Deck 16: Carboxylic Acids and Esters
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 16: Carboxylic Acids and Esters
1
What is the common name of the compound? O 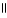 CH₃ - CH2 - CH2 - C - OH is
CH₃ - CH2 - CH2 - C - OH is
A)acetic acid
B)propanoic acid
C)propionic acid
D)butanoic acid
E)butyric acid
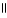 CH₃ - CH2 - CH2 - C - OH is
CH₃ - CH2 - CH2 - C - OH isA)acetic acid
B)propanoic acid
C)propionic acid
D)butanoic acid
E)butyric acid
butyric acid
2
Which of these functional groups is likely to give a sour taste to a food?
A)ester
B)ether
C)ketone
D)carboxylic acid
E)thiol
A)ester
B)ether
C)ketone
D)carboxylic acid
E)thiol
carboxylic acid
3
In the compound below, the hydroxyl group is in which position? O 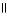 CH₃ - CH2 - CH - C - OH | OH
CH₃ - CH2 - CH - C - OH | OH
A)1
B)2
C)3
D)4
E)5
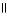 CH₃ - CH2 - CH - C - OH | OH
CH₃ - CH2 - CH - C - OH | OHA)1
B)2
C)3
D)4
E)5
2
4
In the common naming convention for carboxylic acids, what is the correct Greek letter used for the carbon adjacent to the carboxyl group?
A)α
B)β
C)γ
D)δ
E)ε
A)α
B)β
C)γ
D)δ
E)ε

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
W hat is the common name for ethanoic acid?
A)butyric acid
B)formic acid
C)citric acid
D)stearic acid
E)acetic acid
A)butyric acid
B)formic acid
C)citric acid
D)stearic acid
E)acetic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
What therapeutic use is made of α-hydroxy acids?
A)ulcer treatment
B)fever reduction
C)antibiotic
D)reduction of skin pigmentation
E)sunscreen
A)ulcer treatment
B)fever reduction
C)antibiotic
D)reduction of skin pigmentation
E)sunscreen

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
What α-hydroxy acid is found predominantly in grapes?
A)tartaric acid
B)citric acid
C)lactic acid
D)glycolic acid
E)benzoic acid
A)tartaric acid
B)citric acid
C)lactic acid
D)glycolic acid
E)benzoic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name for this compound? CH₃ O | 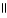 CH₃ - CH - CH2 - C - OH
CH₃ - CH - CH2 - C - OH
A)pentanoic acid
B)2-methylbutanoic acid
C)3-methylbutanoic acid
D)2-methyl butyric acid
E)2-methyl-4-butanoic acid
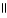 CH₃ - CH - CH2 - C - OH
CH₃ - CH - CH2 - C - OHA)pentanoic acid
B)2-methylbutanoic acid
C)3-methylbutanoic acid
D)2-methyl butyric acid
E)2-methyl-4-butanoic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Which functional group is a carboxylic acid?
A)- OH
B)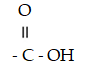
C)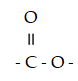
D)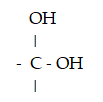
E) - CH2 - OH
A)- OH
B)
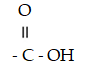
C)
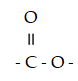
D)
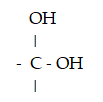
E) - CH2 - OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
What is the irritating acid found in ant and bee stings?
A)acetic acid
B)formic acid
C)citric acid
D)butyric acid
E)stearic acid
A)acetic acid
B)formic acid
C)citric acid
D)butyric acid
E)stearic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
The functional group in acetic acid is called the
A)hydroxyl group.
B)aldehyde group.
C)carbonyl group.
D)carboxyl group.
E)ester group.
A)hydroxyl group.
B)aldehyde group.
C)carbonyl group.
D)carboxyl group.
E)ester group.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
Which organic acid accounts for the odor of rancid butter?
A)propionic acid
B)acetic acid
C)formic acid
D)butyric acid
E)ethanoic acid
A)propionic acid
B)acetic acid
C)formic acid
D)butyric acid
E)ethanoic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
What is the IUPAC name of the following compound? 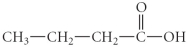
A)propyl butanoate
B)butanoic acid
C)1-butanal
D)1-butanoate
E)propyl methanoate
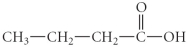
A)propyl butanoate
B)butanoic acid
C)1-butanal
D)1-butanoate
E)propyl methanoate

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
What kind of taste do carboxylic acids have?
A)sweet
B)sour
C)fruity
D)slippery
E)oily
A)sweet
B)sour
C)fruity
D)slippery
E)oily

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name for this compound? CH₃ O | 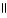 CH₃ - CH - CH2 - C - OH
CH₃ - CH - CH2 - C - OH
A)pentanoic acid
B)γ-methylbutanoic acid
C)3-methylbutanoic acid
D)γ-methyl butyric acid
E)2-methyl-4-butanoic acid
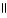 CH₃ - CH - CH2 - C - OH
CH₃ - CH - CH2 - C - OHA)pentanoic acid
B)γ-methylbutanoic acid
C)3-methylbutanoic acid
D)γ-methyl butyric acid
E)2-methyl-4-butanoic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
What is the method of preparing carboxylic acids from alcohols or aldehydes?
A)reduction
B)hydration
C)oxidation
D)saponification
E)hydrolysis
A)reduction
B)hydration
C)oxidation
D)saponification
E)hydrolysis

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
What significant side effect is seen with α-hydroxy acid use?
A)UV sensitivity
B)increased thirst
C)nausea
D)increased susceptibility to infection
E)gastric irritation
A)UV sensitivity
B)increased thirst
C)nausea
D)increased susceptibility to infection
E)gastric irritation

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
A carboxylic acid is prepared from an aldehyde by
A)oxidation.
B)reduction.
C)hydrolysis.
D)neutralization.
E)hydrogenation.
A)oxidation.
B)reduction.
C)hydrolysis.
D)neutralization.
E)hydrogenation.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
The compound below is named 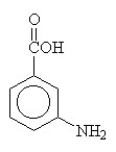
A)benzoic acid amine.
B)3-aminobenzoic acid.
C)2-acid aniline.
D)benzamide.
E)2-aminobenzoic acid.

A)benzoic acid amine.
B)3-aminobenzoic acid.
C)2-acid aniline.
D)benzamide.
E)2-aminobenzoic acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is found in vinegar?
A)nitric acid
B)formic acid
C)acetic acid
D)propionic acid
E)butyric acid
A)nitric acid
B)formic acid
C)acetic acid
D)propionic acid
E)butyric acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
What is the product of the reaction of pentanoic acid with ethanol in the presence of a strong acid?
A)pentyl ethanoate
B)ethyl pentanoate
C)pentyl acetate
D)heptanoic acid
A)pentyl ethanoate
B)ethyl pentanoate
C)pentyl acetate
D)heptanoic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds is most soluble in water?
A)CH₃
- CH2 - CH₃
B)CH₃
- CH2 - CH2 - O - CH₃
C)CH₃
- CH2 - CH2 - CH2 - OH
D) O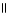
CH₃
- CH2 - CH2 - CH2 - C - OH
E) O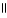
CH₃
- C - OH
A)CH₃
- CH2 - CH₃
B)CH₃
- CH2 - CH2 - O - CH₃
C)CH₃
- CH2 - CH2 - CH2 - OH
D) O
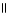
CH₃
- CH2 - CH2 - CH2 - C - OH
E) O
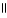
CH₃
- C - OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
What is the common use of monosodium glutamate?
A)as a preservative
B)as a disinfectant
C)as an anti-pyretic
D)as a flavor enhancer
E)as a spoilage inhibitor
A)as a preservative
B)as a disinfectant
C)as an anti-pyretic
D)as a flavor enhancer
E)as a spoilage inhibitor

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
Why do carboxylic acids have higher boiling points than similar alcohols or aldehydes?
A)They form dimers that are relatively stable.
B)They are more water soluble.
C)They have higher molecular weights.
D)They have an additional oxygen atom.
E)The carboxylic acid chain is not linear.
A)They form dimers that are relatively stable.
B)They are more water soluble.
C)They have higher molecular weights.
D)They have an additional oxygen atom.
E)The carboxylic acid chain is not linear.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
A carboxylic acid is named in the IUPAC system by replacing the -e in the name of the parent alkane with
A)-oic acid.
B)-oic.
C)-carboxylic acid.
D)acid.
E)-oate.
A)-oic acid.
B)-oic.
C)-carboxylic acid.
D)acid.
E)-oate.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Which carboxylic acid in the list below is an aromatic carboxylic acid?
A)acetic acid
B)benzoic acid
C)butyric acid
D)benzene
E)citric acid
A)acetic acid
B)benzoic acid
C)butyric acid
D)benzene
E)citric acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
What metabolic product of pyruvic acid is formed anaerobically during exercise?
A)lactic acid
B)citric acid
C)malic acid
D)acetic acid
E)β-ketoglutaric acid
A)lactic acid
B)citric acid
C)malic acid
D)acetic acid
E)β-ketoglutaric acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
What kind of intermolecular bonding occurs between carboxylic acids?
A)ionic bonding
B)nonpolar bonding
C)covalent bonding
D)hydrogen bonding
E)charge-transfer bonding
A)ionic bonding
B)nonpolar bonding
C)covalent bonding
D)hydrogen bonding
E)charge-transfer bonding

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
What common use is made of sodium propionate and sodium benzoate?
A)flavor enhancer
B)preservative
C)pH adjuster
D)disinfectant
E)decongestant
A)flavor enhancer
B)preservative
C)pH adjuster
D)disinfectant
E)decongestant

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the reaction for the ionization of β-hydroxypropanoic acid in water?
A) O OH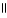
|
HO - CH2 - CH2 - C - OH + H2O ↔ HO - CH2 - CH2 - CH - OH + H+
B) O O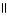
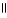
HO - CH2 - CH2 - C - OH + H2O ↔ HO - CH2 - CH2 - C - O- + H₃
O+
C) O O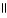
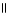
CH₃
- CH - C - OH + H2O ↔ CH₃
- CH - C - O- + H₃
O+
| |
OH OH
D) O O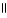
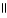
HO - CH2 - CH2 - C - OH + 2 H2O ↔ -OCH2CH2 C O- + 2 H₃
O+
E) O O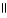
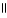
CH₃
CH C OH + H2O ↔ CH₃
- CH - C - OH + H₃
O+
| |
OH O-
A) O OH
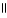
|
HO - CH2 - CH2 - C - OH + H2O ↔ HO - CH2 - CH2 - CH - OH + H+
B) O O
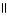
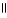
HO - CH2 - CH2 - C - OH + H2O ↔ HO - CH2 - CH2 - C - O- + H₃
O+
C) O O
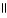
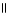
CH₃
- CH - C - OH + H2O ↔ CH₃
- CH - C - O- + H₃
O+
| |
OH OH
D) O O
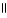
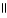
HO - CH2 - CH2 - C - OH + 2 H2O ↔ -OCH2CH2 C O- + 2 H₃
O+
E) O O
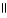
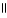
CH₃
CH C OH + H2O ↔ CH₃
- CH - C - OH + H₃
O+
| |
OH O-

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
The reactants that will form an ester in the presence of an acid catalyst are
A)two carboxylic acids.
B)two alcohols.
C)a carboxylic acid and an alcohol.
D)an aldehyde and an alcohol.
E)two aldehydes.
A)two carboxylic acids.
B)two alcohols.
C)a carboxylic acid and an alcohol.
D)an aldehyde and an alcohol.
E)two aldehydes.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is the reaction for the neutralization of β-hydroxybutyric acid with NaOH?
A) OH O OH O
|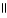
|
CH₃
- CH - CH2 - C- OH + NaOH → CH₃
- CH - CH2 - C - O- Na+ + H2O
B) OH O OH O
|
|
CH₃
- CH - CH2 - C- OH + NaOH → CH₃
- C - CH2 - C - OH + NaH
|
OH
C) OH O OH O
|
|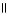
CH₃
- CH2 - CH - C- OH + NaOH → CH₃
- CH2 - CH - C - O- Na+ + H2O
D) OH O O- Na+ O
|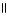
|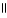
CH₃
- CH - CH2 - C- OH + 2 NaOH → CH₃
- CH - CH2 - C - O- Na+ + 2 H2O
E) O O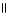
CH₃
- CH2 - CH - C- OH + 2 NaOH → CH₃
- CH2 - CH - C - O- Na+ + 2 H2O
| |
OH OH
A) OH O OH O
|
|
CH₃
- CH - CH2 - C- OH + NaOH → CH₃
- CH - CH2 - C - O- Na+ + H2O
B) OH O OH O
|
|
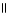
CH₃
- CH - CH2 - C- OH + NaOH → CH₃
- C - CH2 - C - OH + NaH
|
OH
C) OH O OH O
|
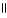
|
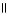
CH₃
- CH2 - CH - C- OH + NaOH → CH₃
- CH2 - CH - C - O- Na+ + H2O
D) OH O O- Na+ O
|
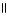
|
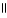
CH₃
- CH - CH2 - C- OH + 2 NaOH → CH₃
- CH - CH2 - C - O- Na+ + 2 H2O
E) O O
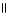
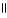
CH₃
- CH2 - CH - C- OH + 2 NaOH → CH₃
- CH2 - CH - C - O- Na+ + 2 H2O
| |
OH OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
Which of these is an acid formed in the citric acid cycle?
A)acetic acid
B)propionic acid
C)α-ketoglutaric acid
D)benzoic acid
E)palmitic acid
A)acetic acid
B)propionic acid
C)α-ketoglutaric acid
D)benzoic acid
E)palmitic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
What happens to water solubility as chain length increases in carboxylic acids?
A)It increases.
B)It decreases.
C)It stays the same.
A)It increases.
B)It decreases.
C)It stays the same.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
The structural formula of the carboxylic acid produced by the oxidation of 2,2-dimethyl-1-propanol is
A) CH₃
O
|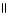
CH₃
C - CH
|
CH₃
B) CH₃
O CH₃
|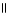
|
CH₃
C - C - O C CH₃
| |
CH₃
CH₃
C) O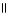
CH₃
CH2CH2CH2 C OH
D) CH₃
O
|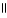
CH₃
C - C OH
|
CH₃
E) CH₃
|
CH₃
C - OH
|
CH₃
A) CH₃
O
|
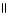
CH₃
C - CH
|
CH₃
B) CH₃
O CH₃
|
|
CH₃
C - C - O C CH₃
| |
CH₃
CH₃
C) O
CH₃
CH2CH2CH2 C OH
D) CH₃
O
|
CH₃
C - C OH
|
CH₃
E) CH₃
|
CH₃
C - OH
|
CH₃

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
Which compound below contains an ester functional group?
A)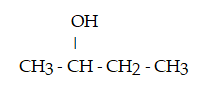
B)CH₃- CH2 - O -CH2- CH₃
C)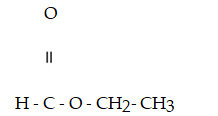
D)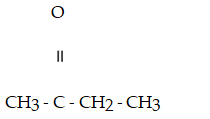
E)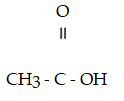
A)
B)CH₃- CH2 - O -CH2- CH₃
C)
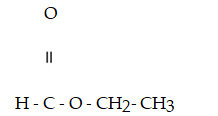
D)
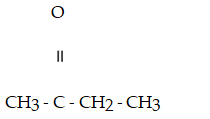
E)
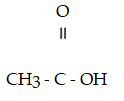

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
In water solution, how does dilute acetic acid behave?
A)as a strong acid
B)as a weak acid
C)as a strong base
D)as a weak base
E)as a neutral compound
A)as a strong acid
B)as a weak acid
C)as a strong base
D)as a weak base
E)as a neutral compound

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
When compared to sulfuric acid, how strong are carboxylic acids?
A)stronger
B)just as strong
C)weaker
D)not acidic at all
A)stronger
B)just as strong
C)weaker
D)not acidic at all

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
The neutralization of formic acid by NaOH produces
A)sodium formate as the only product.
B)formate ion and hydronium ion.
C)sodium formaldehyde.
D)methyl alcohol.
E)sodium formate and H2O.
A)sodium formate as the only product.
B)formate ion and hydronium ion.
C)sodium formaldehyde.
D)methyl alcohol.
E)sodium formate and H2O.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
This functional group is known as a(n) O 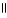 - C - O - C
- C - O - C
A)ester.
B)carboxylic acid.
C)alcohol.
D)aldehyde.
E)acetal.
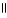 - C - O - C
- C - O - CA)ester.
B)carboxylic acid.
C)alcohol.
D)aldehyde.
E)acetal.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Derivatives of which aromatic carboxylic acid have been used as analgesics, antipyretics, and anti-inflammatory agents?
A)benzoic acid
B)anthranilic acid
C)naphthenic acid
D)p-toluenesulfonic acid
E)salicylic acid
A)benzoic acid
B)anthranilic acid
C)naphthenic acid
D)p-toluenesulfonic acid
E)salicylic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
Which of these compounds is the ester formed from the reaction of acetic acid and 1-propanol?
A) O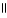
CH₃
- CH2 - C - O - CH2- CH₃
B) OH
|
CH₃
- C - OH
|
O - CH2 - CH2 - CH₃
C) OH
|
CH₃
- CH2 - C - OH
|
O - CH2 - CH₃
D) O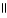
CH₃
- C - O - CH2 - CH2 - CH₃
E) O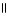
CH₃
- CH2 - CH2 - O - CH2- C - OH
A) O
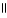
CH₃
- CH2 - C - O - CH2- CH₃
B) OH
|
CH₃
- C - OH
|
O - CH2 - CH2 - CH₃
C) OH
|
CH₃
- CH2 - C - OH
|
O - CH2 - CH₃
D) O
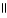
CH₃
- C - O - CH2 - CH2 - CH₃
E) O
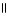
CH₃
- CH2 - CH2 - O - CH2- C - OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is the reaction for the acid hydrolysis of ethyl formate?
A) O O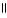
CH₃
- C - O - CH₃
+ NaOH → CH₃
- C - O- Na+ + CH₃
- OH
B) O O
CH₃
- C - O - CH₃
+ H2O → CH₃
- C - OH + CH₃
- OH
C) O O
H - C - O - CH₃
+ H2O → H - C - OH + CH₃
- OH
D) O O
H - C - O - CH2 - CH₃
+ H2O → H - C - OH + CH₃
- CH2 - OH
E) O OH
|
H - C - O - CH2 - CH₃
+ H2O → H - C - O - CH2 - CH₃
|
OH
A) O O
CH₃
- C - O - CH₃
+ NaOH → CH₃
- C - O- Na+ + CH₃
- OH
B) O O
CH₃
- C - O - CH₃
+ H2O → CH₃
- C - OH + CH₃
- OH
C) O O
H - C - O - CH₃
+ H2O → H - C - OH + CH₃
- OH
D) O O
H - C - O - CH2 - CH₃
+ H2O → H - C - OH + CH₃
- CH2 - OH
E) O OH
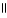
|
H - C - O - CH2 - CH₃
+ H2O → H - C - O - CH2 - CH₃
|
OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
The alcohol and carboxylic acid required to form propyl ethanoate are
A)methanol and propionic acid.
B)ethanol and propionic acid.
C)propanol and propanoic acid.
D)1-propanol and ethanoic acid.
E)2-propanol and ethanoic acid.
A)methanol and propionic acid.
B)ethanol and propionic acid.
C)propanol and propanoic acid.
D)1-propanol and ethanoic acid.
E)2-propanol and ethanoic acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
The splitting apart of an ester in the presence of a strong acid and water is called
A)hydrolysis.
B)saponification.
C)neutralization.
D)esterification.
E)reduction.
A)hydrolysis.
B)saponification.
C)neutralization.
D)esterification.
E)reduction.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
Carboxylic acids are responsible for the sweet taste of fruits and vegetables.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
What is the product of the reaction of an alcohol and a carboxylic acid when reacted together under acidic conditions?
A)an ether
B)an ester
C)a salt
D)a ketone
E)an aldehyde
A)an ether
B)an ester
C)a salt
D)a ketone
E)an aldehyde

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
Which part of a soap is responsible for its ability to dissolve fats and oily dirt?
A)the hydrophilic end
B)the hydrophobic end
C)the carboxylate
D)the ionized oxygen
E)the carbonyl group
A)the hydrophilic end
B)the hydrophobic end
C)the carboxylate
D)the ionized oxygen
E)the carbonyl group

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
What chemical process is responsible for the smell of vinegar in an old bottle of aspirin?
A)reduction
B)hydration
C)hydrolysis
D)esterification
E)dissolution
A)reduction
B)hydration
C)hydrolysis
D)esterification
E)dissolution

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
What is the common name of this compound? O 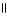 CH₃ - C - O - CH2 - CH₃
CH₃ - C - O - CH2 - CH₃
A)ethyl methyl ester
B)diethyl ester
C)ethyl methanoate
D)2-ether-2-butanone
E)ethyl acetate
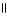 CH₃ - C - O - CH2 - CH₃
CH₃ - C - O - CH2 - CH₃A)ethyl methyl ester
B)diethyl ester
C)ethyl methanoate
D)2-ether-2-butanone
E)ethyl acetate

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
Give the IUPAC name for the following compound. 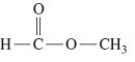
A)methyl formyl ether
B)methyl methanoate
C)methyl ethanoate
D)methyl formate
E)ethanoate
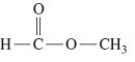
A)methyl formyl ether
B)methyl methanoate
C)methyl ethanoate
D)methyl formate
E)ethanoate

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
Give the IUPAC name for the following compound. 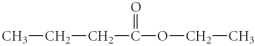
A)ethyl propyl ether
B)ethyl propanoate
C)ethyl butanoate
D)hexanoic acid
E)4-hexanal
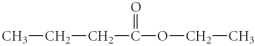
A)ethyl propyl ether
B)ethyl propanoate
C)ethyl butanoate
D)hexanoic acid
E)4-hexanal

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
What is the name of the structure formed when a soap coats an oily particle to make it water soluble?
A)micelle
B)cluster
C)liposome
D)dimer
E)lipid
A)micelle
B)cluster
C)liposome
D)dimer
E)lipid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
The reaction of an ester with NaOH is known as
A)esterification.
B)neutralization.
C)saponification.
D)reduction.
E)oxidation.
A)esterification.
B)neutralization.
C)saponification.
D)reduction.
E)oxidation.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
It is always safe to use any commercial skin care product without doing a test patch first.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
The major acidic component of vinegar is formic acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
From what component is the first part of the IUPAC name of an ester (such as methyl acetate) derived?
A)the carboxylic acid
B)the alcohol
C)the ether
D)the ester
E)the amide
A)the carboxylic acid
B)the alcohol
C)the ether
D)the ester
E)the amide

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
Alpha-hydroxy acids should be used at concentrations under 10% in skin care products.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is the reaction for the saponification of methyl acetate?
A) O O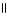
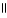
CH₃
- C - O - CH₃
+ NaOH → CH₃
- C - OH + CH₃
- O- Na+
B) O O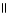
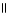
CH₃
- C - O - CH₃
+ NaOH → CH₃
- C - O- Na+ + CH₃
- OH
C) O O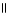
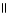
H - C - O - CH₃
+ H2O → H - C - O- + CH₃
- OH
D) O O
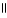
H - C - O - CH₃
+ NaOH → H - C - O- Na+ + CH₃
- OH
E) O O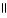
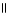
CH₃
- C - O - CH₃
+ H2O → CH₃
- C - O- + CH₃
- OH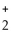
A) O O
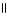
CH₃
- C - O - CH₃
+ NaOH → CH₃
- C - OH + CH₃
- O- Na+
B) O O
CH₃
- C - O - CH₃
+ NaOH → CH₃
- C - O- Na+ + CH₃
- OH
C) O O
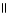
H - C - O - CH₃
+ H2O → H - C - O- + CH₃
- OH
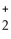
D) O O
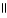
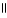
H - C - O - CH₃
+ NaOH → H - C - O- Na+ + CH₃
- OH
E) O O
CH₃
- C - O - CH₃
+ H2O → CH₃
- C - O- + CH₃
- OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Many of the fragrances of flowers and the flavors of fruits are due to
A)ethers.
B)carboxylic acids.
C)esters.
D)amines.
E)amides.
A)ethers.
B)carboxylic acids.
C)esters.
D)amines.
E)amides.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
When benzoic acid is neutralized by sodium hydroxide, sodium benzoate is formed.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
The Krebs cycle is a process that the cell uses to produce energy.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Sodium benzoate is a common preservative.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
Citric acid is an important part of glycolysis.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
Esters are formed from the reaction of an ether with a carboxylic acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
Sodium propionate is a common disinfectant.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
Butyl alcohol is one of the reactants used to make methyl butyrate.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
Polyesters are plastics that are used to make fabrics, bottles, and medical devices such as heart valves.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
Benzoic acid is an aromatic carboxylic acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
The boiling points of carboxylic acids are lower than the corresponding alcohols.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Carboxylic acids with four or fewer carbons are very water soluble.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
Methyl salicylate (oil of wintergreen) is used therapeutically as a counter-irritant.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
The Krebs cycle and the citric acid cycle are different processes.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
When in solution, carboxylic acids are mostly in their ionized forms.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
The IUPAC name of this compound is propanoic acid. 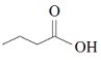


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
An ester is derived from an alcohol and a carboxylic acid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
The ester formed from butyl alcohol and acetic acid is called butyl acetate.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
The IUPAC name of this compound is methyl butanoate. 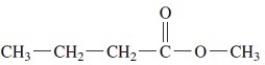
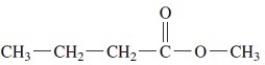

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Carboxylic acids are strong acids.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Carboxylic acids with more than five carbons are very water soluble.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


