Deck 40: All About Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/76
Play
Full screen (f)
Deck 40: All About Atoms
1
The number of values of the orbital quantum number 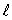 associated with the principal quantum number n = 3 is:
associated with the principal quantum number n = 3 is:
A) 1
B) 2
C) 3
D) 4
E) 7
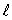 associated with the principal quantum number n = 3 is:
associated with the principal quantum number n = 3 is:A) 1
B) 2
C) 3
D) 4
E) 7
3
2
Possible values of the principal quantum number n for an electron in an atom are:
A) only 0 and 1
B) only 0,1,2,...,
C) only 0,1,...,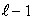
D) only 1/2 and -1/2
E) only 1,2,3,...,
A) only 0 and 1
B) only 0,1,2,...,
C) only 0,1,...,
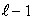
D) only 1/2 and -1/2
E) only 1,2,3,...,
only 1,2,3,...,
3
The electron states in an atom which constitute a single subshell all have:
A) only the same value of n
B) only the same value of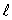
C) only the same value of n and the same value of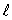
D) only the same value of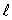 and the same value of
and the same value of 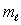
E) the same set of all four quantum numbers
A) only the same value of n
B) only the same value of
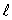
C) only the same value of n and the same value of
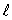
D) only the same value of
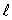 and the same value of
and the same value of 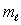
E) the same set of all four quantum numbers
only the same value of n and the same value of 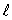
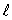
4
The number of possible values of the magnetic quantum number 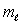 associated with a given value of the orbital quantum number
associated with a given value of the orbital quantum number  is:
is:
A) 1
B) 2
C)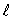
D)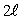
E)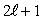
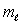 associated with a given value of the orbital quantum number
associated with a given value of the orbital quantum number 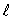 is:
is:A) 1
B) 2
C)
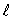
D)
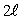
E)
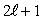

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
5
The number of states in a subshell with orbital quantum number 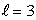 is:
is:
A) 2
B) 3
C) 7
D) 9
E) 14
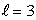 is:
is:A) 2
B) 3
C) 7
D) 9
E) 14

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
6
The number of states in a shell with principal quantum number n = 3 is:
A) 3
B) 9
C) 15
D) 18
E) 25
A) 3
B) 9
C) 15
D) 18
E) 25

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
7
The electron states in an atom which constitute a single shell all have:
A) the same value of n
B) the same value of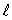
C) the same value of n and the same value of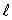
D) the same value of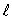 and the same value of
and the same value of 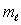
E) the same set of all four quantum numbers
A) the same value of n
B) the same value of
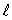
C) the same value of n and the same value of
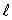
D) the same value of
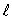 and the same value of
and the same value of 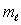
E) the same set of all four quantum numbers

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
8
The quantum number ms is most closely associated with what property of the electron in an atom?
A) Magnitude of the orbital angular momentum
B) Energy
C) z component of the spin angular momentum
D) z component of the orbital angular momentum
E) Radius of the orbit
A) Magnitude of the orbital angular momentum
B) Energy
C) z component of the spin angular momentum
D) z component of the orbital angular momentum
E) Radius of the orbit

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
9
The possible values for the magnetic quantum number ms of an electron in an atom:
A) depend on n
B) depend on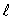
C) depend on both n and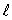
D) depend on whether or not there is an external magnetic field present
E) are 1/2
A) depend on n
B) depend on
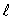
C) depend on both n and
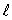
D) depend on whether or not there is an external magnetic field present
E) are 1/2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
10
An electron in an atom is in a state with principal quantum number n = 4. The possible values of the orbital quantum number 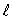 are:
are:
A) 1, 2, 3
B) 1, 2, 3, 4
C) -3, -2, -1, 0, 1, 2, 3
D) 0, 1, 2, 3
E) 0, 1, 2
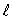 are:
are:A) 1, 2, 3
B) 1, 2, 3, 4
C) -3, -2, -1, 0, 1, 2, 3
D) 0, 1, 2, 3
E) 0, 1, 2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
11
An electron in an atom is in a state with 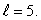 The minimum angle between
The minimum angle between 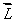 and the z axis is:
and the z axis is:
A) 0
B) 18.0
C) 24.1
D) 36.7
E) 33.6
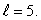 The minimum angle between
The minimum angle between 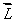 and the z axis is:
and the z axis is:A) 0
B) 18.0
C) 24.1
D) 36.7
E) 33.6

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
12
The magnitude of the orbital angular momentum of an electron is what multiple of 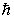 ? (
? ( 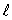 is a positive integer.)
is a positive integer.)
A) 1
B) 1/2
C)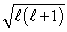
D)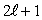
E)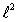
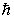 ? (
? ( 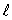 is a positive integer.)
is a positive integer.)A) 1
B) 1/2
C)
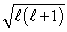
D)
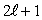
E)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
13
Space quantization means that:
A) space is quantized
B) Lz can have only certain discrete values
C)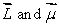 are in the same direction
are in the same direction
D)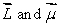 are in opposite directions
are in opposite directions
E) an electron has a magnetic dipole moment
A) space is quantized
B) Lz can have only certain discrete values
C)
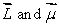 are in the same direction
are in the same directionD)
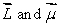 are in opposite directions
are in opposite directionsE) an electron has a magnetic dipole moment

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
14
The total number of electron states with n = 2 and 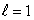 for an atom is:
for an atom is:
A) two
B) four
C) six
D) eight
E) ten
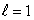 for an atom is:
for an atom is:A) two
B) four
C) six
D) eight
E) ten

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
15
An atom is in a state with orbital quantum number 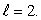 Possible values of the magnetic quantum number
Possible values of the magnetic quantum number 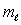 are:
are:
A) 1, 2
B) 0, 1, 2
C) 0, 1
D) -1, 0, 1
E) -2, -1, 0, 1, 2
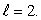 Possible values of the magnetic quantum number
Possible values of the magnetic quantum number 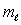 are:
are:A) 1, 2
B) 0, 1, 2
C) 0, 1
D) -1, 0, 1
E) -2, -1, 0, 1, 2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
16
An electron is in a quantum state for which there are seven allowed values of the z component of the angular momentum. The magnitude of the angular momentum is:
A)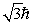
B)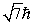
C)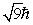
D)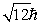
E)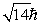
A)
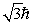
B)
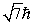
C)
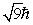
D)
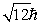
E)
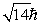

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
17
In the relation 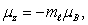 the quantity B is:
the quantity B is:
A) the Bohr magneton
B) the component of the dipole moment along the magnetic field
C) the permeability of the material
D) a friction coefficient
E) none of the above
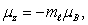 the quantity B is:
the quantity B is:A) the Bohr magneton
B) the component of the dipole moment along the magnetic field
C) the permeability of the material
D) a friction coefficient
E) none of the above

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
18
The magnetic quantum number 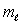 is most closely associated with what property of the electron in an atom?
is most closely associated with what property of the electron in an atom?
A) Magnitude of the orbital angular momentum
B) Energy
C) z component of the spin angular momentum
D) z component of the orbital angular momentum
E) Radius of the orbit
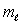 is most closely associated with what property of the electron in an atom?
is most closely associated with what property of the electron in an atom?A) Magnitude of the orbital angular momentum
B) Energy
C) z component of the spin angular momentum
D) z component of the orbital angular momentum
E) Radius of the orbit

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
19
An electron in an atom is in a state with 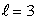 and
and 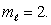 The angle between
The angle between 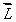 and the z axis is:
and the z axis is:
A) 48.2
B) 60
C) 30
D) 35.3
E) 54.7
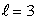 and
and 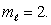 The angle between
The angle between 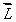 and the z axis is:
and the z axis is:A) 48.2
B) 60
C) 30
D) 35.3
E) 54.7

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
20
An electron is in a quantum state for which the magnitude of the orbital angular momentum is 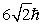 . How many allowed values of the z component of the angular momentum are there?
. How many allowed values of the z component of the angular momentum are there?
A) 7
B) 8
C) 16
D) 17
E) 20
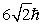 . How many allowed values of the z component of the angular momentum are there?
. How many allowed values of the z component of the angular momentum are there?A) 7
B) 8
C) 16
D) 17
E) 20

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
21
Electrons are in a two-dimensional square potential energy well with sides of length L. The potential energy is infinite at the sides and zero inside. The single-particle energies are given by 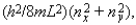 where nx and ny are integers. The number of single-particle states with energy 5(h2/8mL2) is:
where nx and ny are integers. The number of single-particle states with energy 5(h2/8mL2) is:
A) 1
B) 2
C) 3
D) 4
E) 5
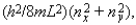 where nx and ny are integers. The number of single-particle states with energy 5(h2/8mL2) is:
where nx and ny are integers. The number of single-particle states with energy 5(h2/8mL2) is:A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
22
The minimum energy principle tells us that:
A) the energy of an atom with a high atomic number is less than the energy of an atom with a low atomic number
B) the energy of an atom with a low atomic number is less than the energy of an atom with high atomic number
C) when an atom makes an upward transition the energy of the absorbed photon is the least possible
D) the ground state configuration of any atom is the one with the least energy
E) the ground state configuration of any atom is the one with the least ionization energy
A) the energy of an atom with a high atomic number is less than the energy of an atom with a low atomic number
B) the energy of an atom with a low atomic number is less than the energy of an atom with high atomic number
C) when an atom makes an upward transition the energy of the absorbed photon is the least possible
D) the ground state configuration of any atom is the one with the least energy
E) the ground state configuration of any atom is the one with the least ionization energy

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
23
The Stern-Gerlach experiment makes use of:
A) a strong uniform magnetic field
B) a strong non-uniform magnetic field
C) a strong uniform electric field
D) a strong non-uniform electric field
E) strong perpendicular electric and magnetic fields
A) a strong uniform magnetic field
B) a strong non-uniform magnetic field
C) a strong uniform electric field
D) a strong non-uniform electric field
E) strong perpendicular electric and magnetic fields

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following subshells cannot exist?
A) 3p
B) 2p
C) 4d
D) 3d
E) 2d
A) 3p
B) 2p
C) 4d
D) 3d
E) 2d

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
25
A magnetic dipole 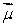 is placed in a strong uniform magnetic field
is placed in a strong uniform magnetic field 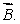 The associated force exerted on the dipole is:
The associated force exerted on the dipole is:
A) along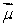
B) along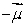
C) along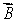
D) along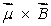
E) zero
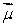 is placed in a strong uniform magnetic field
is placed in a strong uniform magnetic field 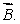 The associated force exerted on the dipole is:
The associated force exerted on the dipole is:A) along
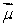
B) along
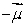
C) along
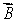
D) along
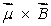
E) zero

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following 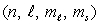 combinations is impossible for an electron in an atom?
combinations is impossible for an electron in an atom?
A) 3, 1, 1, -1/2
B) 6, 2, 0, 1/2
C) 3, 2, -2, -1/2
D) 3, 1, -2, 1/2
E) 1, 0, 0, -1/2
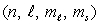 combinations is impossible for an electron in an atom?
combinations is impossible for an electron in an atom?A) 3, 1, 1, -1/2
B) 6, 2, 0, 1/2
C) 3, 2, -2, -1/2
D) 3, 1, -2, 1/2
E) 1, 0, 0, -1/2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
27
When a lithium atom is made from a helium atom by adding a proton (and neutron) to the nucleus and an electron outside, the electron goes into an n = 2, 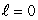 state rather than an n = 1,
state rather than an n = 1, 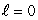 state. This is an indication that electrons:
state. This is an indication that electrons:
A) obey the Pauli exclusion principle
B) obey the minimum energy principle
C) undergo the Zeeman effect
D) are diffracted
E) and protons are interchangeable
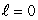 state rather than an n = 1,
state rather than an n = 1, 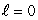 state. This is an indication that electrons:
state. This is an indication that electrons:A) obey the Pauli exclusion principle
B) obey the minimum energy principle
C) undergo the Zeeman effect
D) are diffracted
E) and protons are interchangeable

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
28
For any atom other that hydrogen and helium all electrons in the same shell have:
A) the same energy
B) the same magnitude of angular momentum
C) the same magnetic quantum number
D) the same spin quantum number
E) none of the above
A) the same energy
B) the same magnitude of angular momentum
C) the same magnetic quantum number
D) the same spin quantum number
E) none of the above

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
29
To observe the Zeeman effect one uses:
A) a strong uniform magnetic field
B) a strong non-uniform magnetic field
C) a strong uniform electric field
D) a strong non-uniform electric field
E) mutually perpendicular electric and magnetic fields
A) a strong uniform magnetic field
B) a strong non-uniform magnetic field
C) a strong uniform electric field
D) a strong non-uniform electric field
E) mutually perpendicular electric and magnetic fields

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
30
If electrons did not have intrinsic angular momentum (spin) but still obeyed the Pauli exclusion principle the states occupied by electrons in the ground state of helium would be:
A) (n = 1,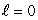 ); (n = 1,
); (n = 1, 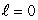 )
)
B) (n = 1,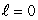 ); (n = 1,
); (n = 1, 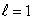 )
)
C) (n = 1,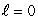 ); (n = 2,
); (n = 2, 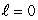 )
)
D) (n = 2,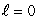 ); (n = 2,
); (n = 2, 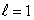 )
)
E) (n = 2,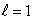 ); (n = 2,
); (n = 2, 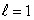 )
)
A) (n = 1,
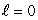 ); (n = 1,
); (n = 1, 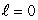 )
)B) (n = 1,
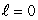 ); (n = 1,
); (n = 1, 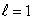 )
)C) (n = 1,
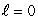 ); (n = 2,
); (n = 2, 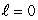 )
)D) (n = 2,
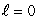 ); (n = 2,
); (n = 2, 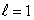 )
)E) (n = 2,
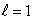 ); (n = 2,
); (n = 2, 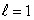 )
)
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
31
When a lithium atom in its ground state is made from a helium atom by adding a proton (and neutron) to the nucleus and an electron outside, the electron goes into an n = 2, 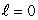 state rather than an n = 3,
state rather than an n = 3, 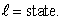 This is an indication that electrons:
This is an indication that electrons:
A) obey the Pauli exclusion principle
B) obey the minimum energy principle
C) undergo the Zeeman effect
D) are diffracted
E) and protons are interchangeable
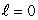 state rather than an n = 3,
state rather than an n = 3, 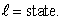 This is an indication that electrons:
This is an indication that electrons:A) obey the Pauli exclusion principle
B) obey the minimum energy principle
C) undergo the Zeeman effect
D) are diffracted
E) and protons are interchangeable

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
32
A magnetic dipole is placed between the poles of a magnet as shown. The direction of the associated force exerted on the dipole is: 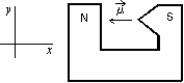
A) positive x
B) positive y
C) negative x
D) negative y
E) into or out of the page

A) positive x
B) positive y
C) negative x
D) negative y
E) into or out of the page

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
33
Five electrons are in a two-dimensional square potential energy well with sides of length L. The potential energy is infinite at the sides and zero inside. The single-particle energies are given by 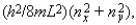 where nx and ny are integers. In units of (h2/8mL2) the energy of the ground state of the system is
where nx and ny are integers. In units of (h2/8mL2) the energy of the ground state of the system is
A) 0
B) 10
C) 19
D) 24
E) 48
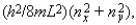 where nx and ny are integers. In units of (h2/8mL2) the energy of the ground state of the system is
where nx and ny are integers. In units of (h2/8mL2) the energy of the ground state of the system isA) 0
B) 10
C) 19
D) 24
E) 48

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
34
The force exerted on a magnetic dipole as it moves with velocity 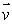 through a Stern-Gerlach apparatus is:
through a Stern-Gerlach apparatus is:
A) proportional to v
B) proportional to 1/v
C) zero
D) proportional to v2
E) independent of v
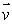 through a Stern-Gerlach apparatus is:
through a Stern-Gerlach apparatus is:A) proportional to v
B) proportional to 1/v
C) zero
D) proportional to v2
E) independent of v

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
35
The Pauli exclusion principle is obeyed by:
A) all particles
B) all charged particles
C) all particles with spin quantum numbers of 1/2
D) all particles with spin quantum numbers of 1
E) all particles with mass
A) all particles
B) all charged particles
C) all particles with spin quantum numbers of 1/2
D) all particles with spin quantum numbers of 1
E) all particles with mass

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
36
Five electrons are in a two-dimensional square potential energy well with sides of length L. The potential energy is infinite at the sides and zero inside. The single-particle energies are given by 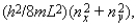 where nx and ny are integers. In units of (h2/8mL2) the energy of the first excited state of the system is:
where nx and ny are integers. In units of (h2/8mL2) the energy of the first excited state of the system is:
A) 13
B) 22
C) 24
D) 25
E) 27
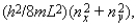 where nx and ny are integers. In units of (h2/8mL2) the energy of the first excited state of the system is:
where nx and ny are integers. In units of (h2/8mL2) the energy of the first excited state of the system is:A) 13
B) 22
C) 24
D) 25
E) 27

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
37
Six electrons are in a two-dimensional square potential energy well with sides of length L. The potential energy is infinite at the sides and zero inside. The single-particle energies are given by 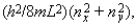 where nx and ny are integers. If a seventh electron is added to the system when it is in its ground state the least energy the additional electron can have is:
where nx and ny are integers. If a seventh electron is added to the system when it is in its ground state the least energy the additional electron can have is:
A) 2(h2/8mL2)
B) 5(h2/8mL2)
C) 10(h2/8mL2)
D) 13(h2/8mL2)
E) 18(h2/8mL2)
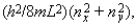 where nx and ny are integers. If a seventh electron is added to the system when it is in its ground state the least energy the additional electron can have is:
where nx and ny are integers. If a seventh electron is added to the system when it is in its ground state the least energy the additional electron can have is:A) 2(h2/8mL2)
B) 5(h2/8mL2)
C) 10(h2/8mL2)
D) 13(h2/8mL2)
E) 18(h2/8mL2)

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
38
No state in an atom can be occupied by more than one electron. This is most closely related to the:
A) wave nature of matter
B) finite value for the speed of light
C) Bohr magneton
D) Pauli exclusion principle
E) the Einstein-de Haas effect
A) wave nature of matter
B) finite value for the speed of light
C) Bohr magneton
D) Pauli exclusion principle
E) the Einstein-de Haas effect

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
39
The magnetic field 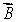 is along the z axis in a Stern-Gerlach experiment. The force it exerts on a magnetic dipole with dipole moment
is along the z axis in a Stern-Gerlach experiment. The force it exerts on a magnetic dipole with dipole moment 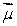 is proportional to:
is proportional to:
A)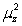
B) B2
C) dB/dz
D) d2B/dz2
E) B dz
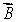 is along the z axis in a Stern-Gerlach experiment. The force it exerts on a magnetic dipole with dipole moment
is along the z axis in a Stern-Gerlach experiment. The force it exerts on a magnetic dipole with dipole moment 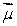 is proportional to:
is proportional to:A)
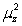
B) B2
C) dB/dz
D) d2B/dz2
E) B dz

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
40
Electrons are in a two-dimensional square potential energy well with sides of length L. The potential energy is infinite at the sides and zero inside. The single-particle energies are given by 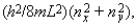 where nx and ny are integers. At most the number of electrons that can have energy 8(h2/8mL2) is:
where nx and ny are integers. At most the number of electrons that can have energy 8(h2/8mL2) is:
A) 1
B) 2
C) 3
D) 4
E) any number
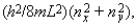 where nx and ny are integers. At most the number of electrons that can have energy 8(h2/8mL2) is:
where nx and ny are integers. At most the number of electrons that can have energy 8(h2/8mL2) is:A) 1
B) 2
C) 3
D) 4
E) any number

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
41
An electron in an L shell of an atom has the principal quantum number:
A) n = 0
B) n = 1
C) n = 2
D) n = 3
E) n =
A) n = 0
B) n = 1
C) n = 2
D) n = 3
E) n =

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
42
The states being filled from the beginning to end of the lanthanide series of atoms are:
A) n = 3,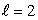 states
states
B) n = 4,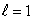 states
states
C) n = 4,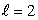 states
states
D) n = 4,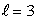 states
states
E) n = 5,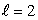 states
states
A) n = 3,
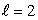 states
statesB) n = 4,
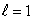 states
statesC) n = 4,
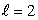 states
statesD) n = 4,
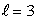 states
statesE) n = 5,
 states
states
Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
43
Suppose the energy required to ionize an argon atom is i, the energy to excite it is e, and its thermal energy at room temperature is t. In increasing order, these three energies are:
A) i, e, t
B) t, i, e
C) e, t, i
D) i, t, e
E) t, e, i
A) i, e, t
B) t, i, e
C) e, t, i
D) i, t, e
E) t, e, i

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
44
An electron in a K shell of an atom has the principal quantum number:
A) n = 0
B) n = 1
C) n = 2
D) n = 3
E) n =
A) n = 0
B) n = 1
C) n = 2
D) n = 3
E) n =

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
45
The K x rays arixing from a cobalt (Z = 27) target have a wavelength of about 179 pm. The atomic number of a target that gives rise to K x rays with a wavelength one-third as great ( 60pm) is:
A) Z = 9
B) Z = 10
C) Z = 12
D) Z = 16
E) Z = 46
A) Z = 9
B) Z = 10
C) Z = 12
D) Z = 16
E) Z = 46

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
46
In connection with x-ray emission the symbol L refers to:
A) a beta particle radiation
B) an atomic state of angular momentum h/2
C) the inductance associated with an orbiting electron
D) x-radiation associated with an electron going from n = 4 to n = 2
E) none of the above
A) a beta particle radiation
B) an atomic state of angular momentum h/2
C) the inductance associated with an orbiting electron
D) x-radiation associated with an electron going from n = 4 to n = 2
E) none of the above

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
47
In connection with x-ray emission the symbol K refers to:
A) an alpha particle radiation
B) an effect of the dielectric constant on energy levels
C) x-ray radiation from potassium
D) x-ray radiation associated with an electron going from n = to n = 1
E) x-ray radiation associated with an electron going from n = 2 to n = 1
A) an alpha particle radiation
B) an effect of the dielectric constant on energy levels
C) x-ray radiation from potassium
D) x-ray radiation associated with an electron going from n = to n = 1
E) x-ray radiation associated with an electron going from n = 2 to n = 1

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
48
The most energetic electron in any atom at the beginning of a period of the periodic table is in:
A) an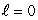 state
state
B) an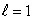 state
state
C) an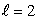 state
state
D) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum
A) an
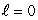 state
stateB) an
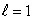 state
stateC) an
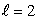 state
stateD) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
49
The most energetic electron in any atom at the end of a period of the periodic table is in:
A) an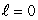 state
state
B) an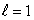 state
state
C) an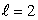 state
state
D) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum
A) an
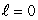 state
stateB) an
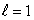 state
stateC) an
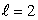 state
stateD) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
50
The group of atoms at the ends of periods of the periodic table are called:
A) alkali metals
B) rare earths
C) transition metal atoms
D) alkaline atoms
E) inert gas atoms
A) alkali metals
B) rare earths
C) transition metal atoms
D) alkaline atoms
E) inert gas atoms

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
51
The group of atoms at the beginning of periods of the periodic table are called:
A) alkali metal atoms
B) rare earth atoms
C) transition metal atoms
D) alkaline atoms
E) inert gas atoms
A) alkali metal atoms
B) rare earth atoms
C) transition metal atoms
D) alkaline atoms
E) inert gas atoms

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
52
Characteristic K x-radiation of an element is caused by:
A) stoppage of electrons by the nucleus
B) scattering of the incident radiation with a change of wavelength
C) ejection of an electron from an outer shell
D) transition of an electron to the innermost orbit
E) none of the above
A) stoppage of electrons by the nucleus
B) scattering of the incident radiation with a change of wavelength
C) ejection of an electron from an outer shell
D) transition of an electron to the innermost orbit
E) none of the above

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
53
In a Moseley graph:
A) the x-ray frequency is plotted as a function of atomic number
B) the square of the x-ray frequency is plotted as a function of atomic number
C) the square root of the x-ray frequency is plotted as a function of atomic number
D) the x-ray frequency is plotted as a function of the square root of atomic number
E) the square root of the x-ray frequency is plotted as a function of atomic mass
A) the x-ray frequency is plotted as a function of atomic number
B) the square of the x-ray frequency is plotted as a function of atomic number
C) the square root of the x-ray frequency is plotted as a function of atomic number
D) the x-ray frequency is plotted as a function of the square root of atomic number
E) the square root of the x-ray frequency is plotted as a function of atomic mass

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
54
The effective charge acting on a single valence electron outside a closed shell is about Ne, where N is:
A) the atomic number of the nucleus
B) the atomic mass of the atom
C) usually between 1 and 3
D) half the atomic number
E) less than 1
A) the atomic number of the nucleus
B) the atomic mass of the atom
C) usually between 1 and 3
D) half the atomic number
E) less than 1

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
55
A photon with the smallest wavelength in the continuous z-ray spectrum is emitted when:
A) an electron is knocked from a K shell
B) a valence electron is knocked from the atom
C) the incident electron becomes bound to the atom
D) the atom has the greatest recoil energy
E) the incident electron loses all its energy in a single decelerating event
A) an electron is knocked from a K shell
B) a valence electron is knocked from the atom
C) the incident electron becomes bound to the atom
D) the atom has the greatest recoil energy
E) the incident electron loses all its energy in a single decelerating event

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
56
Radiation with the minimum wavelength as well as the K x-ray lines are detected for a certain target. The energy of the incident electrons is then doubled, with the result that:
A) the minimum wavelength increases and the wavelengths of the K lines remain the same
B) the minimum wavelength decreases and the wavelengths of the K lines remain the same
C) the minimum wavelength and the wavelengths of the K lines all increase
D) the minimum wavelength and the wavelengths of the K lines all decrease
E) the minimum wavelength increases and the wavelengths of the K lines all decrease
A) the minimum wavelength increases and the wavelengths of the K lines remain the same
B) the minimum wavelength decreases and the wavelengths of the K lines remain the same
C) the minimum wavelength and the wavelengths of the K lines all increase
D) the minimum wavelength and the wavelengths of the K lines all decrease
E) the minimum wavelength increases and the wavelengths of the K lines all decrease

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
57
The most energetic photon in a continuous x-ray spectrum has an energy approximately equal to:
A) the energy of all the electrons in a target atom
B) the kinetic energy of an incident-beam electron
C) the rest energy, mc2, of an electron
D) the total energy of a K-electron in the target atom
E) the kinetic energy of a K-electron in the target atom
A) the energy of all the electrons in a target atom
B) the kinetic energy of an incident-beam electron
C) the rest energy, mc2, of an electron
D) the total energy of a K-electron in the target atom
E) the kinetic energy of a K-electron in the target atom

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
58
Two different electron beams are incident on two different targets and both produce x rays. The cutoff wavelength for target 1 is shorter than the cutoff wavelength for target 2. We can conclude that:
A) target 2 has a higher atomic number than target 1
B) target 2 has a lower atomic number than target 1
C) the electrons in beam 1 have greater kinetic energy than those in beam 2
D) the electrons in beam 1 have less kinetic energy than those in beam 2
E) target 1 is thicker than target 2
A) target 2 has a higher atomic number than target 1
B) target 2 has a lower atomic number than target 1
C) the electrons in beam 1 have greater kinetic energy than those in beam 2
D) the electrons in beam 1 have less kinetic energy than those in beam 2
E) target 1 is thicker than target 2

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
59
The transition shown gives rise to an x-ray. The correct label for this is: 
A) K
B) K
C) L
D) L
E) KL

A) K
B) K
C) L
D) L
E) KL

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
60
The ionization energy of an atom in its ground state is:
A) the energy required to remove the least energetic electron
B) the energy required to remove the most energetic electron
C) the energy difference between the most energetic electron and the least energetic electron
D) the same as the energy of a K photon
E) the same as the excitation energy of the most energetic electron
A) the energy required to remove the least energetic electron
B) the energy required to remove the most energetic electron
C) the energy difference between the most energetic electron and the least energetic electron
D) the same as the energy of a K photon
E) the same as the excitation energy of the most energetic electron

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
61
A laser must be pumped to achieve:
A) a metastable state
B) fast response
C) stimulated emission
D) population inversion
E) the same wavelength for all photons
A) a metastable state
B) fast response
C) stimulated emission
D) population inversion
E) the same wavelength for all photons

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
62
A metastable state is important for the generation of a laser beam because it assures that:
A) spontaneous emission does not occur more often than stimulated emission
B) photons do not split too rapidly
C) more photons are emitted than are absorbed
D) photons do not collide with each other
E) photons do not make upward transitions
A) spontaneous emission does not occur more often than stimulated emission
B) photons do not split too rapidly
C) more photons are emitted than are absorbed
D) photons do not collide with each other
E) photons do not make upward transitions

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
63
Photons in a laser beam are produced by:
A) transitions from a metastable state
B) transitions from a state that decays rapidly
C) splitting of other photons
D) pumping
E) reflection from mirrors
A) transitions from a metastable state
B) transitions from a state that decays rapidly
C) splitting of other photons
D) pumping
E) reflection from mirrors

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is essential for the laser action to occur between two energy levels of an atom?
A) the lower level is metastable
B) there are more atoms in the upper level than in the lower level
C) there are more atoms in the lower level than in the upper level
D) the lower level is the ground state
E) the lasing material is a gas
A) the lower level is metastable
B) there are more atoms in the upper level than in the lower level
C) there are more atoms in the lower level than in the upper level
D) the lower level is the ground state
E) the lasing material is a gas

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
65
Photons in a laser beam have the same energy, wavelength, polarization direction, and phase because:
A) each is produced in an emission that is stimulated by another
B) all come from the same atom
C) the lasing material has only two quantum states
D) all photons are alike, no matter what their source
E) none of the above
A) each is produced in an emission that is stimulated by another
B) all come from the same atom
C) the lasing material has only two quantum states
D) all photons are alike, no matter what their source
E) none of the above

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
66
The ratio of wavelength of K x-ray line for Nb (Z = 41) to that of Ga (Z = 31) is:
A) 9/16
B) 16/9
C) 3/4
D) 4/3
E) 1.15
A) 9/16
B) 16/9
C) 3/4
D) 4/3
E) 1.15

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
67
Electrons in a certain laser make transitions from a metastable state to the ground state. Initially there are 6 * 1020 atoms in the metastable state and 2 * 1020 atoms in the ground state. The number of photons that can be produced in a single burst is about:
A) 2 *1020
B) 3 * 1020
C) 4 *1020
D) 6 *1020
E) 8 * 1020
A) 2 *1020
B) 3 * 1020
C) 4 *1020
D) 6 *1020
E) 8 * 1020

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
68
The "e" in laser stands for:
A) electric
B) emf
C) energy
D) emission
E) entropy
A) electric
B) emf
C) energy
D) emission
E) entropy

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
69
In calculating the x-ray energy levels the effective charge of the nucleus is taken to be Z - b, where Z is the atomic number. The parameter b enters because:
A) an electron is removed from the inner shell
B) a proton is removed from the nucleus
C) the quantum mechanical force between two charges is less than the classical force
D) the nucleus is screened by electrons
E) the Pauli exclusion principle must be obeyed
A) an electron is removed from the inner shell
B) a proton is removed from the nucleus
C) the quantum mechanical force between two charges is less than the classical force
D) the nucleus is screened by electrons
E) the Pauli exclusion principle must be obeyed

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
70
In a laser:
A) excited atoms are stimulated to emit photons by radiation external to the laser
B) the transitions for laser emission are directly to the ground state
C) the states which give rise to laser emission are usually very unstable states that decay rapidly
D) the state in which an atom is initially excited is never between two states that are involved in the stimulated emission
E) a minimum of two energy levels are required.
A) excited atoms are stimulated to emit photons by radiation external to the laser
B) the transitions for laser emission are directly to the ground state
C) the states which give rise to laser emission are usually very unstable states that decay rapidly
D) the state in which an atom is initially excited is never between two states that are involved in the stimulated emission
E) a minimum of two energy levels are required.

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
71
Population inversion is important for the generation of a laser beam because it assures that:
A) spontaneous emission does not occur more often than stimulated emission
B) photons do not split too rapidly
C) more photons are emitted than are absorbed
D) photons do not collide with each other
E) photons do not make upward transitions
A) spontaneous emission does not occur more often than stimulated emission
B) photons do not split too rapidly
C) more photons are emitted than are absorbed
D) photons do not collide with each other
E) photons do not make upward transitions

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is essential for laser action to occur between two energy levels of an atom?
A) the lower level is metastable
B) the upper level is metastable
C) the lower level is the ground state
D) the are more atoms in the lower level than in the upper level
E) the lasing material is a gas
A) the lower level is metastable
B) the upper level is metastable
C) the lower level is the ground state
D) the are more atoms in the lower level than in the upper level
E) the lasing material is a gas

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
73
In a helium-neon laser, the laser light arises from a transition from a _________ state to a _________ state:
A) He, He
B) Ne, Ne
C) He, Ne
D) Ne, He
E) N, He
A) He, He
B) Ne, Ne
C) He, Ne
D) Ne, He
E) N, He

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
74
A laser beam can be sharply focused because it is:
A) highly coherent
B) plane polarized
C) intense
D) circularly polarized
E) highly directional
A) highly coherent
B) plane polarized
C) intense
D) circularly polarized
E) highly directional

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
75
The purpose of the mirrors at the ends of a helium-neon laser is:
A) to assure that no laser light leaks out
B) to increase the number of stimulated emissions
C) to absorb some of the photons
D) to keep the light used for pumping inside the laser
E) to double the effective length of the laser
A) to assure that no laser light leaks out
B) to increase the number of stimulated emissions
C) to absorb some of the photons
D) to keep the light used for pumping inside the laser
E) to double the effective length of the laser

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck
76
A group of electromagnetic waves might  Which of these describe the waves from a laser?
Which of these describe the waves from a laser?
A) I only
B) II only
C) III only
D) I and II only
E) I, II, and III
 Which of these describe the waves from a laser?
Which of these describe the waves from a laser?A) I only
B) II only
C) III only
D) I and II only
E) I, II, and III

Unlock Deck
Unlock for access to all 76 flashcards in this deck.
Unlock Deck
k this deck


