Deck 13: Alcohols, Phenols, Thiols, and Ethers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 13: Alcohols, Phenols, Thiols, and Ethers
1
Thiols are strong-smelling compounds responsible for
A) fruity odors.
B) sharp odors.
C) flowery odors.
D) skunky or bad smelling odors.
E) salty odors.
A) fruity odors.
B) sharp odors.
C) flowery odors.
D) skunky or bad smelling odors.
E) salty odors.
skunky or bad smelling odors.
2
What is the name for this compound? 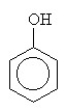
A) cyclopentanol
B) cyclohexanol
C) cyclobenzenol
D) phenol
E) glycerol

A) cyclopentanol
B) cyclohexanol
C) cyclobenzenol
D) phenol
E) glycerol
phenol
3
The condensed structural formula for 2,3-dichloro-4-methylcyclohexanol is
A)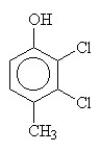
B)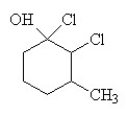
C)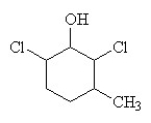
D)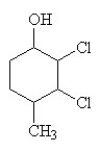
E)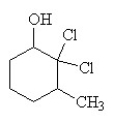
A)

B)

C)

D)

E)


4
The common name of CH3 - CH₂ - O - CH₂ - CH3 is
A) dimethyl ether.
B) diethyl ether.
C) 2-etherbutane.
D) butyl ether.
E) dibutyl ether.
A) dimethyl ether.
B) diethyl ether.
C) 2-etherbutane.
D) butyl ether.
E) dibutyl ether.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
Thiols have structures similar to alcohols except that they contain
A) three alcohol groups.
B) more than one carbon.
C) sulfur in place of oxygen in the functional group.
D) lithium in place of oxygen in the functional group.
E) nitrogen in place of oxygen in the functional group.
A) three alcohol groups.
B) more than one carbon.
C) sulfur in place of oxygen in the functional group.
D) lithium in place of oxygen in the functional group.
E) nitrogen in place of oxygen in the functional group.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following compounds is a thiol?
A)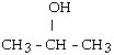
B)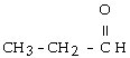
C) CH3 - CH = CH - CH₂ - CH3
D) CH3 - CH₂ - O - CH₂ - CH3
E) CH3 - SH
A)
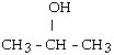
B)
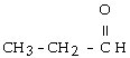
C) CH3 - CH = CH - CH₂ - CH3
D) CH3 - CH₂ - O - CH₂ - CH3
E) CH3 - SH

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following compounds is an alcohol?
A)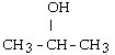
B)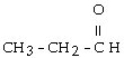
C) CH3 - CH= CH - CH₂ - CH3
D) CH3 - CH₂ - O - CH₂ - CH3
E) CH3 - SH
A)
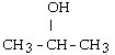
B)
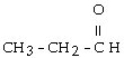
C) CH3 - CH= CH - CH₂ - CH3
D) CH3 - CH₂ - O - CH₂ - CH3
E) CH3 - SH

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name of this compound? 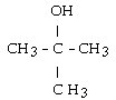
A) butanol
B) propanol
C) 2-propanol
D) 2-methylbutanol
E) 2-methyl-2-propanol

A) butanol
B) propanol
C) 2-propanol
D) 2-methylbutanol
E) 2-methyl-2-propanol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
1,2-ethanediol (ethylene diol) has uses that include
A) antifreeze.
B) solvent for paint.
C) production of synthetic fibers.
D) solvent for ink.
E) All of the above.
A) antifreeze.
B) solvent for paint.
C) production of synthetic fibers.
D) solvent for ink.
E) All of the above.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
What is the name for this compound? 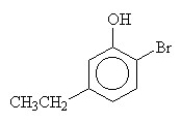
A) o-bromo-m-ethylphenol
B) 2-bromo-5-ethylphenol
C) 4-bromo-1-ethyl-5-phenol
D) 6-bromo-3-ethylphenol
E) 2-bromo-5-methylphenol

A) o-bromo-m-ethylphenol
B) 2-bromo-5-ethylphenol
C) 4-bromo-1-ethyl-5-phenol
D) 6-bromo-3-ethylphenol
E) 2-bromo-5-methylphenol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
A phenol has an -OH group bonded to a(n)
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) aromatic carbon.
E) tetrasubstituted carbon.
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) aromatic carbon.
E) tetrasubstituted carbon.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name of this compound? 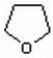
A) pyran
B) tetrahydropyran
C) tetrahydrofuran
D) furan
E) oxycyclopentane

A) pyran
B) tetrahydropyran
C) tetrahydrofuran
D) furan
E) oxycyclopentane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
The common name for the compound CH3 - CH₂ - CH₂ - O - CH₂ - CH3 is
A) 3-pentanol.
B) ethyl propyl ether.
C) 3-hexanol.
D) 3-ether pentane.
E) ethyl propyl ketone.
A) 3-pentanol.
B) ethyl propyl ether.
C) 3-hexanol.
D) 3-ether pentane.
E) ethyl propyl ketone.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name for this compound? 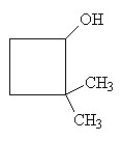
A) methylcyclobutanol
B) o-methylcyclobutanol
C) 2-hydroxy-1, 1-dimethylcyclobutane
D) 2-dimethyl-1-cyclobutanol
E) 2,2-dimethylcyclobutanol

A) methylcyclobutanol
B) o-methylcyclobutanol
C) 2-hydroxy-1, 1-dimethylcyclobutane
D) 2-dimethyl-1-cyclobutanol
E) 2,2-dimethylcyclobutanol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name for CH3 - CH₂ - CH₂ - SH?
A) 1-propanethiol
B) 2-propanethiol
C) 1-butanethiol
D) 2-butanethiol
E) propyl thiol
A) 1-propanethiol
B) 2-propanethiol
C) 1-butanethiol
D) 2-butanethiol
E) propyl thiol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
What is the name of this compound? 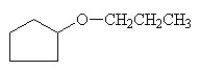
A) cyclopentyl propyl ether
B) cyclopentyl propyl ketone
C) 1-cyclopropyl-1-propylalcohol
D) propylcyclopentanol
E) 3-cyclopentylpropanol
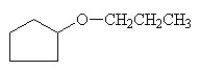
A) cyclopentyl propyl ether
B) cyclopentyl propyl ketone
C) 1-cyclopropyl-1-propylalcohol
D) propylcyclopentanol
E) 3-cyclopentylpropanol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the IUPAC name for the compound below? 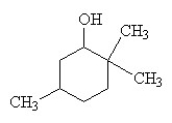
A) 1,1,4-trimethyl-6-cyclohexanol
B) 1,1,4-trimethyl-2-cyclohexanol
C) 2,2,5-trimethylcyclohexanol
D) 2,2,5-trimethylphenol
E) 2-ethyl-5-methylcyclohexanol

A) 1,1,4-trimethyl-6-cyclohexanol
B) 1,1,4-trimethyl-2-cyclohexanol
C) 2,2,5-trimethylcyclohexanol
D) 2,2,5-trimethylphenol
E) 2-ethyl-5-methylcyclohexanol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
The compound CH3 - CH₂ - SH is in the organic family known as
A) ethers.
B) thiols.
C) alcohols.
D) sulfides.
E) amino acids.
A) ethers.
B) thiols.
C) alcohols.
D) sulfides.
E) amino acids.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Methanol is used in all the following except
A) making plastics.
B) racing fuel.
C) alcoholic beverages.
D) solvents.
E) paint remover.
A) making plastics.
B) racing fuel.
C) alcoholic beverages.
D) solvents.
E) paint remover.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
Alcohols contain which functional group?
A) amine
B) amide
C) hydroxyl
D) thiol
A) amine
B) amide
C) hydroxyl
D) thiol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
The dehydration product of CH3 - CH₂ - CH₂ - CH₂ - OH in the presence of acid is
A) CH₂ = C = CH₂.
B) cyclopropane.
C) cyclopropene.
D) propene.
E) propyne.
A) CH₂ = C = CH₂.
B) cyclopropane.
C) cyclopropene.
D) propene.
E) propyne.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
What is the structural formula of the ether formed in this reaction? 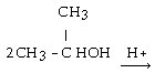
A)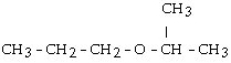
B)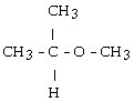
C) CH3 - CH₂ - CH₂ - O - CH₂ - CH₂ - CH3
D)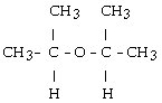
E) CH3 - CH₂ - O - CH₂ - CH3

A)
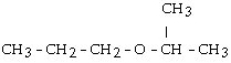
B)

C) CH3 - CH₂ - CH₂ - O - CH₂ - CH₂ - CH3
D)
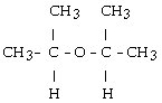
E) CH3 - CH₂ - O - CH₂ - CH3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
What classification of alcohol is resistant to oxidation?
A) primary
B) secondary
C) tertiary
D) quaternary
E) none
A) primary
B) secondary
C) tertiary
D) quaternary
E) none

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
What is the common name of the ether that is a structural isomer of 2-propanol?
A) isopropyl ether
B) diethyl ether
C) dimethyl ether
D) ethyl methyl ether
E) 1-propanol
A) isopropyl ether
B) diethyl ether
C) dimethyl ether
D) ethyl methyl ether
E) 1-propanol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
When 2-methyl-2-butanol undergoes dehydration in acid, one product is
A) 2-methyl-2-butene.
B) 2-methylbutanone.
C) 2-pentanone.
D) 2-methylbutanal.
E) hexene.
A) 2-methyl-2-butene.
B) 2-methylbutanone.
C) 2-pentanone.
D) 2-methylbutanal.
E) hexene.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
Why has diethyl ether been replaced as a general anesthetic?
A) It causes unpleasant side effects in many patients.
B) It is slightly polar.
C) It can hydrogen bond to water.
D) It is not very reactive.
E) It is slightly soluble in water.
A) It causes unpleasant side effects in many patients.
B) It is slightly polar.
C) It can hydrogen bond to water.
D) It is not very reactive.
E) It is slightly soluble in water.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
In a tertiary alcohol, how many alkyl groups are attached to the carbon atom bonded to the -OH group?
A) none
B) one
C) two
D) three
E) four
A) none
B) one
C) two
D) three
E) four

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
Tertiary alcohols cannot be oxidized because
A) there are no oxygen atoms to remove from the alcohol carbon.
B) there are no hydrogen atoms attached to the alcohol carbon.
C) the alcohol carbon is bonded to four groups so no oxygen can be added to it.
D) the alcohol carbon is bonded to four groups so no hydrogen can be added to it.
E) the alcohol carbon is too electronegative to have hydrogen removed from it.
A) there are no oxygen atoms to remove from the alcohol carbon.
B) there are no hydrogen atoms attached to the alcohol carbon.
C) the alcohol carbon is bonded to four groups so no oxygen can be added to it.
D) the alcohol carbon is bonded to four groups so no hydrogen can be added to it.
E) the alcohol carbon is too electronegative to have hydrogen removed from it.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following compounds is a secondary alcohol?
A)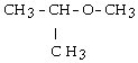
B) CH3OH
C)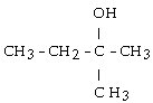
D)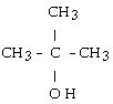
E)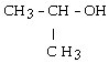
A)
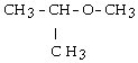
B) CH3OH
C)
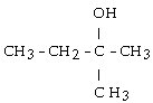
D)

E)
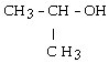

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
A primary alcohol has a hydroxyl group bonded to a(n)
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) aromatic carbon.
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) aromatic carbon.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
The dehydration of an alcohol in the presence of a strong acid yields
A) an alkane.
B) an alkene.
C) a ketone.
D) an alcohol.
E) an aldehyde.
A) an alkane.
B) an alkene.
C) a ketone.
D) an alcohol.
E) an aldehyde.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
What kind of bonds do alcohols form between individual molecules?
A) oxygen bonds
B) hydrogen bonds
C) single bonds
D) carbon bonds
E) ionic bonds
A) oxygen bonds
B) hydrogen bonds
C) single bonds
D) carbon bonds
E) ionic bonds

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
What is the common name of the ether that is a structural isomer of 1-butanol?
A) isopropyl ether
B) diethyl ether
C) dimethyl ether
D) ethyl methyl ether
E) butyl ether
A) isopropyl ether
B) diethyl ether
C) dimethyl ether
D) ethyl methyl ether
E) butyl ether

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
What classification of alcohol undergoes oxidation to yield a ketone?
A) primary alcohol
B) both primary and secondary alcohols
C) secondary alcohol
D) all classes of alcohols
E) both secondary and tertiary alcohols
A) primary alcohol
B) both primary and secondary alcohols
C) secondary alcohol
D) all classes of alcohols
E) both secondary and tertiary alcohols

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following compounds is a weak acid?
A) ethanol
B) ethanal
C) phenol
D) cyclohexanol
E) acetone
A) ethanol
B) ethanal
C) phenol
D) cyclohexanol
E) acetone

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
The alcohol in this list that would be most soluble in water is
A) ethanol.
B) 1-butanol.
C) 1-pentanol.
D) 1-hexanol.
E) 1-heptanol.
A) ethanol.
B) 1-butanol.
C) 1-pentanol.
D) 1-hexanol.
E) 1-heptanol.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
In the oxidation of an alcohol to a ketone, there is
A) a loss of hydrogen.
B) a loss of oxygen.
C) a loss of carbon.
D) a gain of hydrogen.
E) a gain of oxygen.
A) a loss of hydrogen.
B) a loss of oxygen.
C) a loss of carbon.
D) a gain of hydrogen.
E) a gain of oxygen.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
A tertiary alcohol has a hydroxyl group bonded to a(n)
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) triple-bonded carbon.
E) double bonded carbon.
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) triple-bonded carbon.
E) double bonded carbon.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
What is the product when this compound undergoes gentle oxidation? 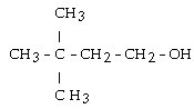
A) hexanal
B) 2,2-dimethylbutanal
C) 2,2-dimethyl-4-butanone
D) 3,3-dimethyl-1-butanone
E) 3,3-dimethylbutanal

A) hexanal
B) 2,2-dimethylbutanal
C) 2,2-dimethyl-4-butanone
D) 3,3-dimethyl-1-butanone
E) 3,3-dimethylbutanal

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
A secondary alcohol has a hydroxyl group bonded to a(n)
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) aromatic carbon.
A) singly substituted or unsubstituted carbon.
B) disubstituted carbon.
C) trisubstituted carbon.
D) aromatic carbon.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Tertiary alcohols are oxidized to
A) secondary alcohols.
B) ketones.
C) aldehydes.
D) carboxylic acids.
E) None of the above.
A) secondary alcohols.
B) ketones.
C) aldehydes.
D) carboxylic acids.
E) None of the above.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Ethers can be formed from the reaction of
A) water and an alkene.
B) water and an alcohol.
C) two alkenes.
D) two alcohols.
E) a ketone and an alkene.
A) water and an alkene.
B) water and an alcohol.
C) two alkenes.
D) two alcohols.
E) a ketone and an alkene.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Secondary alcohols are oxidized to
A) carboxylic acids.
B) ketones.
C) aldehydes.
D) esters.
E) ethers.
A) carboxylic acids.
B) ketones.
C) aldehydes.
D) esters.
E) ethers.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
Which functional group below contains the most highly oxidized carbon?
A) alkane
B) alcohol
C) aldehyde
D) ketone
E) carboxylic acid
A) alkane
B) alcohol
C) aldehyde
D) ketone
E) carboxylic acid

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
In the dehydration of an alcohol to an alkene, what is produced in addition to the alkene?
A) water
B) hydrogen
C) oxygen
D) carbon dioxide
E) carbon monoxide
A) water
B) hydrogen
C) oxygen
D) carbon dioxide
E) carbon monoxide

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Ethylene glycol and methanol are toxic because they are __________ by the liver to carboxylic acids.
A) reduced
B) protonated
C) oxidized
D) conjugated
E) hydrated
A) reduced
B) protonated
C) oxidized
D) conjugated
E) hydrated

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Thiols can be gently oxidized to
A) disulfides.
B) aldehydes.
C) ketones.
D) carboxylic acids.
E) thioethers.
A) disulfides.
B) aldehydes.
C) ketones.
D) carboxylic acids.
E) thioethers.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Methanol, 2-propanol, and ethylene glycol are all toxic when ingested.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
What is the product when the following compound is oxidized? 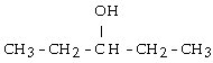
A) diethyl ketone
B) 2-pentene
C) pentanal
D) diethyl ether
E) pentane
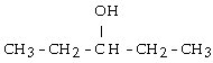
A) diethyl ketone
B) 2-pentene
C) pentanal
D) diethyl ether
E) pentane

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
Alcohols, ethers, and phenols contain oxygen with only single bonds.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Pyran is a cyclic ether.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Ethyl ether has been replaced by halogenated anesthetics, which have fewer side effects.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
Thiols usually have sweet odors.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
The dehydration product of 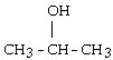 in the presence of acid is
in the presence of acid is
A) CH₂ = C = CH₂.
B) cyclopropane.
C) cyclopropene.
D) propene.
E) propyne.
 in the presence of acid is
in the presence of acid isA) CH₂ = C = CH₂.
B) cyclopropane.
C) cyclopropene.
D) propene.
E) propyne.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
When two molecules of methanol are heated with acid,what is the product?
A) CH3-OH
B) CH3 - O - CH3
C) CH₂ = CH₂
D) CH₂
E) CH3 - CH₂- OH
A) CH3-OH
B) CH3 - O - CH3
C) CH₂ = CH₂
D) CH₂
E) CH3 - CH₂- OH

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
1,2,3-propanetriol is obtained during the manufacture of soap.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Which compound will undergo oxidation to yield cyclopentanone?
A) pentanol
B) cyclopentane
C) cyclopentanol
D) methylcyclobutanol
E) cyclopentene
A) pentanol
B) cyclopentane
C) cyclopentanol
D) methylcyclobutanol
E) cyclopentene

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
When a primary alcohol is completely oxidized, the product is
A) another alcohol.
B) a carboxylic acid.
C) an aldehyde.
D) an alkane.
E) a ketone.
A) another alcohol.
B) a carboxylic acid.
C) an aldehyde.
D) an alkane.
E) a ketone.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Glycerin strongly attracts and holds water, a property that makes it useful as a skin softener in cosmetics.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Alcohols, ethers, and thiols contain oxygen atoms.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
The oxygen atom in alcohols decreases water solubility of the molecule.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
A secondary alcohol can be easily oxidized to a carboxylic acid.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
Dioxanes are aromatic compounds that contain two oxygen atoms in the ring.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
Ethers can only be straight chain compounds.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
Heptanol is a water soluble alcohol.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
Secondary alcohols can be oxidized to ketones.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Alcohols can form hydrogen bonds.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Cycloalkanols are straight chain alcohols.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
Primary alcohols can be oxidized to either aldehydes or ketones.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
Phenols behave as weak acids in water.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
Furan is a compound that contains oxygen in the ring.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Alcohols form hydrogen bonds; this accounts for their higher boiling points when compared to similar-sized alkanes.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
Alcohols can be dehydrated to form alkenes.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
Tertiary alcohols are easily oxidized.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


