Deck 15: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/124
Play
Full screen (f)
Deck 15: Chemical Kinetics
1
The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. ![<strong>The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. What form will the pseudo-rate law have?</strong> A) Rate = kk'[A]<sup>x</sup> B) Rate = k'[A]<sup>x</sup>[B]<sup>y</sup> C) Rate = kk'[B]<sup>y</sup> D) Rate = k'[B]<sup>y</sup> E) Rate = k'[A]<sup>x</sup>](https://storage.examlex.com/TB6422/11eaaf91_9de8_f4b2_892c_937fe19b9050_TB6422_00_TB6422_00_TB6422_00.jpg)
What form will the pseudo-rate law have?
A) Rate = kk'[A]x
B) Rate = k'[A]x[B]y
C) Rate = kk'[B]y
D) Rate = k'[B]y
E) Rate = k'[A]x
![<strong>The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. What form will the pseudo-rate law have?</strong> A) Rate = kk'[A]<sup>x</sup> B) Rate = k'[A]<sup>x</sup>[B]<sup>y</sup> C) Rate = kk'[B]<sup>y</sup> D) Rate = k'[B]<sup>y</sup> E) Rate = k'[A]<sup>x</sup>](https://storage.examlex.com/TB6422/11eaaf91_9de8_f4b2_892c_937fe19b9050_TB6422_00_TB6422_00_TB6422_00.jpg)
What form will the pseudo-rate law have?
A) Rate = kk'[A]x
B) Rate = k'[A]x[B]y
C) Rate = kk'[B]y
D) Rate = k'[B]y
E) Rate = k'[A]x
Rate = k'[A]x
2
For the reaction 2N2O5(g) → 4NO2(g) + O2(g), the following data were collected. 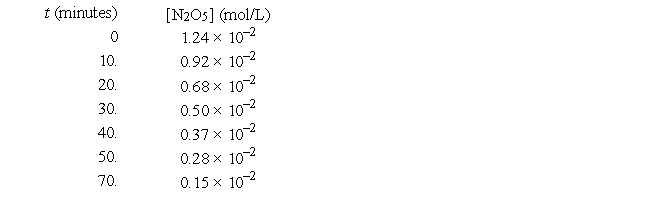 The initial rate of production of NO2 for this reaction is approximately
The initial rate of production of NO2 for this reaction is approximately
A) 6.4 × 10-4 mol/L • min
B) 3.2 × 10-4 mol/L • min
C) 1.24 × 10-2 mol/L • min
D) 1.6 × 10-4 mol/L • min
E) none of these
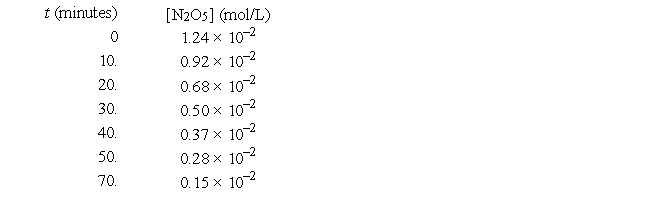 The initial rate of production of NO2 for this reaction is approximately
The initial rate of production of NO2 for this reaction is approximatelyA) 6.4 × 10-4 mol/L • min
B) 3.2 × 10-4 mol/L • min
C) 1.24 × 10-2 mol/L • min
D) 1.6 × 10-4 mol/L • min
E) none of these
6.4 × 10-4 mol/L • min
3
The balanced equation for the reaction of bromate ion with bromide in acidic solution is
BrO+ 5Br- + 6H+ → 3Br2 + 3H2O
At a particular instant in time, the value of -Δ[Br-]/Δt is 2.0 × 10-3 mol/L • s. What is the value of Δ[Br2]/Δt in the same units?
A) 1.2 × 10-3
B) 2.0 × 10-3
C) 6.0 × 10-3
D) 3.3 × 10-5
E) 3.3 × 10-3
BrO+ 5Br- + 6H+ → 3Br2 + 3H2O
At a particular instant in time, the value of -Δ[Br-]/Δt is 2.0 × 10-3 mol/L • s. What is the value of Δ[Br2]/Δt in the same units?
A) 1.2 × 10-3
B) 2.0 × 10-3
C) 6.0 × 10-3
D) 3.3 × 10-5
E) 3.3 × 10-3
1.2 × 10-3
4
A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. 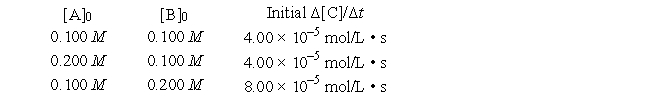
What is the numerical value of the rate constant?
A) 4.00 × 10-1
B) 4.00 × 10-2
C) 4.00 × 10-3
D) 4.00 × 10-4
E) none of these
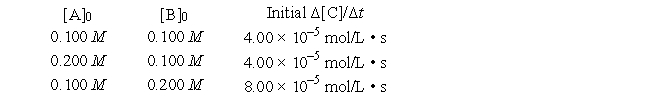
What is the numerical value of the rate constant?
A) 4.00 × 10-1
B) 4.00 × 10-2
C) 4.00 × 10-3
D) 4.00 × 10-4
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
5
The following data were obtained for the reaction of NO with O2. Concentrations are in molecules/cm3 and rates are in molecules/cm3 • s. ![<strong>The following data were obtained for the reaction of NO with O<sub>2</sub>. Concentrations are in molecules/cm<sup>3</sup> and rates are in molecules/cm<sup>3</sup> • s. Which of the following is the correct rate law?</strong> A) Rate = k[NO]<sup>2</sup> B) Rate = k[NO]<sup>2</sup>[O<sub>2</sub>]<sup>2</sup> C) Rate = k[NO][O<sub>2</sub>]<sup>2</sup> D) Rate = k[NO][O<sub>2</sub>] E) Rate = k[NO]<sup>2</sup>[O<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9de9_69e4_892c_45c6b34bb401_TB6422_00.jpg) Which of the following is the correct rate law?
Which of the following is the correct rate law?
A) Rate = k[NO]2
B) Rate = k[NO]2[O2]2
C) Rate = k[NO][O2]2
D) Rate = k[NO][O2]
E) Rate = k[NO]2[O2]
![<strong>The following data were obtained for the reaction of NO with O<sub>2</sub>. Concentrations are in molecules/cm<sup>3</sup> and rates are in molecules/cm<sup>3</sup> • s. Which of the following is the correct rate law?</strong> A) Rate = k[NO]<sup>2</sup> B) Rate = k[NO]<sup>2</sup>[O<sub>2</sub>]<sup>2</sup> C) Rate = k[NO][O<sub>2</sub>]<sup>2</sup> D) Rate = k[NO][O<sub>2</sub>] E) Rate = k[NO]<sup>2</sup>[O<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9de9_69e4_892c_45c6b34bb401_TB6422_00.jpg) Which of the following is the correct rate law?
Which of the following is the correct rate law?A) Rate = k[NO]2
B) Rate = k[NO]2[O2]2
C) Rate = k[NO][O2]2
D) Rate = k[NO][O2]
E) Rate = k[NO]2[O2]

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
6
A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. 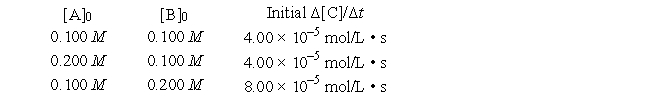
What is the order of the reaction with respect to A?
A) 1
B) 0
C) 4
D) 3
E) 2
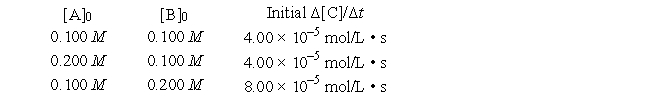
What is the order of the reaction with respect to A?
A) 1
B) 0
C) 4
D) 3
E) 2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
7
The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. ![<strong>The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. The rate law for the reaction is Rate = k[A]<sup>x</sup>[B]<sup>y</sup>. What are the values of x and y?</strong> A) x = 1 y = 0 B) x = 1 y = 1 C) x = 0 y = 1 D) x = 1 y = 2 E) x = 2 y = 1](https://storage.examlex.com/TB6422/11eaaf91_9de8_f4b2_892c_937fe19b9050_TB6422_00_TB6422_00_TB6422_00.jpg)
The rate law for the reaction is Rate = k[A]x[B]y. What are the values of x and y?
A) x = 1 y = 0
B) x = 1 y = 1
C) x = 0 y = 1
D) x = 1 y = 2
E) x = 2 y = 1
![<strong>The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. The rate law for the reaction is Rate = k[A]<sup>x</sup>[B]<sup>y</sup>. What are the values of x and y?</strong> A) x = 1 y = 0 B) x = 1 y = 1 C) x = 0 y = 1 D) x = 1 y = 2 E) x = 2 y = 1](https://storage.examlex.com/TB6422/11eaaf91_9de8_f4b2_892c_937fe19b9050_TB6422_00_TB6422_00_TB6422_00.jpg)
The rate law for the reaction is Rate = k[A]x[B]y. What are the values of x and y?
A) x = 1 y = 0
B) x = 1 y = 1
C) x = 0 y = 1
D) x = 1 y = 2
E) x = 2 y = 1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction
H2SeO3(aq) + 6I-(aq) + 4H+(aq) → 2I3-(aq) + 3H2O(l) + Se(s)
was studied at 0°C by the method of initial rates:![<strong>The reaction H<sub>2</sub>SeO<sub>3</sub>(aq) + 6I<sup>-</sup>(aq) + 4H<sup>+</sup>(aq) → 2I<sub>3</sub><sup>-</sup>(aq) + 3H<sub>2</sub>O(l) + Se(s) was studied at 0°C by the method of initial rates: What is the rate law?</strong> A) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>]<sup>2</sup> B) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>3</sup> C) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>] D) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>] E) Rate = k[H<sub>2</sub>SeO<sub>3</sub>]<sup>2</sup>[H<sup>+</sup>][I<sup>-</sup>]](https://storage.examlex.com/TB6422/11eaaf91_9de9_90f5_892c_19a35e2fb5b9_TB6422_00_TB6422_00.jpg)
What is the rate law?
A) Rate = k[H2SeO3][H+][I-]2
B) Rate = k[H2SeO3][H+]2[I-]3
C) Rate = k[H2SeO3][H+][I-]
D) Rate = k[H2SeO3][H+]2[I-]
E) Rate = k[H2SeO3]2[H+][I-]
H2SeO3(aq) + 6I-(aq) + 4H+(aq) → 2I3-(aq) + 3H2O(l) + Se(s)
was studied at 0°C by the method of initial rates:
![<strong>The reaction H<sub>2</sub>SeO<sub>3</sub>(aq) + 6I<sup>-</sup>(aq) + 4H<sup>+</sup>(aq) → 2I<sub>3</sub><sup>-</sup>(aq) + 3H<sub>2</sub>O(l) + Se(s) was studied at 0°C by the method of initial rates: What is the rate law?</strong> A) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>]<sup>2</sup> B) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>3</sup> C) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>] D) Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>] E) Rate = k[H<sub>2</sub>SeO<sub>3</sub>]<sup>2</sup>[H<sup>+</sup>][I<sup>-</sup>]](https://storage.examlex.com/TB6422/11eaaf91_9de9_90f5_892c_19a35e2fb5b9_TB6422_00_TB6422_00.jpg)
What is the rate law?
A) Rate = k[H2SeO3][H+][I-]2
B) Rate = k[H2SeO3][H+]2[I-]3
C) Rate = k[H2SeO3][H+][I-]
D) Rate = k[H2SeO3][H+]2[I-]
E) Rate = k[H2SeO3]2[H+][I-]

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
9
The oxidation of Cr3+ to CrO42- can be accomplished using Ce4+ in a buffered solution. The following data were obtained: 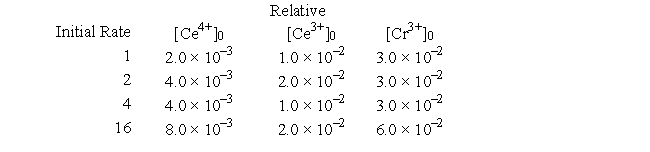
Determine the order in the rate law of the species Ce3+.
A) -1
B) 2
C) 3
D) -2
E) 1
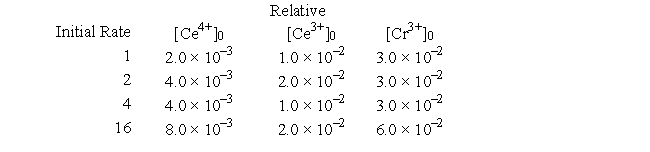
Determine the order in the rate law of the species Ce3+.
A) -1
B) 2
C) 3
D) -2
E) 1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
10
A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. 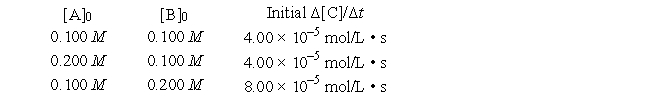
What is the order of the reaction with respect to B?
A) 3
B) 2
C) 4
D) 1
E) 0
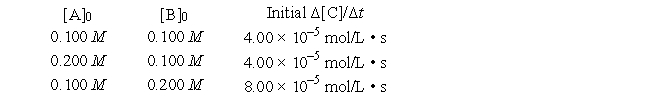
What is the order of the reaction with respect to B?
A) 3
B) 2
C) 4
D) 1
E) 0

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
11
For the reaction
2A + B → products
the following mechanism is proposed:
A + B M
M
A + M → products
A catalyst never appears in a rate law.
2A + B → products
the following mechanism is proposed:
A + B
 M
MA + M → products
A catalyst never appears in a rate law.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
12
The oxidation of Cr3+ to CrO42- can be accomplished using Ce4+ in a buffered solution. The following data were obtained: 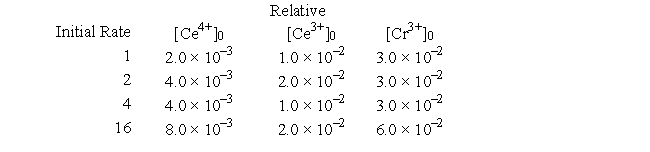
Determine the order in the rate law of the species Cr3+.
A) -2
B) 3
C) 1
D) 2
E) -1
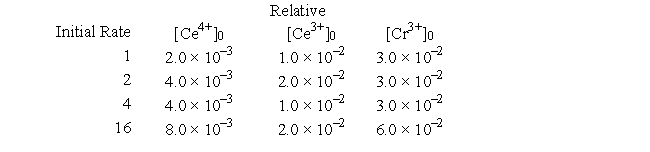
Determine the order in the rate law of the species Cr3+.
A) -2
B) 3
C) 1
D) 2
E) -1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
13
A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. 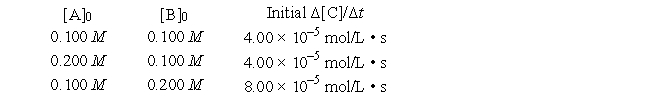
What is the overall order of the reaction?
A) 0
B) 2
C) 3
D) 4
E) 1
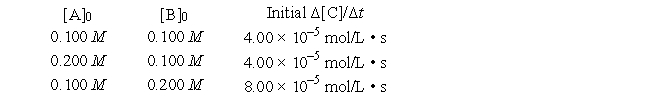
What is the overall order of the reaction?
A) 0
B) 2
C) 3
D) 4
E) 1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
14
The rate constant k is dependent on
A) the concentration of the product.
B) the order of the reaction.
C) the temperature.
D) the concentration of the reactant.
E) none of these
A) the concentration of the product.
B) the order of the reaction.
C) the temperature.
D) the concentration of the reactant.
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
15
A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. ![<strong>A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. For the first of the reactions in the table of data, determine -Δ[B]/Δt.</strong> A) 8.00 × 10<sup>-5</sup> B) 1.60 × 10<sup>-4</sup> C) 4.00 × 10<sup>-5</sup> D) 2.00 × 10<sup>-5</sup> E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9de9_42d3_892c_9b805d38bcd5_TB6422_00_TB6422_00_TB6422_00_TB6422_00_TB6422_00_TB6422_00.jpg)
For the first of the reactions in the table of data, determine -Δ[B]/Δt.
A) 8.00 × 10-5
B) 1.60 × 10-4
C) 4.00 × 10-5
D) 2.00 × 10-5
E) none of these
![<strong>A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. For the first of the reactions in the table of data, determine -Δ[B]/Δt.</strong> A) 8.00 × 10<sup>-5</sup> B) 1.60 × 10<sup>-4</sup> C) 4.00 × 10<sup>-5</sup> D) 2.00 × 10<sup>-5</sup> E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9de9_42d3_892c_9b805d38bcd5_TB6422_00_TB6422_00_TB6422_00_TB6422_00_TB6422_00_TB6422_00.jpg)
For the first of the reactions in the table of data, determine -Δ[B]/Δt.
A) 8.00 × 10-5
B) 1.60 × 10-4
C) 4.00 × 10-5
D) 2.00 × 10-5
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
16
A general reaction written as 2A + 2B → C + 2D is studied and yields the following data. 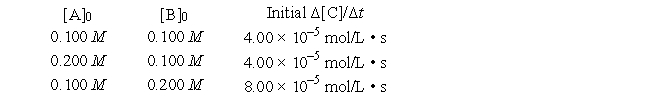
What are the proper units for the rate constant for the reaction?
A) L mol-1 s-1
B) s-1
C) L3 mol-3 s-1
D) mol L-1 s-1
E) L2 mol-2 s-1
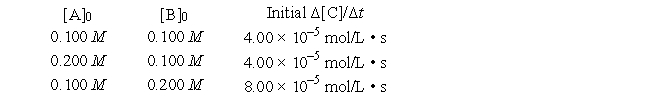
What are the proper units for the rate constant for the reaction?
A) L mol-1 s-1
B) s-1
C) L3 mol-3 s-1
D) mol L-1 s-1
E) L2 mol-2 s-1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
17
A first-order reaction is 58% complete at the end of 11 min. What is the value of the rate constant?
A) 0.079 min-1
B) 0.37 min-1
C) 7.9 × 10-2 min-1
D) 3.4 × 10-2 min-1
E) 5.0× 10-2 min-1
A) 0.079 min-1
B) 0.37 min-1
C) 7.9 × 10-2 min-1
D) 3.4 × 10-2 min-1
E) 5.0× 10-2 min-1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
18
The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. ![<strong>The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. The rate expression for a particular reaction is Rate = k[A][B]<sup>3</sup>. If the initial concentration of B is increased from 0.2 M to 0.6 M, the initial rate will increase by which of the following factors?</strong> A) 6 B) 3 C) 27 D) 4 E) 12](https://storage.examlex.com/TB6422/11eaaf91_9de8_f4b2_892c_937fe19b9050_TB6422_00_TB6422_00_TB6422_00.jpg)
The rate expression for a particular reaction is Rate = k[A][B]3. If the initial concentration of B is increased from 0.2 M to 0.6 M, the initial rate will increase by which of the following factors?
A) 6
B) 3
C) 27
D) 4
E) 12
![<strong>The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. The rate expression for a particular reaction is Rate = k[A][B]<sup>3</sup>. If the initial concentration of B is increased from 0.2 M to 0.6 M, the initial rate will increase by which of the following factors?</strong> A) 6 B) 3 C) 27 D) 4 E) 12](https://storage.examlex.com/TB6422/11eaaf91_9de8_f4b2_892c_937fe19b9050_TB6422_00_TB6422_00_TB6422_00.jpg)
The rate expression for a particular reaction is Rate = k[A][B]3. If the initial concentration of B is increased from 0.2 M to 0.6 M, the initial rate will increase by which of the following factors?
A) 6
B) 3
C) 27
D) 4
E) 12

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
19
The oxidation of Cr3+ to CrO42- can be accomplished using Ce4+ in a buffered solution. The following data were obtained: 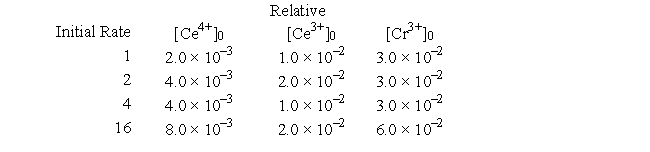
Determine the order in the rate law of the species Ce4+.
A) 1
B) -2
C) 3
D) -1
E) 2
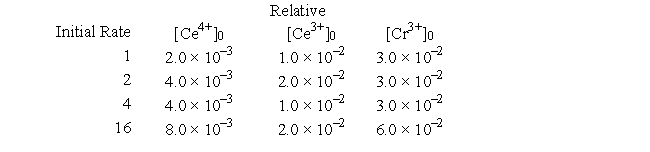
Determine the order in the rate law of the species Ce4+.
A) 1
B) -2
C) 3
D) -1
E) 2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
20
The average rate of disappearance of ozone in the reaction 2O3(g) → 3O2(g) is found to be 8.8 × 10-3 atm over a certain interval of time. What is the rate of appearance of O2 during this interval?
A) 5.9 × 10-3 atm/time
B) 2.6 × 10-2 atm/time
C) 1.8 × 10-2 atm/time
D) 1.3 × 10-2 atm/time
E) 8.8 × 10-3 atm/time
A) 5.9 × 10-3 atm/time
B) 2.6 × 10-2 atm/time
C) 1.8 × 10-2 atm/time
D) 1.3 × 10-2 atm/time
E) 8.8 × 10-3 atm/time

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
21
The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assume the form of the rate law is
Rate![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and O<sub>2</sub>(g) obeys first-order kinetics. Assume the form of the rate law is Rate where k = 3.4 × 10<sup>-5</sup> s<sup>-1</sup> at 25°C. What is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 5.0 × 10<sup>-2</sup> M?</strong> A) 3.4 × 10<sup>-5</sup> mol/L • s B) 5.0 × 10<sup>-2</sup> mol/L • s C) 6.8 × 10<sup>-4</sup> mol/L • s D) 1.7 × 10<sup>-6</sup> mol/L • s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_f09a_892c_a9519fd66c83_TB6422_11_TB6422_11_TB6422_11.jpg) where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.
What is the initial rate of reaction at 25°C where [N2O5]0 = 5.0 × 10-2 M?
A) 3.4 × 10-5 mol/L • s
B) 5.0 × 10-2 mol/L • s
C) 6.8 × 10-4 mol/L • s
D) 1.7 × 10-6 mol/L • s
E) none of these
Rate
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and O<sub>2</sub>(g) obeys first-order kinetics. Assume the form of the rate law is Rate where k = 3.4 × 10<sup>-5</sup> s<sup>-1</sup> at 25°C. What is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 5.0 × 10<sup>-2</sup> M?</strong> A) 3.4 × 10<sup>-5</sup> mol/L • s B) 5.0 × 10<sup>-2</sup> mol/L • s C) 6.8 × 10<sup>-4</sup> mol/L • s D) 1.7 × 10<sup>-6</sup> mol/L • s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_f09a_892c_a9519fd66c83_TB6422_11_TB6422_11_TB6422_11.jpg) where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.What is the initial rate of reaction at 25°C where [N2O5]0 = 5.0 × 10-2 M?
A) 3.4 × 10-5 mol/L • s
B) 5.0 × 10-2 mol/L • s
C) 6.8 × 10-4 mol/L • s
D) 1.7 × 10-6 mol/L • s
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
22
The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assume the form of the rate law is
Rate![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and O<sub>2</sub>(g) obeys first-order kinetics. Assume the form of the rate law is Rate where k = 3.4 × 10<sup>-5</sup> s<sup>-1</sup> at 25°C. The reaction A → B + C is known to be zero order in A with a rate constant of 3.8× 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the rate after 6.5 minutes?</strong> A) 1.0 × 10<sup>-3</sup> mol/L • s B) 3.8 × 10<sup>-2</sup> mol/L • s C) 3.8 × 10<sup>-5</sup> mol/L • s D) 3.7 × 10<sup>-4</sup> mol/L • s E) 1.5 × 10<sup>-11</sup> mol/L • s](https://storage.examlex.com/TB6422/11eaaf91_9dea_f09a_892c_a9519fd66c83_TB6422_11_TB6422_11_TB6422_11.jpg) where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.
The reaction A → B + C is known to be zero order in A with a rate constant of 3.8× 10-2 mol/L • s at 25° C. An experiment was run at 25°C where [A]0 = 1.0 × 10-3 M. What is the rate after 6.5 minutes?
A) 1.0 × 10-3 mol/L • s
B) 3.8 × 10-2 mol/L • s
C) 3.8 × 10-5 mol/L • s
D) 3.7 × 10-4 mol/L • s
E) 1.5 × 10-11 mol/L • s
Rate
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and O<sub>2</sub>(g) obeys first-order kinetics. Assume the form of the rate law is Rate where k = 3.4 × 10<sup>-5</sup> s<sup>-1</sup> at 25°C. The reaction A → B + C is known to be zero order in A with a rate constant of 3.8× 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the rate after 6.5 minutes?</strong> A) 1.0 × 10<sup>-3</sup> mol/L • s B) 3.8 × 10<sup>-2</sup> mol/L • s C) 3.8 × 10<sup>-5</sup> mol/L • s D) 3.7 × 10<sup>-4</sup> mol/L • s E) 1.5 × 10<sup>-11</sup> mol/L • s](https://storage.examlex.com/TB6422/11eaaf91_9dea_f09a_892c_a9519fd66c83_TB6422_11_TB6422_11_TB6422_11.jpg) where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.The reaction A → B + C is known to be zero order in A with a rate constant of 3.8× 10-2 mol/L • s at 25° C. An experiment was run at 25°C where [A]0 = 1.0 × 10-3 M. What is the rate after 6.5 minutes?
A) 1.0 × 10-3 mol/L • s
B) 3.8 × 10-2 mol/L • s
C) 3.8 × 10-5 mol/L • s
D) 3.7 × 10-4 mol/L • s
E) 1.5 × 10-11 mol/L • s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
23
The following questions refer to the hypothetical reaction A + B → products. The kinetics data given can be analyzed to answer the questions. 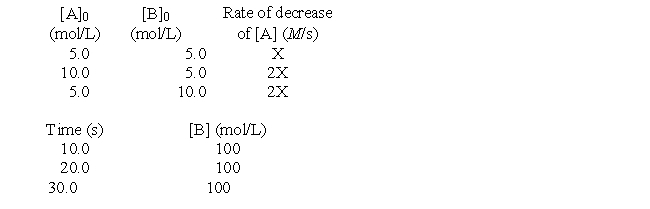
Determine the magnitude of the pseudo-rate constant (k') if the magnitude of X in the rate data is 0.00905.
A) 0.31
B) 1.81 × 10-3
C) 4.3 × 10-3
D) 0.86
E) 1.2 × 10-2

Determine the magnitude of the pseudo-rate constant (k') if the magnitude of X in the rate data is 0.00905.
A) 0.31
B) 1.81 × 10-3
C) 4.3 × 10-3
D) 0.86
E) 1.2 × 10-2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
24
The following initial rate data were found for the reaction
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the integrated rate law?</strong> A) [A] = kt B) [A]<sub>0</sub> - [A] = kt C) D) [A] - [A]<sub>0</sub> = kt E)](https://storage.examlex.com/TB6422/11eaaf91_9dea_7b64_892c_8fe7e6b64e7e_TB6422_00_TB6422_00_TB6422_00_TB6422_00.jpg)
The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10-2 mol/L • s at 25° C. An experiment was run at 25°C where [A]0 = 1.0 × 10-3 M. What is the integrated rate law?
A) [A] = kt
B) [A]0 - [A] = kt
C)![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the integrated rate law?</strong> A) [A] = kt B) [A]<sub>0</sub> - [A] = kt C) D) [A] - [A]<sub>0</sub> = kt E)](https://storage.examlex.com/TB6422/11eaaf91_9dea_a275_892c_9b4016f48bc2_TB6422_11.jpg)
D) [A] - [A]0 = kt
E)![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the integrated rate law?</strong> A) [A] = kt B) [A]<sub>0</sub> - [A] = kt C) D) [A] - [A]<sub>0</sub> = kt E)](https://storage.examlex.com/TB6422/11eaaf91_9dea_a276_892c_3348d944c38a_TB6422_11.jpg)
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O
![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the integrated rate law?</strong> A) [A] = kt B) [A]<sub>0</sub> - [A] = kt C) D) [A] - [A]<sub>0</sub> = kt E)](https://storage.examlex.com/TB6422/11eaaf91_9dea_7b64_892c_8fe7e6b64e7e_TB6422_00_TB6422_00_TB6422_00_TB6422_00.jpg)
The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10-2 mol/L • s at 25° C. An experiment was run at 25°C where [A]0 = 1.0 × 10-3 M. What is the integrated rate law?
A) [A] = kt
B) [A]0 - [A] = kt
C)
![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the integrated rate law?</strong> A) [A] = kt B) [A]<sub>0</sub> - [A] = kt C) D) [A] - [A]<sub>0</sub> = kt E)](https://storage.examlex.com/TB6422/11eaaf91_9dea_a275_892c_9b4016f48bc2_TB6422_11.jpg)
D) [A] - [A]0 = kt
E)
![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O The reaction A → B + C is known to be zero order in A with a rate constant of 5.0 × 10<sup>-2</sup> mol/L • s at 25° C. An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M. What is the integrated rate law?</strong> A) [A] = kt B) [A]<sub>0</sub> - [A] = kt C) D) [A] - [A]<sub>0</sub> = kt E)](https://storage.examlex.com/TB6422/11eaaf91_9dea_a276_892c_3348d944c38a_TB6422_11.jpg)

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
25
The OH radical disproportionates according to the elementary chemical reaction OH + OH → H2O + O. This reaction is second order in OH. The rate constant for the reaction is 2.1 × 10-12 cm3/molecule • s at room temperature. If the initial OH concentration is 1.6 × 1013 molecules/cm3, what is the first half-life for the reaction?
A) 3.3 × 1011 s
B) 0.030 s
C) 3.8 × 1024 s
D) 3.9 s
E) 6.0 s
A) 3.3 × 1011 s
B) 0.030 s
C) 3.8 × 1024 s
D) 3.9 s
E) 6.0 s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
26
Use the following initial rate data for the reaction in aqueous solution to determine the rate law. ![<strong>Use the following initial rate data for the reaction in aqueous solution to determine the rate law. </strong> A) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][H<sup>+</sup>] B) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][Br<sub>2</sub>][H<sup>+</sup>] C) Rate = k[Br<sub>2</sub>][H<sup>+</sup>] D) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][Br<sub>2</sub>][H<sup>+</sup>]<sup>2</sup> E) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][Br<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9de9_90f6_892c_89fc17b9a694_TB6422_00.jpg)
A) Rate = k[CH3COCH3][H+]
B) Rate = k[CH3COCH3][Br2][H+]
C) Rate = k[Br2][H+]
D) Rate = k[CH3COCH3][Br2][H+]2
E) Rate = k[CH3COCH3][Br2]
![<strong>Use the following initial rate data for the reaction in aqueous solution to determine the rate law. </strong> A) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][H<sup>+</sup>] B) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][Br<sub>2</sub>][H<sup>+</sup>] C) Rate = k[Br<sub>2</sub>][H<sup>+</sup>] D) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][Br<sub>2</sub>][H<sup>+</sup>]<sup>2</sup> E) Rate = k[CH<sub>3</sub>COCH<sub>3</sub>][Br<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9de9_90f6_892c_89fc17b9a694_TB6422_00.jpg)
A) Rate = k[CH3COCH3][H+]
B) Rate = k[CH3COCH3][Br2][H+]
C) Rate = k[Br2][H+]
D) Rate = k[CH3COCH3][Br2][H+]2
E) Rate = k[CH3COCH3][Br2]

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
27
Two isomers (A and B) of a given compound dimerize as follows: ![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.</strong> A) 4.0 × 10<sup>2</sup> s B) 1.8 × 10<sup>2</sup> s C) 2.5 × 10<sup>3</sup> D) 1.7 × 10<sup>1</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_a277_892c_5da26164625f_TB6422_11.jpg)
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.</strong> A) 4.0 × 10<sup>2</sup> s B) 1.8 × 10<sup>2</sup> s C) 2.5 × 10<sup>3</sup> D) 1.7 × 10<sup>1</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_c988_892c_b72e80f49c66_TB6422_11.jpg)
Both processes are known to be second order in reactant, and k1 is known to be 0.25 L/mol • s at 25° C, where
Rate![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.</strong> A) 4.0 × 10<sup>2</sup> s B) 1.8 × 10<sup>2</sup> s C) 2.5 × 10<sup>3</sup> D) 1.7 × 10<sup>1</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_c989_892c_1bf3707bca83_TB6422_11.jpg)
In a particular experiment, A and B were placed in separate containers at 25° C, where [A]0 = 1.0 × 10-2 M and [B]0 = 2.5 × 10-2 M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.
A) 4.0 × 102 s
B) 1.8 × 102 s
C) 2.5 × 103
D) 1.7 × 101 s
E) none of these
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.</strong> A) 4.0 × 10<sup>2</sup> s B) 1.8 × 10<sup>2</sup> s C) 2.5 × 10<sup>3</sup> D) 1.7 × 10<sup>1</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_a277_892c_5da26164625f_TB6422_11.jpg)
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.</strong> A) 4.0 × 10<sup>2</sup> s B) 1.8 × 10<sup>2</sup> s C) 2.5 × 10<sup>3</sup> D) 1.7 × 10<sup>1</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_c988_892c_b72e80f49c66_TB6422_11.jpg)
Both processes are known to be second order in reactant, and k1 is known to be 0.25 L/mol • s at 25° C, where
Rate
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.</strong> A) 4.0 × 10<sup>2</sup> s B) 1.8 × 10<sup>2</sup> s C) 2.5 × 10<sup>3</sup> D) 1.7 × 10<sup>1</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dea_c989_892c_1bf3707bca83_TB6422_11.jpg)
In a particular experiment, A and B were placed in separate containers at 25° C, where [A]0 = 1.0 × 10-2 M and [B]0 = 2.5 × 10-2 M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.Calculate the half-life for the reaction involving A.
A) 4.0 × 102 s
B) 1.8 × 102 s
C) 2.5 × 103
D) 1.7 × 101 s
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
28
For the reaction A + B → products, the following data were obtained. ![<strong>For the reaction A + B → products, the following data were obtained. What is the experimental rate law?</strong> A) Rate = k[A]<sup>2</sup>[B] B) Rate = k[B] C) Rate = k[A][B] D) Rate = k[A] E) Rate = k[A][B] <sup>2</sup>](https://storage.examlex.com/TB6422/11eaaf91_9de9_df1a_892c_d98a5cd0d9eb_TB6422_00.jpg) What is the experimental rate law?
What is the experimental rate law?
A) Rate = k[A]2[B]
B) Rate = k[B]
C) Rate = k[A][B]
D) Rate = k[A]
E) Rate = k[A][B] 2
![<strong>For the reaction A + B → products, the following data were obtained. What is the experimental rate law?</strong> A) Rate = k[A]<sup>2</sup>[B] B) Rate = k[B] C) Rate = k[A][B] D) Rate = k[A] E) Rate = k[A][B] <sup>2</sup>](https://storage.examlex.com/TB6422/11eaaf91_9de9_df1a_892c_d98a5cd0d9eb_TB6422_00.jpg) What is the experimental rate law?
What is the experimental rate law?A) Rate = k[A]2[B]
B) Rate = k[B]
C) Rate = k[A][B]
D) Rate = k[A]
E) Rate = k[A][B] 2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
29
For which of the following is the half-life directly dependent on the concentration of the reactant?
A) zero-order reaction
B) second-order reaction
C) first-order reaction
D) two of these
E) all of these
A) zero-order reaction
B) second-order reaction
C) first-order reaction
D) two of these
E) all of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
30
The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assume the form of the rate law is
Rate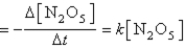 where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.
What is the half-life for the reaction described?
A) 2.4 × 10-5 s
B) 7.4 × 102 s
C) 5.9 × 105 s
D) 2.0 × 104 s
E) none of these
Rate
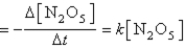 where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.What is the half-life for the reaction described?
A) 2.4 × 10-5 s
B) 7.4 × 102 s
C) 5.9 × 105 s
D) 2.0 × 104 s
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
31
Tabulated below are initial rate data for the reaction
2Fe(CN)63- + 2I- → 2Fe(CN)64- + I2![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d3d_892c_49d2835d8b2a_TB6422_00.jpg)
What is the experimental rate law?
A)![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d3e_892c_6959bf94a16e_TB6422_11.jpg) k[Fe(CN)63-][I-]2
k[Fe(CN)63-][I-]2
B)![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d3f_892c_a3e115c1b060_TB6422_11.jpg) k[Fe(CN)63-][I-] [Fe(CN)64-]
k[Fe(CN)63-][I-] [Fe(CN)64-]
C)![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d40_892c_8b40cf397931_TB6422_11.jpg) k[Fe(CN)63-]2[I-]
k[Fe(CN)63-]2[I-]
D)![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d41_892c_7b707521c872_TB6422_11.jpg) k[Fe(CN)63-]2[I-]2[Fe(CN)64-]2[I2]
k[Fe(CN)63-]2[I-]2[Fe(CN)64-]2[I2]
E)![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_5452_892c_a1ad42751418_TB6422_11.jpg) k[Fe(CN)63-]2[I-][Fe(CN)64-][I2]
k[Fe(CN)63-]2[I-][Fe(CN)64-][I2]
2Fe(CN)63- + 2I- → 2Fe(CN)64- + I2
![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d3d_892c_49d2835d8b2a_TB6422_00.jpg)
What is the experimental rate law?
A)
![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d3e_892c_6959bf94a16e_TB6422_11.jpg) k[Fe(CN)63-][I-]2
k[Fe(CN)63-][I-]2B)
![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d3f_892c_a3e115c1b060_TB6422_11.jpg) k[Fe(CN)63-][I-] [Fe(CN)64-]
k[Fe(CN)63-][I-] [Fe(CN)64-]C)
![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d40_892c_8b40cf397931_TB6422_11.jpg) k[Fe(CN)63-]2[I-]
k[Fe(CN)63-]2[I-]D)
![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_2d41_892c_7b707521c872_TB6422_11.jpg) k[Fe(CN)63-]2[I-]2[Fe(CN)64-]2[I2]
k[Fe(CN)63-]2[I-]2[Fe(CN)64-]2[I2]E)
![<strong>Tabulated below are initial rate data for the reaction 2Fe(CN)<sub>6</sub><sup>3-</sup> + 2I<sup>-</sup> → 2Fe(CN)<sub>6</sub><sup>4-</sup> + I<sub>2</sub> What is the experimental rate law?</strong> A) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>]<sup>2</sup> B) k[Fe(CN)<sub>6</sub><sup>3-</sup>][I<sup>-</sup>] [Fe(CN)<sub>6</sub><sup>4-</sup>] C) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>] D) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[Fe(CN)<sub>6</sub><sup>4-</sup>]<sup>2</sup>[I<sub>2</sub>] E) k[Fe(CN)<sub>6</sub><sup>3-</sup>]<sup>2</sup>[I<sup>-</sup>][Fe(CN)<sub>6</sub><sup>4-</sup>][I<sub>2</sub>]](https://storage.examlex.com/TB6422/11eaaf91_9dea_5452_892c_a1ad42751418_TB6422_11.jpg) k[Fe(CN)63-]2[I-][Fe(CN)64-][I2]
k[Fe(CN)63-]2[I-][Fe(CN)64-][I2]
Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
32
For the reaction in which A and B react to form C, the following initial rate data were obtained. ![<strong>For the reaction in which A and B react to form C, the following initial rate data were obtained. What is the rate law for the reaction?</strong> A) Rate = k[A]<sup>2</sup>[B]<sup>2</sup> B) Rate = k[A]<sup>2</sup>[B] C) Rate = k[A][B]<sup>2</sup> D) Rate = k[A][B] E) Rate = k[A]<sup>3</sup>](https://storage.examlex.com/TB6422/11eaaf91_9dea_062c_892c_43898ac1bb53_TB6422_00.jpg) What is the rate law for the reaction?
What is the rate law for the reaction?
A) Rate = k[A]2[B]2
B) Rate = k[A]2[B]
C) Rate = k[A][B]2
D) Rate = k[A][B]
E) Rate = k[A]3
![<strong>For the reaction in which A and B react to form C, the following initial rate data were obtained. What is the rate law for the reaction?</strong> A) Rate = k[A]<sup>2</sup>[B]<sup>2</sup> B) Rate = k[A]<sup>2</sup>[B] C) Rate = k[A][B]<sup>2</sup> D) Rate = k[A][B] E) Rate = k[A]<sup>3</sup>](https://storage.examlex.com/TB6422/11eaaf91_9dea_062c_892c_43898ac1bb53_TB6422_00.jpg) What is the rate law for the reaction?
What is the rate law for the reaction?A) Rate = k[A]2[B]2
B) Rate = k[A]2[B]
C) Rate = k[A][B]2
D) Rate = k[A][B]
E) Rate = k[A]3

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
33
Initial rate data have been determined at a certain temperature for the gaseous reaction
2NO + 2H2 → N2 + 2H2O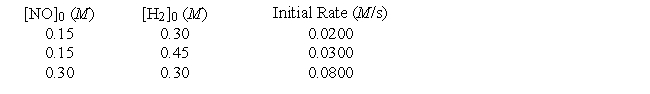
What is the numerical value of the rate constant?
A) 1.5
B) 9.9
C) 3.0
D) 0.13
E) 0.44
2NO + 2H2 → N2 + 2H2O
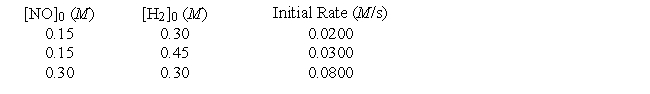
What is the numerical value of the rate constant?
A) 1.5
B) 9.9
C) 3.0
D) 0.13
E) 0.44

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
34
The reaction of (CH3)3CBr with hydroxide ion proceeds with the formation of (CH3)3COH.(CH3)3CBr (aq) + OH- (aq) → (CH3)3COH (aq) + Br- (aq)
The following data were obtained at 55°C.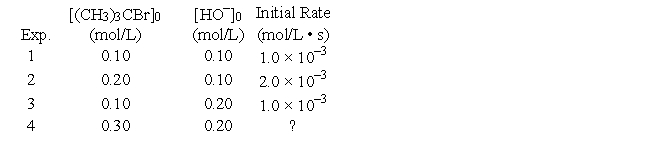
What will the initial rate (in mol/L • s) be in Experiment 4?
A) 18 × 10-3
B) 6.0 × 10-3
C) 9.0 × 10-3
D) 3.0 × 10-3
E) none of these
The following data were obtained at 55°C.
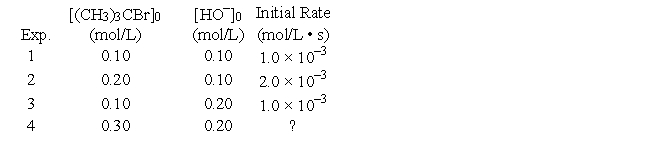
What will the initial rate (in mol/L • s) be in Experiment 4?
A) 18 × 10-3
B) 6.0 × 10-3
C) 9.0 × 10-3
D) 3.0 × 10-3
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
35
The reaction
H2SeO3(aq) + 6I-(aq) + 4H+(aq) → 2I3-(aq) + 3H2O(l) + Se(s)
was studied at 0°C by the method of initial rates: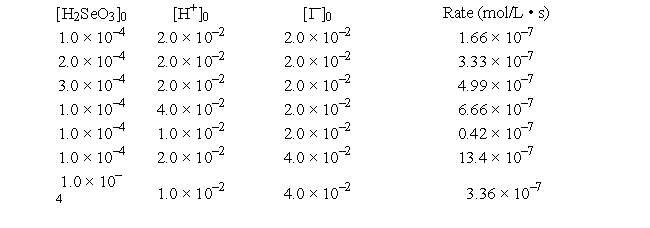
What is the numerical value of the rate constant?
A) 4.2
B) 2.1 × 102
C) 5.2 × 105
D) 1.9 × 10-6
E) none of these
H2SeO3(aq) + 6I-(aq) + 4H+(aq) → 2I3-(aq) + 3H2O(l) + Se(s)
was studied at 0°C by the method of initial rates:
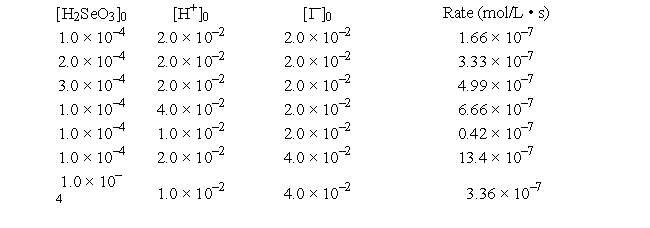
What is the numerical value of the rate constant?
A) 4.2
B) 2.1 × 102
C) 5.2 × 105
D) 1.9 × 10-6
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
36
The following initial rate data were found for the reaction
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O Which of the following is the correct rate law?</strong> A) Rate = k[MnO<sub>4</sub><sup>-</sup>][H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] B) Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] C) Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>] D) Rate = k[MnO<sub>4</sub><sup>-</sup>][H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>2</sup> E) Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>5</sup>[H<sup>+</sup>]<sup>6</sup>](https://storage.examlex.com/TB6422/11eaaf91_9dea_7b64_892c_8fe7e6b64e7e_TB6422_00_TB6422_00_TB6422_00_TB6422_00.jpg)
Which of the following is the correct rate law?
A) Rate = k[MnO4-][H2C2O4][H+]
B) Rate = k[MnO4-]2[H2C2O4][H+]
C) Rate = k[MnO4-]2[H2C2O4]
D) Rate = k[MnO4-][H2C2O4]2
E) Rate = k[MnO4-]2[H2C2O4]5[H+]6
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O
![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> → 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O Which of the following is the correct rate law?</strong> A) Rate = k[MnO<sub>4</sub><sup>-</sup>][H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] B) Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] C) Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>] D) Rate = k[MnO<sub>4</sub><sup>-</sup>][H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>2</sup> E) Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>5</sup>[H<sup>+</sup>]<sup>6</sup>](https://storage.examlex.com/TB6422/11eaaf91_9dea_7b64_892c_8fe7e6b64e7e_TB6422_00_TB6422_00_TB6422_00_TB6422_00.jpg)
Which of the following is the correct rate law?
A) Rate = k[MnO4-][H2C2O4][H+]
B) Rate = k[MnO4-]2[H2C2O4][H+]
C) Rate = k[MnO4-]2[H2C2O4]
D) Rate = k[MnO4-][H2C2O4]2
E) Rate = k[MnO4-]2[H2C2O4]5[H+]6

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
37
Tabulated below are initial rate data for the reaction
2Fe(CN)63- + 2I- → 2Fe(CN)64- + I2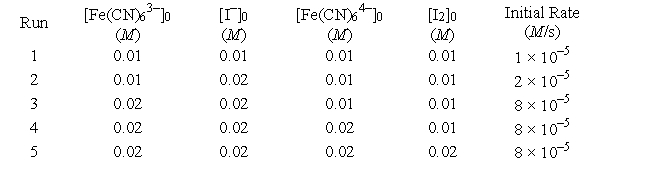
What is the value of k?
A) 103 M-3 s-1
B) 50 M-2 s-1
C) 10 M-2 s-1
D) 107 M-5 s-1
E) none of these
2Fe(CN)63- + 2I- → 2Fe(CN)64- + I2
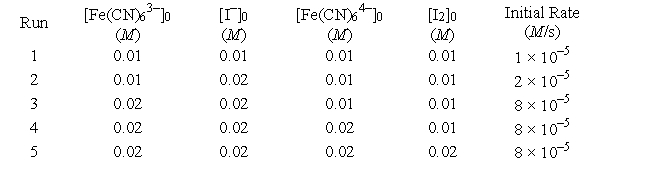
What is the value of k?
A) 103 M-3 s-1
B) 50 M-2 s-1
C) 10 M-2 s-1
D) 107 M-5 s-1
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
38
The following initial rate data were found for the reaction
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O
What is the value of the rate constant?
A) 2 × 105 M • s-1
B) 2 × 105 M-2 • s-1
C) 200 M-2 • s-1
D) 2 × 10-4 M • s-1
E) 200 M-1 • s-1
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O

What is the value of the rate constant?
A) 2 × 105 M • s-1
B) 2 × 105 M-2 • s-1
C) 200 M-2 • s-1
D) 2 × 10-4 M • s-1
E) 200 M-1 • s-1

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
39
What is the rate law for the following reaction, given the data below?
2NO + H2 → N2O + H2O![<strong>What is the rate law for the following reaction, given the data below? 2NO + H<sub>2</sub> → N<sub>2</sub>O + H<sub>2</sub>O </strong> A) Rate = k[N<sub>2</sub>O][H<sub>2</sub>O] B) Rate = k[NO][H<sub>2</sub>] C) Rate = k[NO]<sup>2</sup>[H<sub>2</sub>] D) Rate = k[NO] E) Rate = k[NO]<sup>2</sup>](https://storage.examlex.com/TB6422/11eaaf91_9de9_b808_892c_250285936f30_TB6422_00.jpg)
A) Rate = k[N2O][H2O]
B) Rate = k[NO][H2]
C) Rate = k[NO]2[H2]
D) Rate = k[NO]
E) Rate = k[NO]2
2NO + H2 → N2O + H2O
![<strong>What is the rate law for the following reaction, given the data below? 2NO + H<sub>2</sub> → N<sub>2</sub>O + H<sub>2</sub>O </strong> A) Rate = k[N<sub>2</sub>O][H<sub>2</sub>O] B) Rate = k[NO][H<sub>2</sub>] C) Rate = k[NO]<sup>2</sup>[H<sub>2</sub>] D) Rate = k[NO] E) Rate = k[NO]<sup>2</sup>](https://storage.examlex.com/TB6422/11eaaf91_9de9_b808_892c_250285936f30_TB6422_00.jpg)
A) Rate = k[N2O][H2O]
B) Rate = k[NO][H2]
C) Rate = k[NO]2[H2]
D) Rate = k[NO]
E) Rate = k[NO]2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
40
The following initial rate data were found for the reaction
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O
For which order reaction is the half-life of the reaction independent of the initial concentration of the reactant(s)?
A) zero order
B) first order
C) second order
D) all of these
E) none of these
2MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2 + 8H2O

For which order reaction is the half-life of the reaction independent of the initial concentration of the reactant(s)?
A) zero order
B) first order
C) second order
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
41
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
[A] is constant.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these
[A] is constant.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
42
For the reaction 2N2O5(g) → 4NO2(g) + O2(g), the following data were collected. 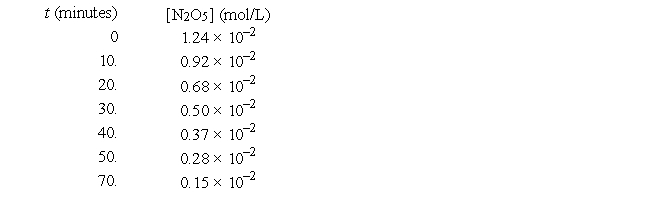
The half-life of this reaction is approximately
A) 18 min
B) 36 min
C) 15 min
D) 23 min
E) 45 min
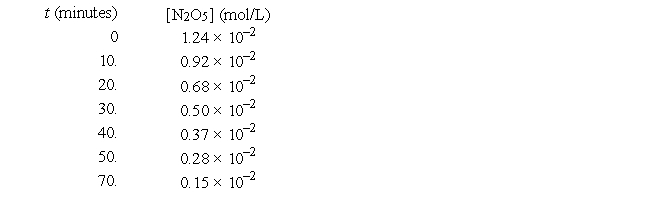
The half-life of this reaction is approximately
A) 18 min
B) 36 min
C) 15 min
D) 23 min
E) 45 min

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
43
For the reaction 2N2O5(g) → 4NO2(g) + O2(g), the following data were collected. 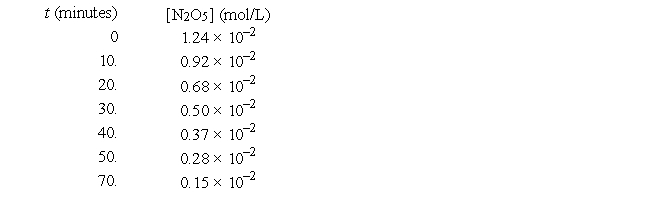
The concentration N2O5 at 100 min will be approximately
A) 0.10 × 10-2 mol/L
B) 0.01 × 10-2 mol/L
C) 0.06 × 10-2 mol/L
D) 0.03 × 10-2 mol/L
E) none of these
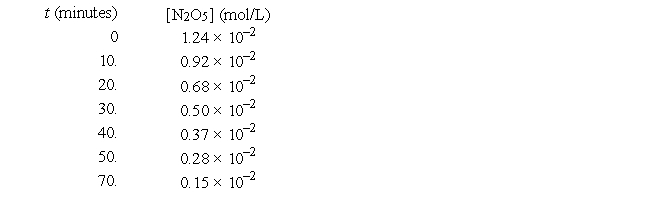
The concentration N2O5 at 100 min will be approximately
A) 0.10 × 10-2 mol/L
B) 0.01 × 10-2 mol/L
C) 0.06 × 10-2 mol/L
D) 0.03 × 10-2 mol/L
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
The reaction A → B + C is known to be zero order in A with a rate constant of 5.2 × 10-2 mol/L • s at 25° C. An experiment was run at 25°C where [A]0 = 3.2 × 10-3 M. What is the half-life for the reaction?
A) 1.3× 101 s
B) 2.6× 10-2 s
C) 8.3× 10-2 s
D) 6.0× 104 s
E) 3.1× 10-2 s
The reaction A → B + C is known to be zero order in A with a rate constant of 5.2 × 10-2 mol/L • s at 25° C. An experiment was run at 25°C where [A]0 = 3.2 × 10-3 M. What is the half-life for the reaction?
A) 1.3× 101 s
B) 2.6× 10-2 s
C) 8.3× 10-2 s
D) 6.0× 104 s
E) 3.1× 10-2 s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
45
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
The rate is constant over time.
A) zero order in A
B) first order in A
C) second order in A
D) all of these
E) none of these
The rate is constant over time.
A) zero order in A
B) first order in A
C) second order in A
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
46
If the reaction 2HI → H2 + I2 is second order, which of the following will yield a linear plot?
A) log [HI] vs. time
B) [HI] vs. time
C) ln [HI] vs. time
D) 1/[HI] vs. time
A) log [HI] vs. time
B) [HI] vs. time
C) ln [HI] vs. time
D) 1/[HI] vs. time

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
47
The reaction 2NO → N2 + O2 has the following rate law: 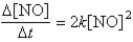 After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
A) 4.0 × 10-4 M-1/s
B) 7.2 × 10-2 M-1/s
C) 1.7 × 10-4 M-1/s
D) 4.0 × 10-7 M-1/s
E) 3.6 × 10-2 M-1/s
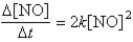 After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?A) 4.0 × 10-4 M-1/s
B) 7.2 × 10-2 M-1/s
C) 1.7 × 10-4 M-1/s
D) 4.0 × 10-7 M-1/s
E) 3.6 × 10-2 M-1/s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
48
The reaction
2NOBr → 2NO + Br2
exhibits the rate law
Rate = k[NOBr]2![<strong>The reaction 2NOBr → 2NO + Br<sub>2</sub><sub> </sub>exhibits the rate law Rate = k[NOBr]<sup>2</sup> where k = 1.0 × 10<sup>-5</sup> M<sup>-1</sup> • s<sup>-1</sup> at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>) is 1.00 × 10<sup>-1</sup> M. The [NO] after 1.00 h has passed is</strong> A) 9.7 × 10<sup>-3</sup> M B) 9.9 × 10<sup>-3</sup> M C) 1.0 × 10<sup>-3</sup> M D) 3.5 × 10<sup>-4</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9deb_65cc_892c_478d3d53b46d_TB6422_11_TB6422_11.jpg) where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.
where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.
The [NO] after 1.00 h has passed is
A) 9.7 × 10-3 M
B) 9.9 × 10-3 M
C) 1.0 × 10-3 M
D) 3.5 × 10-4 M
E) none of these
2NOBr → 2NO + Br2
exhibits the rate law
Rate = k[NOBr]2
![<strong>The reaction 2NOBr → 2NO + Br<sub>2</sub><sub> </sub>exhibits the rate law Rate = k[NOBr]<sup>2</sup> where k = 1.0 × 10<sup>-5</sup> M<sup>-1</sup> • s<sup>-1</sup> at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>) is 1.00 × 10<sup>-1</sup> M. The [NO] after 1.00 h has passed is</strong> A) 9.7 × 10<sup>-3</sup> M B) 9.9 × 10<sup>-3</sup> M C) 1.0 × 10<sup>-3</sup> M D) 3.5 × 10<sup>-4</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9deb_65cc_892c_478d3d53b46d_TB6422_11_TB6422_11.jpg) where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.
where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.The [NO] after 1.00 h has passed is
A) 9.7 × 10-3 M
B) 9.9 × 10-3 M
C) 1.0 × 10-3 M
D) 3.5 × 10-4 M
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
49
For a reaction a A → products, [A]0 = 4.0 M, and the first three successive half-lives are 48, 96, and 192 min.
Calculate k (without units).
A) 5.2 × 10-3
B) 1.4 × 10-2
C) 2.6 × 10-3
D) 4.1 × 10-3
E) none of these
Calculate k (without units).
A) 5.2 × 10-3
B) 1.4 × 10-2
C) 2.6 × 10-3
D) 4.1 × 10-3
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
The half-life is constant.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these
The half-life is constant.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
51
The following question refers to the gas-phase decomposition of chloroethane:
C2H5Cl → products
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.
What is the rate constant for this decomposition?
A) 0.22/s
B) 0.35/s
C) 0.29/s
D) 0.02/s
E) 0.11/s
C2H5Cl → products
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.

What is the rate constant for this decomposition?
A) 0.22/s
B) 0.35/s
C) 0.29/s
D) 0.02/s
E) 0.11/s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
52
For the reaction 2N2O5(g) → 4NO2(g) + O2(g), the following data were collected. 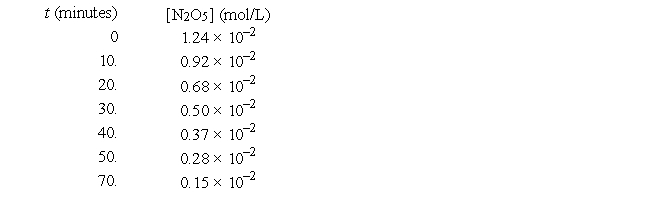
The concentration of O2 at t = 10. min is
A) 0.32 × 10-2 mol/L
B) 2.0 × 10-4 mol/L
C) 0.64 × 10-2 mol/L
D) 0.16 × 10-2 mol/L
E) none of these
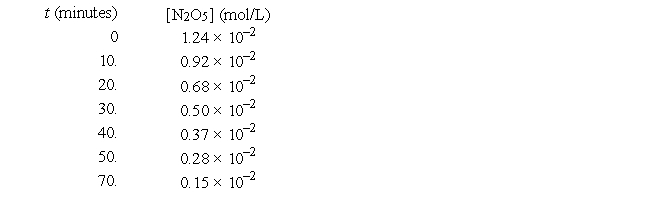
The concentration of O2 at t = 10. min is
A) 0.32 × 10-2 mol/L
B) 2.0 × 10-4 mol/L
C) 0.64 × 10-2 mol/L
D) 0.16 × 10-2 mol/L
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
53
For the reaction 2N2O5(g) → 4NO2(g) + O2(g), the following data were collected. 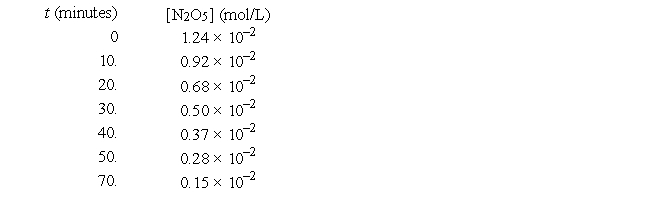
The order of this reaction in N2O5 is
A) 0
B) 1
C) 2
D) 3
E) none of these
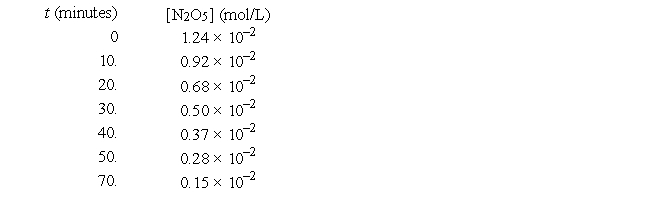
The order of this reaction in N2O5 is
A) 0
B) 1
C) 2
D) 3
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
54
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
A plot of [A] vs. t is a straight line.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these
A plot of [A] vs. t is a straight line.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
55
For a reaction a A → products, [A]0 = 4.0 M, and the first three successive half-lives are 48, 96, and 192 min.
Calculate [A] at t = 81 min.
A) 2.6 M
B) 3.0 M
C) 1.3 M
D) 1.5 M
E) none of these
Calculate [A] at t = 81 min.
A) 2.6 M
B) 3.0 M
C) 1.3 M
D) 1.5 M
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
56
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
A plot of [A]2 vs. t gives a straight line.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these
A plot of [A]2 vs. t gives a straight line.
A) zero order in A
B) second order in A
C) first order in A
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
57
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
A plot of k vs. 1/T gives a straight line.
A) first order in A
B) zero order in A
C) second order in A
D) all of these
E) none of these
A plot of k vs. 1/T gives a straight line.
A) first order in A
B) zero order in A
C) second order in A
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
58
The reaction
2NOBr → 2NO + Br2
exhibits the rate law
Rate = k[NOBr]2![<strong>The reaction 2NOBr → 2NO + Br<sub>2</sub><sub> </sub>exhibits the rate law Rate = k[NOBr]<sup>2</sup> where k = 1.0 × 10<sup>-5</sup> M<sup>-1</sup> • s<sup>-1</sup> at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>) is 1.00 × 10<sup>-1</sup> M. What is one half-life for this experiment?</strong> A) 1.0 × 10<sup>6</sup> s B) 6.9 × 10<sup>4</sup> s C) 5.0 × 10<sup>-1</sup> s D) 1.0 × 10<sup>-5</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9deb_65cc_892c_478d3d53b46d_TB6422_11_TB6422_11.jpg) where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.
where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.
What is one half-life for this experiment?
A) 1.0 × 106 s
B) 6.9 × 104 s
C) 5.0 × 10-1 s
D) 1.0 × 10-5 s
E) none of these
2NOBr → 2NO + Br2
exhibits the rate law
Rate = k[NOBr]2
![<strong>The reaction 2NOBr → 2NO + Br<sub>2</sub><sub> </sub>exhibits the rate law Rate = k[NOBr]<sup>2</sup> where k = 1.0 × 10<sup>-5</sup> M<sup>-1</sup> • s<sup>-1</sup> at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>) is 1.00 × 10<sup>-1</sup> M. What is one half-life for this experiment?</strong> A) 1.0 × 10<sup>6</sup> s B) 6.9 × 10<sup>4</sup> s C) 5.0 × 10<sup>-1</sup> s D) 1.0 × 10<sup>-5</sup> s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9deb_65cc_892c_478d3d53b46d_TB6422_11_TB6422_11.jpg) where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.
where k = 1.0 × 10-5 M-1 • s-1 at 25° C. This reaction is run where the initial concentration of NOBr ([NOBr]0) is 1.00 × 10-1 M.What is one half-life for this experiment?
A) 1.0 × 106 s
B) 6.9 × 104 s
C) 5.0 × 10-1 s
D) 1.0 × 10-5 s
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
59
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
For which order reaction is the half-life of the reaction proportional to 1/k (k is the rate constant)?
A) second order
B) zero order
C) first order
D) all of these
E) none of these
For which order reaction is the half-life of the reaction proportional to 1/k (k is the rate constant)?
A) second order
B) zero order
C) first order
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
60
For the reaction aA → products, select the reaction order(s) that best fit(s) the observations.
The half-life decreases over time.
A) second order in A
B) first order in A
C) zero order in A
D) all of these
E) none of these
The half-life decreases over time.
A) second order in A
B) first order in A
C) zero order in A
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the reaction
3A + B + C → D + E
Where the rate law is defined as![<strong>Consider the reaction 3A + B + C → D + E Where the rate law is defined as An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 2.46× 10<sup>-4</sup> M. After 2.96 min, [A] = 3.20× 10<sup>-5</sup> M. What is the value of k?</strong> A) 8.30× 10<sup>7</sup> L<sup>3</sup>/mol<sup>3</sup> • s B) 4.02× 10<sup>-7</sup> L<sup>3</sup>/mol<sup>3</sup> • s C) 2.14× 10<sup>-5</sup> L<sup>3</sup>/mol<sup>3</sup> • s D) 1.53× 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup> • s E) 9.18× 10<sup>3</sup> L<sup>3</sup>/mol<sup>3</sup> • s](https://storage.examlex.com/TB6422/11eaaf91_9dec_5036_892c_8341544c5dc8_TB6422_11.jpg)
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 2.46× 10-4 M. After 2.96 min, [A] = 3.20× 10-5 M. What is the value of k?
A) 8.30× 107 L3/mol3 • s
B) 4.02× 10-7 L3/mol3 • s
C) 2.14× 10-5 L3/mol3 • s
D) 1.53× 102 L3/mol3 • s
E) 9.18× 103 L3/mol3 • s
3A + B + C → D + E
Where the rate law is defined as
![<strong>Consider the reaction 3A + B + C → D + E Where the rate law is defined as An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 2.46× 10<sup>-4</sup> M. After 2.96 min, [A] = 3.20× 10<sup>-5</sup> M. What is the value of k?</strong> A) 8.30× 10<sup>7</sup> L<sup>3</sup>/mol<sup>3</sup> • s B) 4.02× 10<sup>-7</sup> L<sup>3</sup>/mol<sup>3</sup> • s C) 2.14× 10<sup>-5</sup> L<sup>3</sup>/mol<sup>3</sup> • s D) 1.53× 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup> • s E) 9.18× 10<sup>3</sup> L<sup>3</sup>/mol<sup>3</sup> • s](https://storage.examlex.com/TB6422/11eaaf91_9dec_5036_892c_8341544c5dc8_TB6422_11.jpg)
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 2.46× 10-4 M. After 2.96 min, [A] = 3.20× 10-5 M. What is the value of k?
A) 8.30× 107 L3/mol3 • s
B) 4.02× 10-7 L3/mol3 • s
C) 2.14× 10-5 L3/mol3 • s
D) 1.53× 102 L3/mol3 • s
E) 9.18× 103 L3/mol3 • s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
62
At 760 K, acetaldehyde decomposes to carbon monoxide and methane:
CH3CHO ⎯⎯→ CH4 + CO
A plot of ln [CH3CHO] versus time is linear. After 530 s, [CH3CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?
A)![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_020f_892c_7d2dcbae0afb_TB6422_11.jpg)
B)![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_0210_892c_4d0de4dee0f9_TB6422_11.jpg)
C)![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_0211_892c_c79c7d6bf52f_TB6422_11.jpg)
D)![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_0212_892c_ab8521909784_TB6422_11.jpg)
E)![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_2923_892c_15160e959c7d_TB6422_11.jpg)
CH3CHO ⎯⎯→ CH4 + CO
A plot of ln [CH3CHO] versus time is linear. After 530 s, [CH3CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?
A)
![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_020f_892c_7d2dcbae0afb_TB6422_11.jpg)
B)
![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_0210_892c_4d0de4dee0f9_TB6422_11.jpg)
C)
![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_0211_892c_c79c7d6bf52f_TB6422_11.jpg)
D)
![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_0212_892c_ab8521909784_TB6422_11.jpg)
E)
![<strong>At 760 K, acetaldehyde decomposes to carbon monoxide and methane: CH<sub>3</sub>CHO ⎯⎯→ CH<sub>4</sub> + CO A plot of ln [CH<sub>3</sub>CHO] versus time is linear. After 530 s, [CH<sub>3</sub>CHO] decreases to one half of its initial value of 0.10 M. What is the rate law for the reaction?</strong> A) B) C) D) E)](https://storage.examlex.com/TB6422/11eaaf91_9dec_2923_892c_15160e959c7d_TB6422_11.jpg)

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
63
The reaction
2N2O5(g) → O2(g) + 4NO2(g)
Is first order in N2O5. For this reaction at 45° C, the rate constant k = 1.0 × 10-5 s-1, where the rate law is defined as
Rate![<strong>The reaction 2N<sub>2</sub>O<sub>5</sub>(g) → O<sub>2</sub>(g) + 4NO<sub>2</sub>(g) Is first order in N<sub>2</sub>O<sub>5</sub>. For this reaction at 45° C, the rate constant k = 1.0 × 10<sup>-5</sup> s<sup>-1</sup>, where the rate law is defined as Rate For a particular experiment ([N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M), calculate [N<sub>2</sub>O<sub>5</sub>] after 1.0 × 10<sup>5</sup> s.</strong> A) 0 B) 5.0 × 10<sup>-4</sup> M C) 3.7 × 10<sup>-4</sup> M D) 1.0 × 10<sup>-3</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dec_5035_892c_939d1a242f02_TB6422_11.jpg)
For a particular experiment ([N2O5]0 = 1.0 × 10-3 M), calculate [N2O5] after 1.0 × 105 s.
A) 0
B) 5.0 × 10-4 M
C) 3.7 × 10-4 M
D) 1.0 × 10-3 M
E) none of these
2N2O5(g) → O2(g) + 4NO2(g)
Is first order in N2O5. For this reaction at 45° C, the rate constant k = 1.0 × 10-5 s-1, where the rate law is defined as
Rate
![<strong>The reaction 2N<sub>2</sub>O<sub>5</sub>(g) → O<sub>2</sub>(g) + 4NO<sub>2</sub>(g) Is first order in N<sub>2</sub>O<sub>5</sub>. For this reaction at 45° C, the rate constant k = 1.0 × 10<sup>-5</sup> s<sup>-1</sup>, where the rate law is defined as Rate For a particular experiment ([N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 1.0 × 10<sup>-3</sup> M), calculate [N<sub>2</sub>O<sub>5</sub>] after 1.0 × 10<sup>5</sup> s.</strong> A) 0 B) 5.0 × 10<sup>-4</sup> M C) 3.7 × 10<sup>-4</sup> M D) 1.0 × 10<sup>-3</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dec_5035_892c_939d1a242f02_TB6422_11.jpg)
For a particular experiment ([N2O5]0 = 1.0 × 10-3 M), calculate [N2O5] after 1.0 × 105 s.
A) 0
B) 5.0 × 10-4 M
C) 3.7 × 10-4 M
D) 1.0 × 10-3 M
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the reaction
3A + B + C → D + E
where the rate law is defined as![<strong>Consider the reaction 3A + B + C → D + E where the rate law is defined as k[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 × 10<sup>-4</sup> M. What is the concentration of A after 10.0 min?</strong> A) 9.80 × 10<sup>-6</sup> M B) 2.38 × 10<sup>-6</sup> M C) 1.27 × 10<sup>-5</sup> M D) 1.06 × 10<sup>-9</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dec_7749_892c_a9dd13c197aa_TB6422_11_TB6422_11.jpg) k[A]2[B][C]
k[A]2[B][C]
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 × 10-4 M.
What is the concentration of A after 10.0 min?
A) 9.80 × 10-6 M
B) 2.38 × 10-6 M
C) 1.27 × 10-5 M
D) 1.06 × 10-9 M
E) none of these
3A + B + C → D + E
where the rate law is defined as
![<strong>Consider the reaction 3A + B + C → D + E where the rate law is defined as k[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 × 10<sup>-4</sup> M. What is the concentration of A after 10.0 min?</strong> A) 9.80 × 10<sup>-6</sup> M B) 2.38 × 10<sup>-6</sup> M C) 1.27 × 10<sup>-5</sup> M D) 1.06 × 10<sup>-9</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dec_7749_892c_a9dd13c197aa_TB6422_11_TB6422_11.jpg) k[A]2[B][C]
k[A]2[B][C]An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 × 10-4 M.
What is the concentration of A after 10.0 min?
A) 9.80 × 10-6 M
B) 2.38 × 10-6 M
C) 1.27 × 10-5 M
D) 1.06 × 10-9 M
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
65
The elementary chemical reaction
O + ClO → Cl + O2
is made pseudo-first order in oxygen atoms by using a large excess of ClO radicals. The rate constant for the reaction is 3.5 × 10-11 cm3/molecule • s. If the initial concentration of ClO is 1.0 × 1011 molecules/cm3, how long will it take for the oxygen atoms to decrease to 10.% of their initial concentration?
A) 3.2 × 10-3 s
B) 0.66 s
C) 0.017 s
D) 23 s
E) 2.4 s
O + ClO → Cl + O2
is made pseudo-first order in oxygen atoms by using a large excess of ClO radicals. The rate constant for the reaction is 3.5 × 10-11 cm3/molecule • s. If the initial concentration of ClO is 1.0 × 1011 molecules/cm3, how long will it take for the oxygen atoms to decrease to 10.% of their initial concentration?
A) 3.2 × 10-3 s
B) 0.66 s
C) 0.017 s
D) 23 s
E) 2.4 s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
66
The reaction
3NO → N2O + NO2
Is found to obey the rate law Rate = k[NO]2. If the first half-life of the reaction is found to be 3.5 s, what is the length of the fourth half-life?
A) 21 s
B) 6.6 s
C) 53 s
D) 14 s
E) 56 s
3NO → N2O + NO2
Is found to obey the rate law Rate = k[NO]2. If the first half-life of the reaction is found to be 3.5 s, what is the length of the fourth half-life?
A) 21 s
B) 6.6 s
C) 53 s
D) 14 s
E) 56 s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
67
Two isomers (A and B) of a given compound dimerize as follows: ![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate = k<sub>1</sub>[A]<sup>2</sup><sup> </sup>In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min. Calculate the concentration of A<sub>2</sub> after 3.0 min.</strong> A) 3.1 × 10<sup>-3</sup> M B) 6.9 × 10<sup>-3</sup> M C) 1.6 × 10<sup>-3</sup> M D) 2.8 × 10<sup>-22</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_138a_892c_2350c25637bf_TB6422_11.jpg)
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate = k<sub>1</sub>[A]<sup>2</sup><sup> </sup>In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min. Calculate the concentration of A<sub>2</sub> after 3.0 min.</strong> A) 3.1 × 10<sup>-3</sup> M B) 6.9 × 10<sup>-3</sup> M C) 1.6 × 10<sup>-3</sup> M D) 2.8 × 10<sup>-22</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_138b_892c_395075742182_TB6422_11.jpg) Both processes are known to be second order in reactant, and k1 is known to be 0.25 L/mol • s at 25° C, where
Both processes are known to be second order in reactant, and k1 is known to be 0.25 L/mol • s at 25° C, where
Rate =![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate = k<sub>1</sub>[A]<sup>2</sup><sup> </sup>In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min. Calculate the concentration of A<sub>2</sub> after 3.0 min.</strong> A) 3.1 × 10<sup>-3</sup> M B) 6.9 × 10<sup>-3</sup> M C) 1.6 × 10<sup>-3</sup> M D) 2.8 × 10<sup>-22</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_3a9c_892c_a36b96076f8f_TB6422_11.jpg) k1[A]2
k1[A]2
In a particular experiment, A and B were placed in separate containers at 25° C, where [A]0 = 1.0 × 10-2 M and [B]0 = 2.5 × 10-2 M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.
Calculate the concentration of A2 after 3.0 min.
A) 3.1 × 10-3 M
B) 6.9 × 10-3 M
C) 1.6 × 10-3 M
D) 2.8 × 10-22 M
E) none of these
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate = k<sub>1</sub>[A]<sup>2</sup><sup> </sup>In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min. Calculate the concentration of A<sub>2</sub> after 3.0 min.</strong> A) 3.1 × 10<sup>-3</sup> M B) 6.9 × 10<sup>-3</sup> M C) 1.6 × 10<sup>-3</sup> M D) 2.8 × 10<sup>-22</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_138a_892c_2350c25637bf_TB6422_11.jpg)
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate = k<sub>1</sub>[A]<sup>2</sup><sup> </sup>In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min. Calculate the concentration of A<sub>2</sub> after 3.0 min.</strong> A) 3.1 × 10<sup>-3</sup> M B) 6.9 × 10<sup>-3</sup> M C) 1.6 × 10<sup>-3</sup> M D) 2.8 × 10<sup>-22</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_138b_892c_395075742182_TB6422_11.jpg) Both processes are known to be second order in reactant, and k1 is known to be 0.25 L/mol • s at 25° C, where
Both processes are known to be second order in reactant, and k1 is known to be 0.25 L/mol • s at 25° C, whereRate =
![<strong>Two isomers (A and B) of a given compound dimerize as follows: Both processes are known to be second order in reactant, and k<sub>1</sub> is known to be 0.25 L/mol • s at 25° C, where Rate = k<sub>1</sub>[A]<sup>2</sup><sup> </sup>In a particular experiment, A and B were placed in separate containers at 25° C, where [A]<sub>0</sub> = 1.0 × 10<sup>-2</sup> M and [B]<sub>0</sub> = 2.5 × 10<sup>-2</sup> M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min. Calculate the concentration of A<sub>2</sub> after 3.0 min.</strong> A) 3.1 × 10<sup>-3</sup> M B) 6.9 × 10<sup>-3</sup> M C) 1.6 × 10<sup>-3</sup> M D) 2.8 × 10<sup>-22</sup> M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_3a9c_892c_a36b96076f8f_TB6422_11.jpg) k1[A]2
k1[A]2In a particular experiment, A and B were placed in separate containers at 25° C, where [A]0 = 1.0 × 10-2 M and [B]0 = 2.5 × 10-2 M. It was found that [A] = 3[B] after the reactions progressed for 3.0 min.
Calculate the concentration of A2 after 3.0 min.
A) 3.1 × 10-3 M
B) 6.9 × 10-3 M
C) 1.6 × 10-3 M
D) 2.8 × 10-22 M
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
68
The following questions refer to the gas-phase decomposition of chloroethane:C2H5Cl → products
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.
What was the initial concentration of the ethylene chloride?
A) 0.02 M
B) 0.22 M
C) 0.35 M
D) 0.29 M
E) 0.11 M
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.

What was the initial concentration of the ethylene chloride?
A) 0.02 M
B) 0.22 M
C) 0.35 M
D) 0.29 M
E) 0.11 M

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the reaction
3A + B + C → D + E
where the rate law is defined as![<strong>Consider the reaction 3A + B + C → D + E where the rate law is defined as k[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 × 10<sup>-4</sup> M. What is the concentration of C after 10.0 min?</strong> A) 0.330 M B) 1.10 × 10<sup>-5</sup> M C) 1.00 M D) 0.100 M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dec_7749_892c_a9dd13c197aa_TB6422_11_TB6422_11.jpg) k[A]2[B][C]
k[A]2[B][C]
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 × 10-4 M.
What is the concentration of C after 10.0 min?
A) 0.330 M
B) 1.10 × 10-5 M
C) 1.00 M
D) 0.100 M
E) none of these
3A + B + C → D + E
where the rate law is defined as
![<strong>Consider the reaction 3A + B + C → D + E where the rate law is defined as k[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 × 10<sup>-4</sup> M. What is the concentration of C after 10.0 min?</strong> A) 0.330 M B) 1.10 × 10<sup>-5</sup> M C) 1.00 M D) 0.100 M E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9dec_7749_892c_a9dd13c197aa_TB6422_11_TB6422_11.jpg) k[A]2[B][C]
k[A]2[B][C]An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 × 10-4 M.
What is the concentration of C after 10.0 min?
A) 0.330 M
B) 1.10 × 10-5 M
C) 1.00 M
D) 0.100 M
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
70
The following questions refer to the gas-phase decomposition of chloroethane:C2H5Cl → products
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.
What would the concentration be after 5.0 s?
A) 0.08 M
B) 0.02 M
C) 0.12 M
D) 0.13 M
E) 0.19 M
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.

What would the concentration be after 5.0 s?
A) 0.08 M
B) 0.02 M
C) 0.12 M
D) 0.13 M
E) 0.19 M

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
71
The following questions refer to the gas-phase decomposition of chloroethane:C2H5Cl → products
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.
What is the time to half-life?
A) 8.9 s
B) 0.7 s
C) 6.3 s
D) 1.3 s
E) 2.2 s
Experiment shows that the decomposition is first order. The following data show kinetics information for this reaction.

What is the time to half-life?
A) 8.9 s
B) 0.7 s
C) 6.3 s
D) 1.3 s
E) 2.2 s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
72
The following data were collected for the decay of HO2 radicals. ![<strong>The following data were collected for the decay of HO<sub>2</sub> radicals. Which of the following statements is true?</strong> A) The half-life of the reaction is 2 ms. B) A plot of 1/[HO<sub>2</sub>] versus time gives a straight line. C) The rate of the reaction increases with time. D) The decay of HO<sub>2</sub> occurs by a first-order process. E) A plot of ln [HO<sub>2</sub>] versus time is linear with a slope of -k.](https://storage.examlex.com/TB6422/11eaaf91_9ded_88bf_892c_dbb3148517ed_TB6422_00.jpg) Which of the following statements is true?
Which of the following statements is true?
A) The half-life of the reaction is 2 ms.
B) A plot of 1/[HO2] versus time gives a straight line.
C) The rate of the reaction increases with time.
D) The decay of HO2 occurs by a first-order process.
E) A plot of ln [HO2] versus time is linear with a slope of -k.
![<strong>The following data were collected for the decay of HO<sub>2</sub> radicals. Which of the following statements is true?</strong> A) The half-life of the reaction is 2 ms. B) A plot of 1/[HO<sub>2</sub>] versus time gives a straight line. C) The rate of the reaction increases with time. D) The decay of HO<sub>2</sub> occurs by a first-order process. E) A plot of ln [HO<sub>2</sub>] versus time is linear with a slope of -k.](https://storage.examlex.com/TB6422/11eaaf91_9ded_88bf_892c_dbb3148517ed_TB6422_00.jpg) Which of the following statements is true?
Which of the following statements is true?A) The half-life of the reaction is 2 ms.
B) A plot of 1/[HO2] versus time gives a straight line.
C) The rate of the reaction increases with time.
D) The decay of HO2 occurs by a first-order process.
E) A plot of ln [HO2] versus time is linear with a slope of -k.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
73
In 6 M HCl, the complex ion Ru(NH3)63+ decomposes to a variety of products. The reaction is first order in Ru(NH3)63+ and has a half-life of 14 h at 25°C. Under these conditions, how long will it take for the [Ru(NH3)63+] to decrease to 39.0% of its initial value?
A) 4.3h
B) 10h
C) 8.3h
D) 19h
E) 5.5h
A) 4.3h
B) 10h
C) 8.3h
D) 19h
E) 5.5h

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the second-order reaction aA → products (which has a first half-life of 25 s). If the concentration of A after 15.6s is 0.36M, determine the initial concentration of A.
A) 0.58M
B) 0.26M
C) 0.53M
D) 0.14M
E) 0.16M
A) 0.58M
B) 0.26M
C) 0.53M
D) 0.14M
E) 0.16M

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
75
At a particular temperature, N2O5 decomposes according to a first-order rate law with a half-life of 3.0 s. If the initial concentration of N2O5 is 1.0 × 1016 molecules/cm3, what will be the concentration in molecules/cm3 after 10.0 s?
A) 6.3 × 103
B) 9.9 × 1014
C) 9.4 × 102
D) 7.3 × 109
E) 1.8 × 1012
A) 6.3 × 103
B) 9.9 × 1014
C) 9.4 × 102
D) 7.3 × 109
E) 1.8 × 1012

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
76
The reaction A → B + C is known to be zero order in A with a rate constant of 4.8 × 10-2 mol/L • s at 25° C. An experiment was run at 25°C where [A]0 = 2.0M. What is the concentration of B after 4.0s?
A) 1.8M
B) 5.5× 10-1 M
C) 2.0M
D) 1.9× 10-1 M
E) 1.1× 10-1 M
A) 1.8M
B) 5.5× 10-1 M
C) 2.0M
D) 1.9× 10-1 M
E) 1.1× 10-1 M

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
77
The reaction 2NO2 → 2NO + O2 obeys the rate law ![<strong>The reaction 2NO<sub>2</sub> → 2NO + O<sub>2</sub> obeys the rate law 1.40 × 10<sup>-2</sup> [NO<sub>2</sub>]<sup>2</sup> at 500° K.If the initial concentration of NO<sub>2</sub> is 1.00 M, how long will it take for the [NO<sub>2</sub>] to decrease to 25.0% of its initial value?</strong> A) 71.4 s B) 214 s C) 49.5 s D) 1.40 × 10<sup>-2</sup> s E) cannot be determined from these data](https://storage.examlex.com/TB6422/11eaaf91_9ded_61ae_892c_819b291aad27_TB6422_11.jpg) 1.40 × 10-2 [NO2]2 at 500° K.If the initial concentration of NO2 is 1.00 M, how long will it take for the [NO2] to decrease to 25.0% of its initial value?
1.40 × 10-2 [NO2]2 at 500° K.If the initial concentration of NO2 is 1.00 M, how long will it take for the [NO2] to decrease to 25.0% of its initial value?
A) 71.4 s
B) 214 s
C) 49.5 s
D) 1.40 × 10-2 s
E) cannot be determined from these data
![<strong>The reaction 2NO<sub>2</sub> → 2NO + O<sub>2</sub> obeys the rate law 1.40 × 10<sup>-2</sup> [NO<sub>2</sub>]<sup>2</sup> at 500° K.If the initial concentration of NO<sub>2</sub> is 1.00 M, how long will it take for the [NO<sub>2</sub>] to decrease to 25.0% of its initial value?</strong> A) 71.4 s B) 214 s C) 49.5 s D) 1.40 × 10<sup>-2</sup> s E) cannot be determined from these data](https://storage.examlex.com/TB6422/11eaaf91_9ded_61ae_892c_819b291aad27_TB6422_11.jpg) 1.40 × 10-2 [NO2]2 at 500° K.If the initial concentration of NO2 is 1.00 M, how long will it take for the [NO2] to decrease to 25.0% of its initial value?
1.40 × 10-2 [NO2]2 at 500° K.If the initial concentration of NO2 is 1.00 M, how long will it take for the [NO2] to decrease to 25.0% of its initial value?A) 71.4 s
B) 214 s
C) 49.5 s
D) 1.40 × 10-2 s
E) cannot be determined from these data

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the reaction
3A + B + C → D + E
Where the rate law is defined as![<strong>Consider the reaction 3A + B + C → D + E Where the rate law is defined as (1.96× 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup> • s)[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 2.80 × 10<sup>-4</sup> M. What is the half-life for this experiment?</strong> A) 1.40× 10<sup>-5</sup> s B) 3.54× 10<sup>-3</sup> s C) 1.96× 10<sup>2</sup> s D) 7.14× 10<sup>-7</sup> s E) 1.82× 10<sup>1</sup> s](https://storage.examlex.com/TB6422/11eaaf91_9dec_7747_892c_5d49ae88a17c_TB6422_11.jpg)
![<strong>Consider the reaction 3A + B + C → D + E Where the rate law is defined as (1.96× 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup> • s)[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 2.80 × 10<sup>-4</sup> M. What is the half-life for this experiment?</strong> A) 1.40× 10<sup>-5</sup> s B) 3.54× 10<sup>-3</sup> s C) 1.96× 10<sup>2</sup> s D) 7.14× 10<sup>-7</sup> s E) 1.82× 10<sup>1</sup> s](https://storage.examlex.com/TB6422/11eaaf91_9dec_7748_892c_91073019a153_TB6422_11.jpg)
(1.96× 102 L3/mol3 • s)[A]2[B][C]
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 2.80 × 10-4 M. What is the half-life for this experiment?
A) 1.40× 10-5 s
B) 3.54× 10-3 s
C) 1.96× 102 s
D) 7.14× 10-7 s
E) 1.82× 101 s
3A + B + C → D + E
Where the rate law is defined as
![<strong>Consider the reaction 3A + B + C → D + E Where the rate law is defined as (1.96× 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup> • s)[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 2.80 × 10<sup>-4</sup> M. What is the half-life for this experiment?</strong> A) 1.40× 10<sup>-5</sup> s B) 3.54× 10<sup>-3</sup> s C) 1.96× 10<sup>2</sup> s D) 7.14× 10<sup>-7</sup> s E) 1.82× 10<sup>1</sup> s](https://storage.examlex.com/TB6422/11eaaf91_9dec_7747_892c_5d49ae88a17c_TB6422_11.jpg)
![<strong>Consider the reaction 3A + B + C → D + E Where the rate law is defined as (1.96× 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup> • s)[A]<sup>2</sup>[B][C] An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 2.80 × 10<sup>-4</sup> M. What is the half-life for this experiment?</strong> A) 1.40× 10<sup>-5</sup> s B) 3.54× 10<sup>-3</sup> s C) 1.96× 10<sup>2</sup> s D) 7.14× 10<sup>-7</sup> s E) 1.82× 10<sup>1</sup> s](https://storage.examlex.com/TB6422/11eaaf91_9dec_7748_892c_91073019a153_TB6422_11.jpg)
(1.96× 102 L3/mol3 • s)[A]2[B][C]
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 2.80 × 10-4 M. What is the half-life for this experiment?
A) 1.40× 10-5 s
B) 3.54× 10-3 s
C) 1.96× 102 s
D) 7.14× 10-7 s
E) 1.82× 101 s

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
79
At a particular temperature, the half-life of a zero-order reaction is 29.0min. How long will it take for the reactant concentration to be depleted by a factor of 8?
A) 87.0min
B) 58.0min
C) 50.8min
D) 232min
E) 203min
A) 87.0min
B) 58.0min
C) 50.8min
D) 232min
E) 203min

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the value of k2 where
Rate =![<strong>Calculate the value of k<sub>2</sub> where Rate = K<sub>2</sub>[B]<sup>2</sup></strong> A) 0.75 L/mol • s B) 2.2 L/mol • s C) 0.21 L/mol • s D) 1.9 L/mol • s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_61ad_892c_3fae435f3156_TB6422_11.jpg)
K2[B]2
A) 0.75 L/mol • s
B) 2.2 L/mol • s
C) 0.21 L/mol • s
D) 1.9 L/mol • s
E) none of these
Rate =
![<strong>Calculate the value of k<sub>2</sub> where Rate = K<sub>2</sub>[B]<sup>2</sup></strong> A) 0.75 L/mol • s B) 2.2 L/mol • s C) 0.21 L/mol • s D) 1.9 L/mol • s E) none of these](https://storage.examlex.com/TB6422/11eaaf91_9ded_61ad_892c_3fae435f3156_TB6422_11.jpg)
K2[B]2
A) 0.75 L/mol • s
B) 2.2 L/mol • s
C) 0.21 L/mol • s
D) 1.9 L/mol • s
E) none of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck


