Deck 4: Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 4: Atoms
1
silver
A)S
B)Si
C)Ag
D)Au
E)AG
A)S
B)Si
C)Ag
D)Au
E)AG
Ag
2
sodium
A)So
B)Na
C)No
D)Sm
E)Au
A)So
B)Na
C)No
D)Sm
E)Au
Na
3
Which of the following is a characteristic of nonmetals?
A)shiny
B)malleable
C)good conductors of heat
D)low melting points
E)good conductors of electricity
A)shiny
B)malleable
C)good conductors of heat
D)low melting points
E)good conductors of electricity
low melting points
4
The Group 8A(18) elements
A)are unreactive and are rarely found in combination with other elements.
B)are good conductors of electricity.
C)melt at high temperatures.
D)are liquids at room temperature.
E)react vigorously with water.
A)are unreactive and are rarely found in combination with other elements.
B)are good conductors of electricity.
C)melt at high temperatures.
D)are liquids at room temperature.
E)react vigorously with water.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the noble gas in the following list.
A)helium
B)nitrogen
C)oxygen
D)gold
E)chlorine
A)helium
B)nitrogen
C)oxygen
D)gold
E)chlorine

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
What elements are in hydroxyapatite, Ca5 (PO₄)3OH, a major compound in human bones and teeth?
A)carbon, potassium, oxygen, hydrogen
B)calcium, phosphorus, oxygen, hydrogen
C)carbon, phosphorus, oxygen, helium
D)calcium, phosphorus, oxygen, helium
E)carbon, potassium, oxygen, helium
A)carbon, potassium, oxygen, hydrogen
B)calcium, phosphorus, oxygen, hydrogen
C)carbon, phosphorus, oxygen, helium
D)calcium, phosphorus, oxygen, helium
E)carbon, potassium, oxygen, helium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following elements is a nonmetal?
A)nitrogen
B)sodium
C)iron
D)silver
E)calcium
A)nitrogen
B)sodium
C)iron
D)silver
E)calcium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
potassium
A)P
B)Po
C)Pt
D)K
E)Ko
A)P
B)Po
C)Pt
D)K
E)Ko

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
Ca is the symbol for
A)calcium.
B)carbon.
C)cobalt.
D)copper.
E)cadmium.
A)calcium.
B)carbon.
C)cobalt.
D)copper.
E)cadmium.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following elements is a noble gas?
A)oxygen
B)chlorine
C)bromine
D)argon
E)nitrogen
A)oxygen
B)chlorine
C)bromine
D)argon
E)nitrogen

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following properties is NOT a characteristic of the Group 1A(1) elements (alkali metals)?
A)They are shiny.
B)They are good conductors of heat.
C)They react vigorously with water.
D)Most of them are liquids at room temperature.
E)They are good conductors of electricity.
A)They are shiny.
B)They are good conductors of heat.
C)They react vigorously with water.
D)Most of them are liquids at room temperature.
E)They are good conductors of electricity.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
The primary substances of which all other things are composed are
A)molecules.
B)compounds.
C)elements.
D)electrons.
E)protons.
A)molecules.
B)compounds.
C)elements.
D)electrons.
E)protons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
What is the symbol of the element in Period 4 and Group 2?
A)Be
B)Mg
C)Ca
D)C
E)Si
A)Be
B)Mg
C)Ca
D)C
E)Si

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
What is the symbol of the element in Group 4A(14) and Period 2?
A)Be
B)Mg
C)Ca
D)C
E)Si
A)Be
B)Mg
C)Ca
D)C
E)Si

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is a characteristic of the modern periodic table?
A)A group is a horizontal row on the periodic table.
B)A period is a column on the periodic table.
C)The elements in each group have similar chemical properties.
D)The B groups contain the representative elements.
E)The A groups contain the transition elements.
A)A group is a horizontal row on the periodic table.
B)A period is a column on the periodic table.
C)The elements in each group have similar chemical properties.
D)The B groups contain the representative elements.
E)The A groups contain the transition elements.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Au is the symbol for
A)gold.
B)silver.
C)argon.
D)aluminum.
E)sodium.
A)gold.
B)silver.
C)argon.
D)aluminum.
E)sodium.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following elements is a metal?
A)nitrogen
B)fluorine
C)argon
D)strontium
E)phosphorus
A)nitrogen
B)fluorine
C)argon
D)strontium
E)phosphorus

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
aluminum
A)Al
B)Am
C)Au
D)Sn
E)Ag
A)Al
B)Am
C)Au
D)Sn
E)Ag

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
Which element would have physical and chemical properties similar to chlorine?
A)Ar
B)Br
C)S
D)O
E)P
A)Ar
B)Br
C)S
D)O
E)P

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
iron
A)Ir
B)Fs
C)Fe
D)In
E)FE
A)Ir
B)Fs
C)Fe
D)In
E)FE

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
How many protons are in an isotope of sodium with a mass number of 25?
A)11
B)14
C)15
D)25
E)32
A)11
B)14
C)15
D)25
E)32

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Consider an isotope of sodium with a mass number of 25. The number of neutrons in this isotope of sodium is
A)11.
B)14.
C)16.
D)25.
E)32.
A)11.
B)14.
C)16.
D)25.
E)32.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following gives the correct numbers of protons, neutrons, and electrons in a neutral atom of 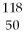 Sn?
Sn?
A)118 protons, 50 neutrons, 118 electrons
B)118 protons, 118 neutrons, 50 electrons
C)50 protons, 68 neutrons, 50 electrons
D)68 protons, 68 neutrons, 50 electrons
E)50 protons, 50 neutrons, 50 electrons
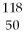 Sn?
Sn?A)118 protons, 50 neutrons, 118 electrons
B)118 protons, 118 neutrons, 50 electrons
C)50 protons, 68 neutrons, 50 electrons
D)68 protons, 68 neutrons, 50 electrons
E)50 protons, 50 neutrons, 50 electrons

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following descriptions of a subatomic particle is correct?
A)A proton has a positive charge and a mass of approximately 1 amu.
B)An electron has a negative charge and a mass of approximately 1 amu.
C)A neutron has no charge and its mass is negligible.
D)A proton has a positive charge and a negligible mass.
E)A neutron has a positive charge and a mass of approximately 1 amu.
A)A proton has a positive charge and a mass of approximately 1 amu.
B)An electron has a negative charge and a mass of approximately 1 amu.
C)A neutron has no charge and its mass is negligible.
D)A proton has a positive charge and a negligible mass.
E)A neutron has a positive charge and a mass of approximately 1 amu.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
Semiconductors are located in the periodic table on (or in) the
A)left side of the table.
B)right side of the table.
C)line dividing metals from nonmetals in the table.
D)first period of the table.
E)last period of the table.
A)left side of the table.
B)right side of the table.
C)line dividing metals from nonmetals in the table.
D)first period of the table.
E)last period of the table.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
The correct symbol for the isotope of potassium with 22 neutrons is
A)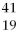
K.
B)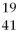
K.
C)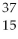
P.
D)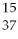
P.
E)
K.
A)
K.
B)
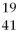
K.
C)
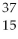
P.
D)
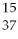
P.
E)
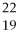
K.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
The element in this list with chemical properties similar to magnesium is
A)sodium.
B)boron.
C)carbon.
D)strontium.
E)chlorine.
A)sodium.
B)boron.
C)carbon.
D)strontium.
E)chlorine.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Consider a neutral atom with 30 protons and 34 neutrons. The number of electrons in this atom is
A)30.
B)32.
C)34.
D)64.
E)94.
A)30.
B)32.
C)34.
D)64.
E)94.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Given the following: 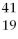 X,
X,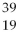 X,
X, 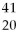 X, and
X, and 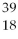 X. Which are isotopes of each other?
X. Which are isotopes of each other?
A)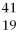 X and
X and 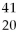 X are isotopes of each other; and
X are isotopes of each other; and 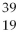 Xand
Xand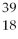 X are isotopes of each other.
X are isotopes of each other.
B)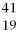 X and
X and 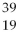 X are isotopes of each other.
X are isotopes of each other.
C)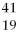 X,
X,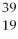 X,
X,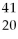 X,and
X,and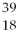 X are isotopes of each other.
X are isotopes of each other.
D)None are isotopes of each other.
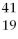 X,
X,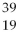 X,
X, 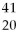 X, and
X, and 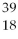 X. Which are isotopes of each other?
X. Which are isotopes of each other?A)
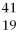 X and
X and 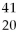 X are isotopes of each other; and
X are isotopes of each other; and 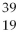 Xand
Xand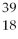 X are isotopes of each other.
X are isotopes of each other.B)
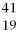 X and
X and 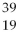 X are isotopes of each other.
X are isotopes of each other.C)
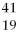 X,
X,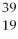 X,
X,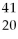 X,and
X,and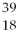 X are isotopes of each other.
X are isotopes of each other.D)None are isotopes of each other.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
Isotopes are atoms of the same element that have
A)different atomic numbers.
B)the same atomic numbers but different numbers of protons.
C)the same atomic numbers but different numbers of electrons.
D)the same atomic number but different numbers of neutrons.
E)the same atomic mass but different numbers of protons.
A)different atomic numbers.
B)the same atomic numbers but different numbers of protons.
C)the same atomic numbers but different numbers of electrons.
D)the same atomic number but different numbers of neutrons.
E)the same atomic mass but different numbers of protons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
The number of neutrons in an atom is equal to
A)the atomic number.
B)the mass number.
C)the mass number + the atomic number.
D)the mass number - the atomic number.
E)the number of protons.
A)the atomic number.
B)the mass number.
C)the mass number + the atomic number.
D)the mass number - the atomic number.
E)the number of protons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
The mass number of an atom can be calculated from
A)the number of electrons.
B)the number of protons plus neutrons.
C)the number of protons.
D)the number of electrons plus protons.
E)the number of neutrons.
A)the number of electrons.
B)the number of protons plus neutrons.
C)the number of protons.
D)the number of electrons plus protons.
E)the number of neutrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
Consider a neutral atom with 30 protons and 34 neutrons. The atomic number of the element is
A)30.
B)32.
C)34.
D)64.
E)94.
A)30.
B)32.
C)34.
D)64.
E)94.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
Identify the metalloid in the following list.
A)sulfur
B)fluorine
C)silver
D)copper
E)germanium
A)sulfur
B)fluorine
C)silver
D)copper
E)germanium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Consider a neutral atom with 30 protons and 34 neutrons. The mass number for this atom is
A)30.
B)32.
C)34.
D)64.
E)94.
A)30.
B)32.
C)34.
D)64.
E)94.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
The atomic number of an atom is equal to the number of
A)nuclei.
B)neutrons.
C)neutrons plus protons.
D)electrons plus protons.
E)protons.
A)nuclei.
B)neutrons.
C)neutrons plus protons.
D)electrons plus protons.
E)protons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
What is the mass number of an atom of potassium that has 20 neutrons?
A)15
B)19
C)35
D)39
E)59
A)15
B)19
C)35
D)39
E)59

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
In an atom, the nucleus contains
A)an equal number of protons and electrons.
B)all the protons and neutrons.
C)all the protons and electrons.
D)only neutrons.
E)only protons.
A)an equal number of protons and electrons.
B)all the protons and neutrons.
C)all the protons and electrons.
D)only neutrons.
E)only protons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
According to the Atomic Theory,
A)all atoms are different.
B)atoms are neither created nor destroyed during a chemical reaction.
C)atoms of the same element combine to form compounds.
D)all matter is made up of tiny particles called electrons.
E)a compound can contain different numbers of atoms as long as it has the same kinds of atoms.
A)all atoms are different.
B)atoms are neither created nor destroyed during a chemical reaction.
C)atoms of the same element combine to form compounds.
D)all matter is made up of tiny particles called electrons.
E)a compound can contain different numbers of atoms as long as it has the same kinds of atoms.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
The smallest particle of an element that retains the characteristics of the element is a(n)
A)electron.
B)neutron.
C)proton.
D)atom.
E)nucleus.
A)electron.
B)neutron.
C)proton.
D)atom.
E)nucleus.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
What is the electron configuration for aluminum?
A)1s22s22p63s23p1
B)1s22s22p63s23p3
C)1s22s22p63s23p5
D)1s22s22p63s23p6
E)1s22s22p63s23p8
A)1s22s22p63s23p1
B)1s22s22p63s23p3
C)1s22s22p63s23p5
D)1s22s22p63s23p6
E)1s22s22p63s23p8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
What is the element with the abbreviated electron configuration [Kr]5s24d8?
A)Ni
B)Pd
C)Pt
D)Kr
E)Xe
A)Ni
B)Pd
C)Pt
D)Kr
E)Xe

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
The maximum number of electrons that may occupy the third energy level is
A)2.
B)8.
C)10.
D)18.
E)32.
A)2.
B)8.
C)10.
D)18.
E)32.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is NOT true for the atoms 13N, 14N, and 15N?
A)They all have the same mass number.
B)They are isotopes.
C)They all have the same atomic number.
D)They all have 7 protons.
E)They all have 7 electrons.
A)They all have the same mass number.
B)They are isotopes.
C)They all have the same atomic number.
D)They all have 7 protons.
E)They all have 7 electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
The atomic mass of an element is equal to
A)its mass number.
B)its atomic number.
C)one-twelfth of the mass of a carbon-12 atom.
D)a weighted average mass of all of the naturally occurring isotopes of the element.
E)the average mass of all of the naturally occurring isotopes of the element.
A)its mass number.
B)its atomic number.
C)one-twelfth of the mass of a carbon-12 atom.
D)a weighted average mass of all of the naturally occurring isotopes of the element.
E)the average mass of all of the naturally occurring isotopes of the element.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
What is the element with the electron configuration 1s22s22p63s23p5?
A)Be
B)Cl
C)F
D)S
E)Ar
A)Be
B)Cl
C)F
D)S
E)Ar

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
A sample of silicon has three naturally occurring isotopes: Si-28 (mass 28.0 amu); Si-29 (mass 29.0 amu) and Si-30 (mass = 30.0 amu). If the average atomic mass of silicon is 28.1 amu, which isotope is the most abundant?
A)Si-28
B)Si-29
C)Si-30
D)All isotopes have the same natural abundance.
A)Si-28
B)Si-29
C)Si-30
D)All isotopes have the same natural abundance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
What is the correct electron configuration for the lithium atom?
A)1s3
B)2s1
C)1s12s2
D)1s22s1
E)1s22s5
A)1s3
B)2s1
C)1s12s2
D)1s22s1
E)1s22s5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
The elements sodium, magnesium, and silicon
A)are isotopes of each other.
B)are in the same period of elements.
C)have the same number of neutrons.
D)are in the same group.
E)have the same mass number.
A)are isotopes of each other.
B)are in the same period of elements.
C)have the same number of neutrons.
D)are in the same group.
E)have the same mass number.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
The elements lithium, sodium, and potassium
A)are isotopes of each other.
B)are in the same period of elements.
C)have the same number of neutrons.
D)are in the same group.
E)have the same mass number.
A)are isotopes of each other.
B)are in the same period of elements.
C)have the same number of neutrons.
D)are in the same group.
E)have the same mass number.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
The electron arrangement of any particular atom shows
A)the number of isotopes possible.
B)a description of the shape of each energy level.
C)the number of electrons in each energy level.
D)a diagram of an atomic nucleus.
E)the maximum number of electrons each energy level can hold.
A)the number of isotopes possible.
B)a description of the shape of each energy level.
C)the number of electrons in each energy level.
D)a diagram of an atomic nucleus.
E)the maximum number of electrons each energy level can hold.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
What element has the electron configuration 1s22s22p63s23p2?
A)carbon
B)oxygen
C)sulfur
D)iron
E)silicon
A)carbon
B)oxygen
C)sulfur
D)iron
E)silicon

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Valence electrons are electrons located
A)in the outermost energy level of an atom.
B)in the nucleus of an atom.
C)in the innermost energy level of an atom.
D)throughout the atom.
E) in the first three shells of an atom.
A)in the outermost energy level of an atom.
B)in the nucleus of an atom.
C)in the innermost energy level of an atom.
D)throughout the atom.
E) in the first three shells of an atom.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
The number of electron levels in a magnesium atom is
A)1.
B)2.
C)3.
D)4.
E)5.
A)1.
B)2.
C)3.
D)4.
E)5.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
What is the electron configuration for potassium (atomic number 19)?
A)1s22s22p63s23p7
B)1s22s22p6 3s23p53d2
C)1s22s22p83s23p5
D)1s22s22p63s23p64s1
E)1s22s22p63s23p54s1
A)1s22s22p63s23p7
B)1s22s22p6 3s23p53d2
C)1s22s22p83s23p5
D)1s22s22p63s23p64s1
E)1s22s22p63s23p54s1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
A sample of chlorine has two naturally occurring isotopes. The isotope Cl-35 (mass 35.0 amu) makes up 75.8% of the sample, and the isotope Cl-37 (mass = 37.0 amu) makes up 24.3% of the sample. What is the average atomic mass for chlorine?
A)36.0 amu
B)35 amu
C)36.6 amu
D)35.5 amu
E)35.521 amu
A)36.0 amu
B)35 amu
C)36.6 amu
D)35.5 amu
E)35.521 amu

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
The number of valence electrons found in an atom of a Group A element is equal to
A)its atomic number.
B)its mass number.
C)its group number.
D)eight.
E)eight minus the group number.
A)its atomic number.
B)its mass number.
C)its group number.
D)eight.
E)eight minus the group number.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following electron configurations is impossible?
A)1s22s22p63s23p1
B)1s22s42p63s23p3
C)1s22s22p63s23p5
D)1s22s22p63s23p6
E)1s22s22p63s23p3
A)1s22s22p63s23p1
B)1s22s42p63s23p3
C)1s22s22p63s23p5
D)1s22s22p63s23p6
E)1s22s22p63s23p3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
The number of electrons in the outer energy level of a neutral atom of boron (atomic number 5) is
A)2.
B)3.
C)5.
D)8.
E)10.
A)2.
B)3.
C)5.
D)8.
E)10.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
What is the abbreviated electron configuration for nickel (atomic number 28)?
A)[He]2s22p3
B)[Ar]4s23d8
C)[Kr]4s23d8
D)[Ar]4s24p4
E)[Ar]3d8
A)[He]2s22p3
B)[Ar]4s23d8
C)[Kr]4s23d8
D)[Ar]4s24p4
E)[Ar]3d8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
Of the elements: B, C, F, Li, and Na. The element with the smallest ionization energy is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
Ionization energy is
A)the energy an ion acquires from an electron.
B)the energy needed to remove the least tightly bound electron.
C)highest for metals in Group 1A (1).
D)higher for potassium than for lithium.
E)None of the above.
A)the energy an ion acquires from an electron.
B)the energy needed to remove the least tightly bound electron.
C)highest for metals in Group 1A (1).
D)higher for potassium than for lithium.
E)None of the above.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Write in the electronic configuration for the atom shown.
Magnesium
Magnesium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
How many valence electrons are in the electron-dot structures for the elements in group 3A(13)?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
The atomic size of atoms
A)increases going across a period.
B)decreases going across a period.
C)decreases going down within a group.
D)does not change going across a period.
E)None of the above.
A)increases going across a period.
B)decreases going across a period.
C)decreases going down within a group.
D)does not change going across a period.
E)None of the above.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
Write in the electronic configuration for the atom shown.
Sulfur
Sulfur

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is the correct electron-dot structure for carbon?
A)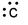
B)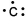
C)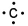
D)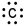
E)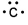
A)
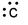
B)
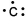
C)
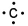
D)
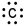
E)
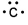

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
Write in the electronic configuration for the atom shown.
Chlorine
Chlorine

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
Of the elements: B, C, F, Li, and Na. The element with the most metallic character is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
Write in the electronic configuration for the atom shown.
Sodium
Sodium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
The number of dots in the electron dot structure of nitrogen is
A)one.
B)two.
C)three.
D)four.
E)five.
A)one.
B)two.
C)three.
D)four.
E)five.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
In an electron-dot structure of an element, the dots are used to represent
A)all of the electrons in the atom.
B)the valence electrons.
C)the electron arrangement.
D)only the electrons that will participate in bond formation.
E)the electrons that the element will gain when it forms a compound.
A)all of the electrons in the atom.
B)the valence electrons.
C)the electron arrangement.
D)only the electrons that will participate in bond formation.
E)the electrons that the element will gain when it forms a compound.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
The number of dots in the electron dot structure of carbon is
A)one.
B)two.
C)three.
D)four.
E)five.
A)one.
B)two.
C)three.
D)four.
E)five.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
Write in the electronic configuration for the atom shown.
Phosphorus
Phosphorus

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Of the elements: B, C, F, Li, and Na, the element with the smallest atomic radius is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
Of the elements: B, C, F, Li, and Na., the element with the largest atomic radius is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
Write in the electronic configuration for the atom shown.
Argon
Argon

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
The ionization energy of atoms
A)decreases going across a period.
B)decreases going down within a group.
C)increases going down within a group.
D)does not change going down within a group.
E)None of the above.
A)decreases going across a period.
B)decreases going down within a group.
C)increases going down within a group.
D)does not change going down within a group.
E)None of the above.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
Of the elements: B, C, F, Li, and Na. The element with the least metallic character is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
Of the elements: B, C, F, Li, and Na. The element with the highest ionization energy is
A)B.
B)C.
C)F.
D)Li.
E)Na.
A)B.
B)C.
C)F.
D)Li.
E)Na.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


