Deck 19: The Kinetic Theory of Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 19: The Kinetic Theory of Gases
1
A sample of an ideal gas is compressed by a piston from 10 m3 to 5 m3 and simultaneously cooled from 273 C to 0 C. As a result there is:
A) an increase in pressure
B) a decrease in pressure
C) a decrease in density
D) no change in volume
E) an increase in density
A) an increase in pressure
B) a decrease in pressure
C) a decrease in density
D) no change in volume
E) an increase in density
an increase in density
2
An automobile tire is pumped up to a gauge pressure of 2.0 * 105 Pa when the temperature is 27 C. What is its gauge pressure after the car has been running on a hot day so that the tire temperature is 77 C? Assume that the volume remains fixed and take atmospheric pressure to be 1.013 *105 Pa.
A) 1.6 *105 Pa
B) 2.6 *105 Pa
C) 3.6 *105 Pa
D) 5.9*105 Pa
E) 7.9 * 105 Pa
A) 1.6 *105 Pa
B) 2.6 *105 Pa
C) 3.6 *105 Pa
D) 5.9*105 Pa
E) 7.9 * 105 Pa
1.6 *105 Pa
3
Air enters a hot-air furnace at 7 C and leaves at 77 C. If the pressure does not change each entering cubic foot of air expands to:
A) 0.80 m3
B) 1.25 m3
C) 1.9 m3
D) 7.0 m3
E) 11 m3
A) 0.80 m3
B) 1.25 m3
C) 1.9 m3
D) 7.0 m3
E) 11 m3
1.25 m3
4
A real gas is changed slowly from state 1 to state 2. During this process no work is done on or by the gas. This process must be:
A) isothermal
B) adiabatic
C) isovolumic
D) isobaric
E) a closed cycle with point 1 coinciding with point 2
A) isothermal
B) adiabatic
C) isovolumic
D) isobaric
E) a closed cycle with point 1 coinciding with point 2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
A 2-m3 weather balloon is loosely filled with helium at 1 atm (76 cm Hg) and at 27 C. At an elevation of 20,000 ft, the atmospheric pressure is down to 38 cm Hg and the helium has expanded, being under no constraint from the confining bag. If the temperature at this elevation is -48 C, the gas volume (in m3) is:
A) 3
B) 4
C) 2
D) 2.5
E) 5.3
A) 3
B) 4
C) 2
D) 2.5
E) 5.3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
An ideal gas occupies 12 liters at 293 K and 1 atm (76 cm Hg). Its temperature is now raised to 373 K and its pressure increased to 215 cm Hg. The new volume is:
A) 0.2 liters
B) 5.4 liters
C) 13.6 liters
D) 20.8 liters
E) none of these
A) 0.2 liters
B) 5.4 liters
C) 13.6 liters
D) 20.8 liters
E) none of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
Two identical rooms in a house are connected by an open doorway. The temperatures in the two rooms are maintained at different values. Which room contains more air?
A) the room with higher temperature
B) the room with lower temperature
C) the room with higher pressure
D) neither because both have the same pressure
E) neither because both have the same volume
A) the room with higher temperature
B) the room with lower temperature
C) the room with higher pressure
D) neither because both have the same pressure
E) neither because both have the same volume

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
An air bubble doubles in volume as it rises from the bottom of a lake (1000 kg/m3). Ignoring any temperature changes, the depth of the lake is:
A) 21 m
B) 0.76 m
C) 4.9 m
D) 10 m
E) 0.99 m
A) 21 m
B) 0.76 m
C) 4.9 m
D) 10 m
E) 0.99 m

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
Over 1 cycle of a cyclic process in which a system does net work on its environment:
A) the change in the pressure of the system cannot be zero
B) the change in the volume of the system cannot be zero
C) the change in the temperature of the system cannot be zero
D) the change in the internal energy of the system cannot be zero
E) none of the above
A) the change in the pressure of the system cannot be zero
B) the change in the volume of the system cannot be zero
C) the change in the temperature of the system cannot be zero
D) the change in the internal energy of the system cannot be zero
E) none of the above

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
273 cm3 of an ideal gas is at 0 C. It is heated at constant pressure to 10 C. It will now occupy:
A) 263 cm3
B) 273 cm3
C) 283 cm3
D) 278 cm3
E) 293 cm3
A) 263 cm3
B) 273 cm3
C) 283 cm3
D) 278 cm3
E) 293 cm3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
An isothermal process for an ideal gas is represented on a p-V diagram by:
A) a horizontal line
B) a vertical line
C) a portion of an ellipse
D) a portion of a parabola
E) a hyperbola
A) a horizontal line
B) a vertical line
C) a portion of an ellipse
D) a portion of a parabola
E) a hyperbola

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
A given mass of gas is enclosed in a suitable container so that it may be maintained at constant volume. Under these conditions, there can be no change is what property of the gas?
A) Pressure
B) Density
C) Molecular kinetic energy
D) Internal energy
E) Temperature
A) Pressure
B) Density
C) Molecular kinetic energy
D) Internal energy
E) Temperature

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
Oxygen (molar mass = 32 g) occupies a volume of 12 liters when its temperature is 20 C and its pressure is 1 atm. Using R = 0.082 liter . atm/mole. K, calculate the mass of the oxygen:
A) 6.4 g
B) 10.7 g
C) 16 g
D) 32 g
E) 64 g
A) 6.4 g
B) 10.7 g
C) 16 g
D) 32 g
E) 64 g

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
A real gas undergoes a process which can be represented as a curve on a p-V diagram. The work done by the gas during this process is:
A) pV
B) p(V2 - V1)
C) (p2 - p1)V
D) p dV
E) V dp
A) pV
B) p(V2 - V1)
C) (p2 - p1)V
D) p dV
E) V dp

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
A quantity of an ideal gas is compressed to half its initial volume. The process may be adiabatic, isothermal or isobaric. Rank those three processes in order of the work required of an external agent, least to greatest.
A) adiabatic, isothermal, isobaric
B) adiabatic, isobaric, isothermal
C) isothermal, adiabatic, isobaric
D) isobaric, adiabatic, isothermal
E) isobaric, isothermal, adiabatic
A) adiabatic, isothermal, isobaric
B) adiabatic, isobaric, isothermal
C) isothermal, adiabatic, isobaric
D) isobaric, adiabatic, isothermal
E) isobaric, isothermal, adiabatic

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
Use R = 8.2 *10-5 m3 . atm/mol . K and NA = 6.02 * 1023 mol-1. The approximate number of air molecules in a 1 m3 volume at room temperature (300 K) and atomospheric pressure is:
A) 41
B) 450
C) 2.5 * 1025
D) 2.7 *1026
E) 5.4 *1026
A) 41
B) 450
C) 2.5 * 1025
D) 2.7 *1026
E) 5.4 *1026

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
The pressures p and volumes V of the five ideal gases,with the same number of molecules, are given below. Which has the highest theperatures?
A) p = 1 * 105 Pa and V = 10cm3
B) p = 3 *105 Pa and V = 6 cm3
C) p = 4 * 105 Pa and V = 4 cm3
D) p = 6 * 105 Pa and V = 2 cm3
E) p = 8 * 105 Pa and V = 2 cm3
A) p = 1 * 105 Pa and V = 10cm3
B) p = 3 *105 Pa and V = 6 cm3
C) p = 4 * 105 Pa and V = 4 cm3
D) p = 6 * 105 Pa and V = 2 cm3
E) p = 8 * 105 Pa and V = 2 cm3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
An ideal gas undergoes an isothermal process starting with a pressure of 2*105 Pa and a volume of 6 cm3. Which of the following might be the pressure and volume of the final state?
A) 1* 105 Pa and 10cm3
B) 3 * 105 Pa and 6 cm3
C) 4 * 105 Pa and 4 cm3
D) 6 * 105 Pa and 2 cm3
E) 8 * 105 Pa and 2 cm3
A) 1* 105 Pa and 10cm3
B) 3 * 105 Pa and 6 cm3
C) 4 * 105 Pa and 4 cm3
D) 6 * 105 Pa and 2 cm3
E) 8 * 105 Pa and 2 cm3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
It is known that 28 grams of a certain ideal gas occupy 22.4 liters at standard conditions (0 C, 1 atm). The volume occupied by 42 grams of this gas at standard conditions is:
A) 14.9 liters
B) 22.4 liters
C) 33.6 liters
D) 42 liters
E) more data are needed
A) 14.9 liters
B) 22.4 liters
C) 33.6 liters
D) 42 liters
E) more data are needed

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
In order that a single process be both isothermal and isobaric:
A) one must use an ideal gas
B) such a process is impossible
C) a change of phase is essential
D) one may use any real gas such as N2
E) one must use a solid
A) one must use an ideal gas
B) such a process is impossible
C) a change of phase is essential
D) one may use any real gas such as N2
E) one must use a solid

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
In a certain gas the molecules are 5.0 *10-9m apart on average, have a mean free path of 5.0 * 10-6m, and have an average speed of 500m/s. The rate at which a molecule has collision with other molecules is about:
A) 10-11 s-1
B) 10-8 s-1
C) 1 s-1
D) 108 s-1
E) 1011 s-1
A) 10-11 s-1
B) 10-8 s-1
C) 1 s-1
D) 108 s-1
E) 1011 s-1

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
The mean free path of molecules in a gas is proportional to:
A) the molecular cross-sectional area
B) the reciprocal of the molecular cross-sectional area
C) the root-mean-square molecular speed
D) the square of the average molecular speed
E) the molar mass
A) the molecular cross-sectional area
B) the reciprocal of the molecular cross-sectional area
C) the root-mean-square molecular speed
D) the square of the average molecular speed
E) the molar mass

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
The pressure of an ideal gas is doubled in an isothermal process. The root-mean-square speed of the molecules:
A) does not change
B)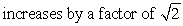
C)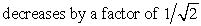
D) increases by a factor of 2
E) decreases by a factor of 1/2
A) does not change
B)
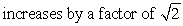
C)
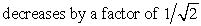
D) increases by a factor of 2
E) decreases by a factor of 1/2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
The mean free path of molecules in a gas is:
A) the average distance they travel before escaping
B) the average distance they travel between collisions
C) the greatest distance they travel between collisions
D) the shortest distance they travel between collisions
E) the average distance they travel before splitting apart
A) the average distance they travel before escaping
B) the average distance they travel between collisions
C) the greatest distance they travel between collisions
D) the shortest distance they travel between collisions
E) the average distance they travel before splitting apart

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
The temperature of a gas is most closely related to:
A) the kinetic energy of translation of its molecules
B) its total molecular kinetic energy
C) the sizes of its molecules
D) the potential energy of its molecules
E) the total energy of its molecules
A) the kinetic energy of translation of its molecules
B) its total molecular kinetic energy
C) the sizes of its molecules
D) the potential energy of its molecules
E) the total energy of its molecules

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
The mean free path of a gas molecule is:
A) the shortest dimension of the containing vessel
B) the cube root of the volume of the containing vessel
C) approximately the diameter of a molecule
D) average distance between adjacent molecules
E) average distance a molecule travels between intermolecular collisions
A) the shortest dimension of the containing vessel
B) the cube root of the volume of the containing vessel
C) approximately the diameter of a molecule
D) average distance between adjacent molecules
E) average distance a molecule travels between intermolecular collisions

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
When an ideal gas undergoes a slow isothermal expansion:
A) the work done by the gas is the same as the energy absorbed as heat
B) the work done by the environment is the same as the energy absorbed as heat
C) the increase in internal energy is the same as the heat absorbed
D) the increase in internal energy is the same as the work done by the gas
E) the increase in internal energy is the same as the work done by the environment
A) the work done by the gas is the same as the energy absorbed as heat
B) the work done by the environment is the same as the energy absorbed as heat
C) the increase in internal energy is the same as the heat absorbed
D) the increase in internal energy is the same as the work done by the gas
E) the increase in internal energy is the same as the work done by the environment

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
A certain ideal gas has a temperature 300 K and a pressure 5.0 *104 Pa. The molecules have a mean free path of 4.0 *10-7m. If the temperature is raised to 350 K and the pressure is reduced to 1.0 * 104 Pa the mean free path is them:
A) 6.9 * 10-8m
B) 9.3 *10 - 8m
C) 3.3 * 10 -7m
D) 1.7 *10-6m
E) 2.3 *10-6m
A) 6.9 * 10-8m
B) 9.3 *10 - 8m
C) 3.3 * 10 -7m
D) 1.7 *10-6m
E) 2.3 *10-6m

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Air is pumped into a bicycle tire at constant temperature. The pressure increases because:
A) more molecules strike the tire wall per second
B) the molecules are larger
C) the molecules are farther apart
D) each molecule is moving faster
E) each molecule has more kinetic energy
A) more molecules strike the tire wall per second
B) the molecules are larger
C) the molecules are farther apart
D) each molecule is moving faster
E) each molecule has more kinetic energy

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
The mean free path of air molecules at room temperature and atmospheric pressure is about:
A) 10-3 m
B) 10-5 m
C) 10-7 m
D) 10-9 m
E) 10-11 m
A) 10-3 m
B) 10-5 m
C) 10-7 m
D) 10-9 m
E) 10-11 m

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
The mean free path of molecules in a gas is proportional to:
A) the molecular diameter
B) the reciprocal of the molecular diameter
C) the molecular concentration
D) the reciprocal of the molecular concentration
E) the average molecular speed
A) the molecular diameter
B) the reciprocal of the molecular diameter
C) the molecular concentration
D) the reciprocal of the molecular concentration
E) the average molecular speed

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
The internal energy of an ideal gas depends on:
A) the temperature only
B) the pressure only
C) the volume only
D) the temperature and pressure only
E) temperature, pressure, and volume
A) the temperature only
B) the pressure only
C) the volume only
D) the temperature and pressure only
E) temperature, pressure, and volume

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
According to the kinetic theory of gases, the pressure of a gas is due to:
A) change of kinetic energy of molecules as they strike the wall
B) change of momentum of molecules as they strike the wall
C) average kinetic energy of the molecules
D) force of repulsion between the molecules
E) rms speed of the molecules
A) change of kinetic energy of molecules as they strike the wall
B) change of momentum of molecules as they strike the wall
C) average kinetic energy of the molecules
D) force of repulsion between the molecules
E) rms speed of the molecules

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
The force on the walls of a vessel of a contained gas is due to:
A) repulsive force between gas molecules
B) slight loss in average speed of a gas molecule after collision with wall
C) change in momentum of a gas molecule due to collision with wall
D) elastic collisions between gas molecules
E) inelastic collisions between gas molecules
A) repulsive force between gas molecules
B) slight loss in average speed of a gas molecule after collision with wall
C) change in momentum of a gas molecule due to collision with wall
D) elastic collisions between gas molecules
E) inelastic collisions between gas molecules

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
A system consists of N gas molecules, each with mass m. Their rms speed is vrms. Their total translational kinetic energy is:
A) (1/2)m(Nvrms)2
B) (1/2)N(mvrms)2
C) (1/2)mv2rms
D) (1/2)Nmv2rms
E) N[(1/2)mvrms]2
A) (1/2)m(Nvrms)2
B) (1/2)N(mvrms)2
C) (1/2)mv2rms
D) (1/2)Nmv2rms
E) N[(1/2)mvrms]2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
A gas is confined to a cylindrical container of radius 1 cm and length 1 m. The pressure exerted on an end face, compared with the pressure exerted on the long curved face, is:
A) smaller because its area is smaller
B) smaller because most molecules cannot traverse the length of the cylinder without undergoing collisions
C) larger because the face is flat
D) larger because the molecules have a greater distance in which to accelerate before they strike the face
E) none of these
A) smaller because its area is smaller
B) smaller because most molecules cannot traverse the length of the cylinder without undergoing collisions
C) larger because the face is flat
D) larger because the molecules have a greater distance in which to accelerate before they strike the face
E) none of these

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
The diagram shows three isotherms for an ideal gas, with T3-T2 the same as T2-T1. It also shows five thermodynamic processes caried out on the gas. Rank the processes in order change in the internal energy of the gas, least to greatest. 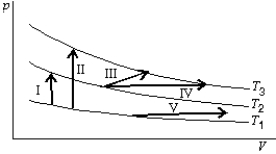
A) I, II, III, IV, V
B) V, then I, III, and IV tied, then II
C) V, I, then I, III, and IV tied, then II
D) IV, V, III, I, II
E) II, I, then III, IV, and V tied

A) I, II, III, IV, V
B) V, then I, III, and IV tied, then II
C) V, I, then I, III, and IV tied, then II
D) IV, V, III, I, II
E) II, I, then III, IV, and V tied

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
If the temperature T of an ideal gas is increased at constant pressure the mean free path:
A) decreases in proportion to 1/T
B) decreases in proportion to 1/T2
C) increases in proportion to T
D) decreases in proportion to T2
E) does not change
A) decreases in proportion to 1/T
B) decreases in proportion to 1/T2
C) increases in proportion to T
D) decreases in proportion to T2
E) does not change

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
The average speeds v and molecular diameters d of five ideal gases are given below. The number of molecules per unit volume is the same for all of them. For which is the collision rate the greatest?
A) v = v0 and d = d0
B) v = 2v0 and d = d0/2
C) v = 3v0 and d = d0
D) v = v0 and d = 2d0
E) v = 4v0 and d = d0/2
A) v = v0 and d = d0
B) v = 2v0 and d = d0/2
C) v = 3v0 and d = d0
D) v = v0 and d = 2d0
E) v = 4v0 and d = d0/2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
Evidence that a gas consists mostly of empty space is the fact that:
A) the density of a gas becomes much greater when it is liquefied
B) gases exert pressure on the walls of their containers
C) gases are transparent
D) heating a gas increases the molecular motion
E) nature abhors a vacuum
A) the density of a gas becomes much greater when it is liquefied
B) gases exert pressure on the walls of their containers
C) gases are transparent
D) heating a gas increases the molecular motion
E) nature abhors a vacuum

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
Two ideal monatomic gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular speeds vA/vB is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
According to the Maxwellian speed distribution, as the temperature increases the average speed:
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
The mass of an oxygen molecule is 16 times that of a hydrogen molecule. At room temperature, the ratio of the rms speed of an oxygen molecule to that of a hydrogen molecule is:
A) 16
B) 4
C) 1
D) 1/4
E) 1/16
A) 16
B) 4
C) 1
D) 1/4
E) 1/16

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
According to the Maxwellian speed distribution, as the temperature increases the number of molecules with speeds within a small interval near the most probable speed:
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
As the pressure in an ideal gas is increased isothermally the average molecular speed:
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
The root-mean-square sped of molecules in a gas is:
A) the most probable speed
B) that speed such that half the molecules are moving faster than vrms and the other half are moving slower
C) the average speed of the molecules
D) the square root of the square of the average speed
E) none of the above
A) the most probable speed
B) that speed such that half the molecules are moving faster than vrms and the other half are moving slower
C) the average speed of the molecules
D) the square root of the square of the average speed
E) none of the above

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
The temperature of low pressure hydrogen is reduced from 100 C to 20 C. The rms speed of its molecules decreases by approximately:
A) 80%
B) 89%
C) 46%
D) 21%
E) 11%
A) 80%
B) 89%
C) 46%
D) 21%
E) 11%

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
According to the Maxwellian speed distribution, as the temperature increases the most probable speed:
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
The speeds of 25 molecules are distributed as follows: 5 in the range from 2 to 3 m/s, 10 in the range from 3 to 4 m/s, 5 in the range from 4 to 5 m/s, 3 in the range from 5 to 6 m/s, 1 in the range from 6 to 7 m/s, and 1 in the range from 7 to 8 m/s. Their average speed is about:
A) 2 m/s
B) 3 m/s
C) 4 m/s
D) 5 m/s
E) 6 m/s
A) 2 m/s
B) 3 m/s
C) 4 m/s
D) 5 m/s
E) 6 m/s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
The Maxwellian speed distribution provides a direct explanation of:
A) thermal expansion
B) the ideal gas law
C) heat
D) evaporation
E) boiling
A) thermal expansion
B) the ideal gas law
C) heat
D) evaporation
E) boiling

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
For a gas at thermal equilibrium the average speed v, the most probable speed vp, and the root-mean-square speed vrms are in the order:
A) vp < vrms < v
B) vrms < vp < v
C) v < vrms < vp
D) vp < v < vrms
E) v < vp < vrms
A) vp < vrms < v
B) vrms < vp < v
C) v < vrms < vp
D) vp < v < vrms
E) v < vp < vrms

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
A sample of argon gas (molar mass 40 g) is at four times the absolute temperature of a sample of hydrogen gas (molar mass 2 g). The ratio of the rms speed of the argon molecules to that of the hydrogen is:
A) 1
B) 5
C) 1/5
D)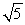
E)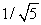
A) 1
B) 5
C) 1/5
D)
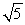
E)
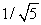

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
Evidence that molecules of a gas are in constant motion is:
A) winds exert pressure
B) two gases interdiffuse quickly
C) warm air rises
D) energy as heat is needed to vaporize a liquid
E) gases are easily compressed
A) winds exert pressure
B) two gases interdiffuse quickly
C) warm air rises
D) energy as heat is needed to vaporize a liquid
E) gases are easily compressed

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
The average speed of air molecules at room temperature is about:
A) zero
B) 2 m/s (walking speed)
C) 30 m/s (fast car)
D) 500 m/s (supersonic airplane)
E) 3 *108 m/s (speed of light)
A) zero
B) 2 m/s (walking speed)
C) 30 m/s (fast car)
D) 500 m/s (supersonic airplane)
E) 3 *108 m/s (speed of light)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
Five molecules have speeds of 2.8, 3.2, 5.8, 7.3, and 7.4 m/s. Their root-mean-square speed is closest to:
A) 5.3 m/s
B) 5.7 m/s
C) 7.3 m/s
D) 28 m/s
E) 32 m/s
A) 5.3 m/s
B) 5.7 m/s
C) 7.3 m/s
D) 28 m/s
E) 32 m/s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
As the volume of an ideal gas is increased at constant pressure the average molecular speed:
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
Ideal monatomic gas A is composed of molecules with mass m while ideal monatomic gas B is composed of molecules with mass 4m. The average molecular speeds are the same if the ratio of the temperatures TA/TB is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
In a system of N gas molecules, the individual speeds are v1, v2, ..., vN. The rms speed of these molecules is:
A)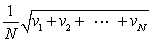
B)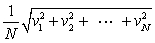
C)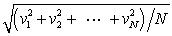
D)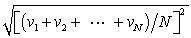
E)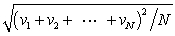
A)
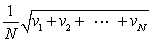
B)
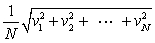
C)
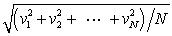
D)

E)


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
The rms speed of an oxygen molecule at 0 C is 460 m/s. If the molar mass of oxygen is 32 g and of helium is 4 g, then the rms speed of a helium molecule at 0 C is:
A) 230 m/s
B) 326 m/s
C) 650 m/s
D) 920 m/s
E) 1300 m/s
A) 230 m/s
B) 326 m/s
C) 650 m/s
D) 920 m/s
E) 1300 m/s

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
If the molecules in a tank of hydrogen have the same rms speed as the molecules in a tank of oxygen, we may be sure that:
A) the pressures are the same
B) the hydrogen is at the higher temperature
C) the hydrogen is at the greater pressure
D) the temperatures are the same
E) the oxygen is at the higher temperature
A) the pressures are the same
B) the hydrogen is at the higher temperature
C) the hydrogen is at the greater pressure
D) the temperatures are the same
E) the oxygen is at the higher temperature

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
An ideal monatomic gas has a molar specific heat Cv at constant volume of:
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
An ideal gas of N monatomic molecules is in thermal equilibrium with an ideal gas of the same number of diatomic molecules and equilibrium is maintained as temperature is increased. The ratio of the changes in the internal energies ΔEdia / Emon is:
A) 1/2
B) 3/5
C) 1
D) 5/3
E) 2
A) 1/2
B) 3/5
C) 1
D) 5/3
E) 2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
Three gases, one consisting of monatomic molecules, the second consisting of diatomic molecules, and the third consisting of polyatomic molecules, are in thermal equilibrium with each other and remains in thermal equilibrium as the temperature is raised. All have the same number of molecules. The gases with the least and greatest internal energy are respectively:
A) polyatomic, monatomic
B) monatomic, polyatomic
C) diatomic, monatomic
D) polyatomic, diatomic
E) monatomic, diatomic
A) polyatomic, monatomic
B) monatomic, polyatomic
C) diatomic, monatomic
D) polyatomic, diatomic
E) monatomic, diatomic

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
The specific heat of a polyatomic gas is greater than the specific heat of a monatomic gas because:
A) the polyatomic gas does more positive work when energy is absorbed as heat
B) the monatomic gas does more positive work when energy is absorbed as heat
C) the energy absorbed by the polyatomic gas is split among more degrees of freedom
D) the pressure is greater in the diatomic gas
E) a monatomic gas cannot hold as much heat
A) the polyatomic gas does more positive work when energy is absorbed as heat
B) the monatomic gas does more positive work when energy is absorbed as heat
C) the energy absorbed by the polyatomic gas is split among more degrees of freedom
D) the pressure is greater in the diatomic gas
E) a monatomic gas cannot hold as much heat

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
An ideal diatomic gas has a molar specific heat at constant pressure, Cp, of:
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
The pressure of an ideal gas of diatomic molecules is doubled by halving the volume. The ratio of the new internal energy to the old, both measured relative to the internal energy at 0 K, is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
The number of degrees of freedom of a triatomic molecule is:
A) 1
B) 3
C) 6
D) 8
E) 9
A) 1
B) 3
C) 6
D) 8
E) 9

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
Two ideal gases, each consisting of N monatomic molecules, are in thermal equilibrium with each other and equilibrium is maintained as the temperature is increased. A molecule of the first gas has mass m and a molecule of the second has mass 4m. The ratio of the internal energies E4m/Em is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following changes when the pressure of an ideal gas is changed isothermally?
A) Mean free path
B) Root-mean-square molecular speed
C) Internal energy
D) Most probable kinetic energy
E) Average speed
A) Mean free path
B) Root-mean-square molecular speed
C) Internal energy
D) Most probable kinetic energy
E) Average speed

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
The ratio of the specific heat of a gas at constant volume to its specific heat at constant pressure is:
A) 1
B) less than 1
C) more than 1
D) has units of pressure/volume
E) has units of volume/pressure
A) 1
B) less than 1
C) more than 1
D) has units of pressure/volume
E) has units of volume/pressure

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
The specific heat Cv at constant volume of a monatomic gas at low pressure is proportional to Tn where the exponent n is:
A) -1
B) 0
C) 1
D) 1/2
E) 2
A) -1
B) 0
C) 1
D) 1/2
E) 2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
An ideal gas has molar specific heat Cp at constant pressure. When the temperature of n moles is increased by T the increase in the internal energy is:
A) nCp T
B) n(Cp + R) T
C) n(Cp - R) T
D) n(2Cp + R) T
E) n(2Cp - R) T
A) nCp T
B) n(Cp + R) T
C) n(Cp - R) T
D) n(2Cp + R) T
E) n(2Cp - R) T

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
The ratio of the specific heat of an ideal gas at constant volume to its specific heat at constant pressure is:
A) R
B) 1/R
C) dependent on the temperature
D) dependent on the pressure
E) different for monatomic, diatomic, and polyatomic gases
A) R
B) 1/R
C) dependent on the temperature
D) dependent on the pressure
E) different for monatomic, diatomic, and polyatomic gases

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
The difference between the molar specific heat at constant pressure and the molar specific heat at constant volume for an ideal gas is:
A) the Boltzmann constant k
B) the universal gas constant R
C) the Avogadro number NA
D) kT
E) RT
A) the Boltzmann constant k
B) the universal gas constant R
C) the Avogadro number NA
D) kT
E) RT

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
The "Principle of equipartition of energy" states that the internal energy of a gas is shared equally:
A) among the molecules
B) between kinetic and potential energy
C) among the relevant degrees of freedom
D) between translational and vibrational kinetic energy
E) between temperature and pressure
A) among the molecules
B) between kinetic and potential energy
C) among the relevant degrees of freedom
D) between translational and vibrational kinetic energy
E) between temperature and pressure

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
Two monatomic ideal gases are in thermal equilibrium with each other. Gas A is composed of molecules with mass m while gas B is composed of molecules with mass 4m. The ratio of the average molecular kinetic energy KA/KB is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
An ideal gas of N diatomic molecules has temperature T. If the number of molecules is doubled without changing the temperature, the internal energy increases by:
A) 0
B) 1/2NkT
C) 3/2NkT
D) 5/2NkT
E) 3NkT
A) 0
B) 1/2NkT
C) 3/2NkT
D) 5/2NkT
E) 3NkT

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
Ideal monatomic gas A is composed of molecules with mass m while ideal monatomic gas B is composed of molecules with mass 4m. The average molecular energies are the same if the ratio of the temperatures TA/TB is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
Both the pressure and volume of an ideal gas of diatomic molecules are doubled. The ratio of the new internal energy to the old both measured relative to the internal energy at 0 K is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
The number of degrees of freedom of a rigid diatomic molecule is:
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


