 β + ?
β + ?
B)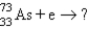
C)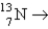 β + ?
β + ?
D)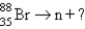
E)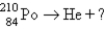
A)
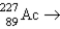 β + ?
β + ?B)
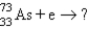
C)
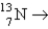 β + ?
β + ?D)
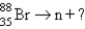
E)
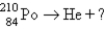

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
The most likely decay mode (or modes) of the unstable nuclide 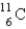 would be
would be
A) positron production.
B) α-particle production.
C) β emission
D) electron capture.
E) either positron production or electron capture or both.
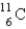 would be
would beA) positron production.
B) α-particle production.
C) β emission
D) electron capture.
E) either positron production or electron capture or both.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
The ratio of the atomic radius to the nuclear radius is approximately
A) 105
B) 10-5
C) 10-15
D) 102
E) 1015
A) 105
B) 10-5
C) 10-15
D) 102
E) 1015

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following processes decreases the atomic number by 2?
A) proton production
B) alpha-particle production
C) gamma-ray production
D) beta production
E) positron-particle production
A) proton production
B) alpha-particle production
C) gamma-ray production
D) beta production
E) positron-particle production

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
What is the most likely decay for the Mn-57 nucleus?
A) positron emission
B) γ-ray emission
C) β decay
D) α decay
E) proton emission
A) positron emission
B) γ-ray emission
C) β decay
D) α decay
E) proton emission

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the missing particle in the following equation: 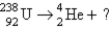
A)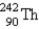
B)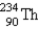
C)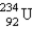
D)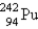
E) none of these
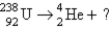
A)
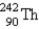
B)
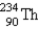
C)
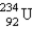
D)
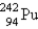
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
What is the number of half-lives needed for a radioactive element to decay to one-fourth of its original activity? (Choose the nearest number.)
A) 1
B) 4
C) 3
D) 5
E) 2
A) 1
B) 4
C) 3
D) 5
E) 2

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following balanced equations is labeled incorrectly?
A) beta production: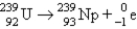
B) bombardment: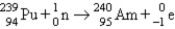
C) alpha production: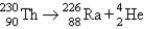
D) alpha emission: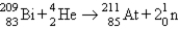
E) fusion: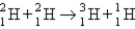
A) beta production:
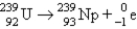
B) bombardment:
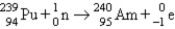
C) alpha production:
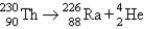
D) alpha emission:
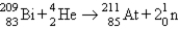
E) fusion:
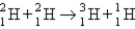

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
Radioactive elements decay via first-order kinetics. Consider a certain type of nucleus that has a rate constant of 1.9× 10-2 h-1. A sample contains 6.6× 108 radioactive nuclides. Calculate the time required to reduce that number to 2.8× 108.
A) 4.5 h
B) 2.0 × 1010 h
C) 4.5 × 101 h
D) 2.0 × 101 h
E) 8.1 × 10-1 h
A) 4.5 h
B) 2.0 × 1010 h
C) 4.5 × 101 h
D) 2.0 × 101 h
E) 8.1 × 10-1 h

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
Breeder reactors are used to convert the nonfissionable nuclide 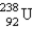 into a fissionable product. Neutron capture of the
into a fissionable product. Neutron capture of the 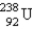
Is followed by two successive beta decays. What is the final fissionable product?
A)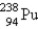
B)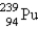
C)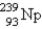
D)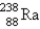
E)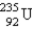
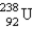 into a fissionable product. Neutron capture of the
into a fissionable product. Neutron capture of the 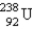
Is followed by two successive beta decays. What is the final fissionable product?
A)
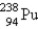
B)
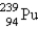
C)
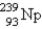
D)
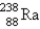
E)
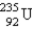

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
The Cs-131 nuclide has a half-life of 30 years. After 120 years, about 3 g remain. The original mass of the Cs-131 sample is closest to
A) 30 g
B) 70 g
C) 60 g
D) 50 g
E) 40 g
A) 30 g
B) 70 g
C) 60 g
D) 50 g
E) 40 g

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
It is desired to determine the blood volume of a live mouse. To do this, 0.10 mL of a saline suspension of red blood cells labeled with 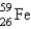 is injected into the tail vein. Before injection the gamma rays were counted for this 0.10-mL solution, and the count rate was found to be 1.0 × 104 cpm. After a sufficient time for the blood to be thoroughly mixed, 0.10 mL of blood is removed and counted. The sample is found to have a count rate of 476 cpm. What is the approximate blood volume of the mouse?
is injected into the tail vein. Before injection the gamma rays were counted for this 0.10-mL solution, and the count rate was found to be 1.0 × 104 cpm. After a sufficient time for the blood to be thoroughly mixed, 0.10 mL of blood is removed and counted. The sample is found to have a count rate of 476 cpm. What is the approximate blood volume of the mouse?
A) 0.48 mL
B) 4.7 mL
C) 21 mL
D) 4.8 mL
E) 2.1 mL
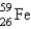 is injected into the tail vein. Before injection the gamma rays were counted for this 0.10-mL solution, and the count rate was found to be 1.0 × 104 cpm. After a sufficient time for the blood to be thoroughly mixed, 0.10 mL of blood is removed and counted. The sample is found to have a count rate of 476 cpm. What is the approximate blood volume of the mouse?
is injected into the tail vein. Before injection the gamma rays were counted for this 0.10-mL solution, and the count rate was found to be 1.0 × 104 cpm. After a sufficient time for the blood to be thoroughly mixed, 0.10 mL of blood is removed and counted. The sample is found to have a count rate of 476 cpm. What is the approximate blood volume of the mouse?A) 0.48 mL
B) 4.7 mL
C) 21 mL
D) 4.8 mL
E) 2.1 mL

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
The rate constant for the decay of a radioactive element is 4.72 × 10-3/day. What is the half-life of this element?
A) 2.36 × 10-3 days
B) 2.12 × 102 days
C) 1.47 × 102 days
D) 1.06 × 102 days
E) 2.19 × 102 days
A) 2.36 × 10-3 days
B) 2.12 × 102 days
C) 1.47 × 102 days
D) 1.06 × 102 days
E) 2.19 × 102 days

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
The half-life of 90Sr is 28 years. How long will it take for a given sample of 90Sr to be 85% decomposed?
A) 3.3 × 101 years
B) 2.9 years
C) 3.3 × 102 years
D) 6.6 years
E) 7.7 × 101 years
A) 3.3 × 101 years
B) 2.9 years
C) 3.3 × 102 years
D) 6.6 years
E) 7.7 × 101 years

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
When the U-235 nucleus is struck with a neutron, the Ce-144 and Sr-90 nuclei are produced, along with some neutrons and beta particles.
How many neutrons are emitted?
A) 5
B) 6
C) 2
D) 3
E) 4
How many neutrons are emitted?
A) 5
B) 6
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
When the U-235 nucleus is struck with a neutron, the Ce-144 and Sr-90 nuclei are produced, along with some neutrons and beta particles.
How many beta particles are emitted?
A) 5
B) 6
C) 3
D) 2
E) 4
How many beta particles are emitted?
A) 5
B) 6
C) 3
D) 2
E) 4

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
When the Pd-106 nucleus is struck with an alpha particle, a proton is produced along with a new element. What is this new element?
A) Cd-109
B) Ag-108
C) Ag-109
D) Cd-112
E) none of these
A) Cd-109
B) Ag-108
C) Ag-109
D) Cd-112
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
What nuclide is necessary to balance the following fission reaction? 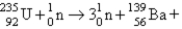 __________
__________
A)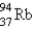
B)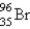
C)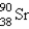
D)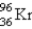
E)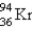
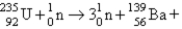 __________
__________A)
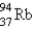
B)
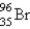
C)
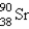
D)
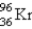
E)
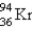

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
Radioactive elements decay via first-order kinetics. Consider a certain type of nucleus that has a rate constant of 2.4 × 10-2 h-1. A sample contains 6.5 × 108 radioactive nuclides. Calculate the time required for 59% of the nuclides to decompose.
A) 1.0 × 101 h
B) 2.2 × 10-1 h
C) 1.6× 101 h
D) 2.2 × 101 h
E) 3.7 × 101 h
A) 1.0 × 101 h
B) 2.2 × 10-1 h
C) 1.6× 101 h
D) 2.2 × 101 h
E) 3.7 × 101 h

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
Radioactive tracers are useful in studying very low concentrations of chemical species. A chemist has a sample of HgI2 in which part of the iodine is the radioactive nuclide of mass 131, so that the count rate is 5.0 × 1011 counts per minute per mole of I. The solid mercuric iodide is placed in water and allowed to come to equilibrium. Then 100 mL of the solution is withdrawn, and its radioactivity is measured and found to give 22 counts per minute. What is the molar concentration of iodide ion in the solution?
A) 1.1 × 10-11
B) 1.1 × 10-9
C) 1.1 × 10-10
D) 4.4 × 10-10
E) 4.4 × 10-11
A) 1.1 × 10-11
B) 1.1 × 10-9
C) 1.1 × 10-10
D) 4.4 × 10-10
E) 4.4 × 10-11

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
When the U-235 nucleus is struck with a neutron, the Zn-72 and Sm-160 nuclei are produced, along with some neutrons. How many neutrons are emitted?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
The number of a certain radioactive nuclide present in a sample decays from2.0 × 102 to 6.0 × 101 in 23 minutes. What is the half-life of this radioactive species?
A) 1.6 × 101 minutes
B) 5.2 × 10-2 minutes
C) 1.3 × 101 minutes
D) 5.3 × 101 minutes
E) 3.0 minutes
A) 1.6 × 101 minutes
B) 5.2 × 10-2 minutes
C) 1.3 × 101 minutes
D) 5.3 × 101 minutes
E) 3.0 minutes

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
A 0.20-mL sample of a solution containing 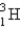 that produces 3.7 × 103 cps is injected into the bloodstream of an animal. After circulatory equilibrium has been established, a 0.20-mL sample of blood is found to have an activity of 20 cps. Calculate the blood volume of the animal.
that produces 3.7 × 103 cps is injected into the bloodstream of an animal. After circulatory equilibrium has been established, a 0.20-mL sample of blood is found to have an activity of 20 cps. Calculate the blood volume of the animal.
A) 18 mL
B) 180 mL
C) 11 mL
D) 37 mL
E) none of these
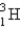 that produces 3.7 × 103 cps is injected into the bloodstream of an animal. After circulatory equilibrium has been established, a 0.20-mL sample of blood is found to have an activity of 20 cps. Calculate the blood volume of the animal.
that produces 3.7 × 103 cps is injected into the bloodstream of an animal. After circulatory equilibrium has been established, a 0.20-mL sample of blood is found to have an activity of 20 cps. Calculate the blood volume of the animal.A) 18 mL
B) 180 mL
C) 11 mL
D) 37 mL
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
A radioactive element has a half-life of 17 min. How many minutes will it take for the number of atoms present to decay to 1/8th of the initial value?
A) 450 min
B) 51 min
C) 2.1 min
D) 136 min
E) 250 min
A) 450 min
B) 51 min
C) 2.1 min
D) 136 min
E) 250 min

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
What is the most likely decay for the Fe-53 nucleus?
A) γ-ray emission
B) α decay
C) β decay
D) positron emission
E) two of these
A) γ-ray emission
B) α decay
C) β decay
D) positron emission
E) two of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
The rate constant for the beta decay of a particular radioactive element is 2.34 × 10-2/day. What is the half-life of this nuclide?
A) 4.27 × 101 days
B) 3.38 × 10-1 days
C) 2.14 × 101 days
D) 8.55 × 102 days
E) 2.96 × 101 days
A) 4.27 × 101 days
B) 3.38 × 10-1 days
C) 2.14 × 101 days
D) 8.55 × 102 days
E) 2.96 × 101 days

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate ΔE in kilojoules per mole for the reaction 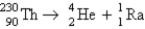 Atomic masses:
Atomic masses: 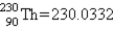
,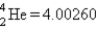
,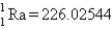
)
A) 0
B) +2.4 × 106 kJ/mol
C) +4.6 × 108 kJ/mol
D) -4.6 × 108 kJ/mol
E) -2.4 × 106 kJ/mol
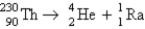 Atomic masses:
Atomic masses: 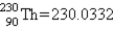
,
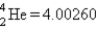
,
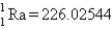
)
A) 0
B) +2.4 × 106 kJ/mol
C) +4.6 × 108 kJ/mol
D) -4.6 × 108 kJ/mol
E) -2.4 × 106 kJ/mol

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement is true about the following reaction? 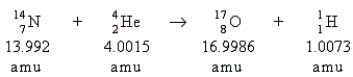
A) No energy change is associated with the reaction.
B) Energy is released in the reaction.
C) Energy is absorbed in the reaction.
D) Not enough information is given for us to determine the energy change.
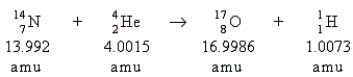
A) No energy change is associated with the reaction.
B) Energy is released in the reaction.
C) Energy is absorbed in the reaction.
D) Not enough information is given for us to determine the energy change.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
If more than one neutron from each fission event causes another fission event, the fission situation is described as
A) supercritical.
B) critical.
C) moderated.
D) subcritical.
E) none of these
A) supercritical.
B) critical.
C) moderated.
D) subcritical.
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is true of the fission of uranium-235?
A) The nuclides produced are individually heavier than the uranium nuclide.
B) The ultimate nuclides produced are more stable than the uranium nuclide.
C) The electron is captured by the nucleus, which becomes unstable.
D) The products include neutrons.
E) two of these
A) The nuclides produced are individually heavier than the uranium nuclide.
B) The ultimate nuclides produced are more stable than the uranium nuclide.
C) The electron is captured by the nucleus, which becomes unstable.
D) The products include neutrons.
E) two of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
The questions below refer to the following:Iron-56, 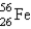 , has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)
, has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)
Determine the amount of energy needed to "decompose" 1 mol of iron-56 nuclei.
A) 4.74 × 1013 J
B) 3.47 × 1011 J
C) 8.90 × 1011 J
D) 1.13 × 1014 J
E) 7.75 × 1013 J
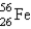 , has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)
, has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)Determine the amount of energy needed to "decompose" 1 mol of iron-56 nuclei.
A) 4.74 × 1013 J
B) 3.47 × 1011 J
C) 8.90 × 1011 J
D) 1.13 × 1014 J
E) 7.75 × 1013 J

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
If a tree dies and the trunk remains undisturbed for 13,750 years, what percentage of the original 14C is still present? (The half-life of 14C is 5730 years.)
A) 2.20%
B) 19.0%
C) 45.0%
D) 5.20%
A) 2.20%
B) 19.0%
C) 45.0%
D) 5.20%

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the change in energy, in kilojoules per mole, for the transmutation of radium from the given molar masses: 
A) -4.7 × 108 kJ/mol
B) -4.7 × 1014 kJ/mol
C) +1.6 × 108 kJ/mol
D) -1.6 kJ/mol
E) -5.2 kJ/mol

A) -4.7 × 108 kJ/mol
B) -4.7 × 1014 kJ/mol
C) +1.6 × 108 kJ/mol
D) -1.6 kJ/mol
E) -5.2 kJ/mol

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
Fresh rainwater or surface water contains enough tritium 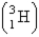 to show 5.5 decompositions per minute per 100. g of water. Tritium has a half-life of 12.3 years. You are asked to check a vintage wine claimed to have been produced in 1946. How many decompositions per minute should you expect to observe in 100. g of that wine?
to show 5.5 decompositions per minute per 100. g of water. Tritium has a half-life of 12.3 years. You are asked to check a vintage wine claimed to have been produced in 1946. How many decompositions per minute should you expect to observe in 100. g of that wine?
A) 1.7
B) 0.035
C) 0.17
D) 181
E) 0.35
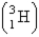 to show 5.5 decompositions per minute per 100. g of water. Tritium has a half-life of 12.3 years. You are asked to check a vintage wine claimed to have been produced in 1946. How many decompositions per minute should you expect to observe in 100. g of that wine?
to show 5.5 decompositions per minute per 100. g of water. Tritium has a half-life of 12.3 years. You are asked to check a vintage wine claimed to have been produced in 1946. How many decompositions per minute should you expect to observe in 100. g of that wine?A) 1.7
B) 0.035
C) 0.17
D) 181
E) 0.35

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
The smallest amount of radioactive material that will support a self-sustained reaction is called the
A) moderator.
B) critical mass.
C) molar mass.
D) supercritical mass.
E) subcritical mass.
A) moderator.
B) critical mass.
C) molar mass.
D) supercritical mass.
E) subcritical mass.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the following process: 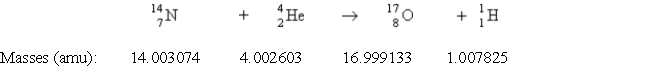 Which statement describes ΔE for the process?
Which statement describes ΔE for the process?
A) 1.15 × 1011 J/mol is released.
B) 1.15 × 1014 J/mol is released.
C) 1.15 × 1018 J/mol is absorbed.
D) 1.15 × 1011 J/mol is absorbed.
E) none of these
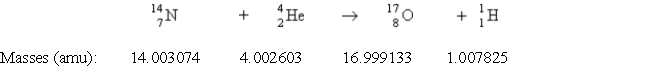 Which statement describes ΔE for the process?
Which statement describes ΔE for the process?A) 1.15 × 1011 J/mol is released.
B) 1.15 × 1014 J/mol is released.
C) 1.15 × 1018 J/mol is absorbed.
D) 1.15 × 1011 J/mol is absorbed.
E) none of these

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
The half-life of 90Sr is 28.1 years. How long will it take a 17.7-g sample of 90Sr to decompose to 0.18 g?
A) 1.1 × 101 years
B) 8.1 × 101 years
C) 1.9× 102 years
D) 2.9 × 10-1 years
E) 2.8 × 103 years
A) 1.1 × 101 years
B) 8.1 × 101 years
C) 1.9× 102 years
D) 2.9 × 10-1 years
E) 2.8 × 103 years

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
The questions below refer to the following:Iron-56, 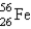 , has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)
, has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)
Determine the difference in mass between 1 mol of iron-56 nuclei and the component nucleons of which it is made.
A) 5.27 × 10-4 kg
B) 2.43 × 10-5 kg
C) 6.65 × 10-5 kg
D) 7.21 × 10-4 kg
E) 9.41 × 10-6 kg
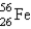 , has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)
, has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 × 10-13 J)Determine the difference in mass between 1 mol of iron-56 nuclei and the component nucleons of which it is made.
A) 5.27 × 10-4 kg
B) 2.43 × 10-5 kg
C) 6.65 × 10-5 kg
D) 7.21 × 10-4 kg
E) 9.41 × 10-6 kg

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
One of the hopes for solving the world's energy problem is to make use of the fusion reaction  How much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? The masses of the atoms and the neutrons are as follows:
How much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? The masses of the atoms and the neutrons are as follows: 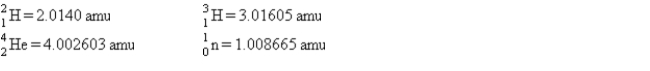
The speed of light is 2.9979 × 108 m/s.
A) 1.69 × 1012 J
B) 5.63 × 108 J
C) 8.44 × 1011 J
D) 7.84 × 1044 J
E) 56.3 J
 How much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? The masses of the atoms and the neutrons are as follows:
How much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? The masses of the atoms and the neutrons are as follows: 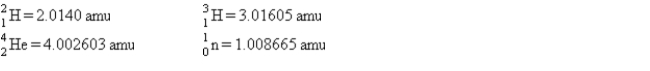
The speed of light is 2.9979 × 108 m/s.
A) 1.69 × 1012 J
B) 5.63 × 108 J
C) 8.44 × 1011 J
D) 7.84 × 1044 J
E) 56.3 J

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
Use the following data to determine the expected 14C activity in the Shroud of Turin. The atmospheric activity of 14C is 15 cpm/gC (counts per minute per gram of carbon). Assume that the cloth was made in the year A.D. 24. The half-life of 14C is 5730 years.
A) 12 cpm/gC
B) 5.1 cpm/gC
C) 28 cpm/gC
D) 7.3 cpm/gC
E) 11 cpm/gC
A) 12 cpm/gC
B) 5.1 cpm/gC
C) 28 cpm/gC
D) 7.3 cpm/gC
E) 11 cpm/gC

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
The half-life for electron capture for 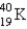 is 1.3 billion years. What will be the
is 1.3 billion years. What will be the 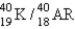
Ratio in a rock that is 4.5 billion years old?
A) 0.10
B) 0.091
C)10
D)11
E) 0.36
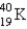 is 1.3 billion years. What will be the
is 1.3 billion years. What will be the 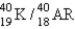
Ratio in a rock that is 4.5 billion years old?
A) 0.10
B) 0.091
C)10
D)11
E) 0.36

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
The Br-82 nucleus has a half-life of about 1.0 × 103 minutes. If you needed at least 2.0 g of Br-82 and had ordered 29 g of NaBr (assuming all of the Br in the NaBr was Br-82), how many days could you wait for delivery?
A) 2.9days
B) 1.1 days
C) 2.7 days
D) 2.4 days
E) 3.5 days
A) 2.9days
B) 1.1 days
C) 2.7 days
D) 2.4 days
E) 3.5 days

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
Radioactive elements decay via first-order kinetics. Consider a certain type of nucleus that has a rate constant of 1.0 × 10-3 h-1. A sample contains 5.0 × 109 radioactive nuclides. Calculate the number of nuclides remaining after 39 days have passed.
A) 64
B) 7.8 × 10-11
C) 2.0 × 109
D) 5.0 × 109
E) 2.5 × 109
A) 64
B) 7.8 × 10-11
C) 2.0 × 109
D) 5.0 × 109
E) 2.5 × 109

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is not a factor in determining the biological effects of radiation exposure?
A) the energy of the radiation
B) the age of the organism when the exposure occurs
C) the penetrating ability of the radiation
D) the ionizing ability of the radiation
E) the chemical properties of the radiation source
A) the energy of the radiation
B) the age of the organism when the exposure occurs
C) the penetrating ability of the radiation
D) the ionizing ability of the radiation
E) the chemical properties of the radiation source

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
A sample of wood from an Egyptian mummy case gives a 14C count of 9.4 cpm/gC (counts per minute per gram of carbon). How old is the wood? (The initial decay rate of 14C is 15.3 cpm/gC, and its half-life is 5730 years.)
A) 3.5 × 103 years
B) 4.0 × 103 years
C) 5.1 × 103 years
D) 1.7 × 103 years
E) 2.8 × 103 years
A) 3.5 × 103 years
B) 4.0 × 103 years
C) 5.1 × 103 years
D) 1.7 × 103 years
E) 2.8 × 103 years

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
The half-life for electron capture for 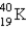 is 1.30 × 109 years. What percent of the original
is 1.30 × 109 years. What percent of the original 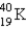
Remains after 3.90 ×109 years?
A) 12.5%
B) 75.0%
C) 25.0%
D) 33.3%
E) 50.0%
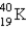 is 1.30 × 109 years. What percent of the original
is 1.30 × 109 years. What percent of the original 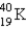
Remains after 3.90 ×109 years?
A) 12.5%
B) 75.0%
C) 25.0%
D) 33.3%
E) 50.0%

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
Explain how a particle accelerator works.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
Discuss and explain the operation of a scintillation counter.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
Distinguish between a cyclotron and a linear accelerator.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
Distinguish alpha-particle production and beta-particle production, and provide an example of each.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
Radiocarbon dating is based on which decay process?
A)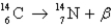
B)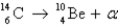
C)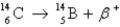
D) All of the above
E) None of the above
A)
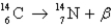
B)
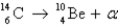
C)
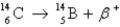
D) All of the above
E) None of the above

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following instruments is/are used to detect and measure radioactivity levels?
1) Geiger counter
2) Richter scale
3) Seismic scale
4) Scintillation counter
A) 1 only
B) 3 only
C) 1 and 4
D) 2 and 3
E) 1, 2, 3, and 4
1) Geiger counter
2) Richter scale
3) Seismic scale
4) Scintillation counter
A) 1 only
B) 3 only
C) 1 and 4
D) 2 and 3
E) 1, 2, 3, and 4

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
Discuss and explain the operation of a nuclear reactor.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
In the presence of a magnetic field in cyclotron, an ion is introduced at the center and is accelerated in an expanding _____ path by use of alternating electric fields.
A) sinusoidal
B) spiral
C) linear
D) Hamilton
E) Euler
A) sinusoidal
B) spiral
C) linear
D) Hamilton
E) Euler

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following statements is/are true of nuclear fission?
1) For the nuclear fission process to be self-sustaining, at least one neutron from each fission event must go on to split another nucleus.2. If more than one neutron from each nuclear fission event causes another fission event, the process dies out and the reaction is said to be subcritical.3. A fission bomb operates by suddenly combining two subcritical masses of fissionable material to form a supercritical mass.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1 and 3
1) For the nuclear fission process to be self-sustaining, at least one neutron from each fission event must go on to split another nucleus.2. If more than one neutron from each nuclear fission event causes another fission event, the process dies out and the reaction is said to be subcritical.3. A fission bomb operates by suddenly combining two subcritical masses of fissionable material to form a supercritical mass.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1 and 3

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following statements is/are true of damage by radiation?
1) Somatic damage is damage to an organism itself, resulting in sickness or death.2. Genetic damage is damage to the genetic machinery, which produces malfunctions in the offspring of the organism.3. The official SI unit for radiation dose is the Gray, which is 10−2 Joule of energy deposited per kg of tissue.
A) 3 only
B) 2 only
C) 1 only
D) 1 and 2
E) 1 and 3
1) Somatic damage is damage to an organism itself, resulting in sickness or death.2. Genetic damage is damage to the genetic machinery, which produces malfunctions in the offspring of the organism.3. The official SI unit for radiation dose is the Gray, which is 10−2 Joule of energy deposited per kg of tissue.
A) 3 only
B) 2 only
C) 1 only
D) 1 and 2
E) 1 and 3

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is not a charged species?
A)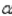 particle
particle
B)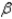 particle
particle
C)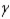 particle
particle
D) all of the above
E) none of the above
A)
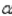 particle
particleB)
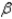 particle
particleC)
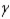 particle
particleD) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
What is the purpose of a moderator surrounding the uranium cylinders in a reactor core?
A) To slow down the neutrons
B) To accelerate the neutrons
C) To produce α particles
D) To produce β particles
E) To absorb β particles
A) To slow down the neutrons
B) To accelerate the neutrons
C) To produce α particles
D) To produce β particles
E) To absorb β particles

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
What is the result of a collision between an electron and a positron?
A) γ rays
B) x-rays
C) antimatter
D) a quark
E) all of the above are possible
A) γ rays
B) x-rays
C) antimatter
D) a quark
E) all of the above are possible

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


