Deck 42: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 42: Nuclear Physics
1
Let Z denote the atomic number and A denote the mass number of a nucleus. The number of neutrons in this nucleus is:
A) Z
B) A - Z
C) A - 2Z
D) A
E) 2A - Z
A) Z
B) A - Z
C) A - 2Z
D) A
E) 2A - Z
A - Z
2
The isotopes of an element:
A) cannot be separated at all
B) occur well separated in nature
C) have similar chemical behavior
D) cannot be separated by physical methods
E) have equal masses
A) cannot be separated at all
B) occur well separated in nature
C) have similar chemical behavior
D) cannot be separated by physical methods
E) have equal masses
have similar chemical behavior
3
Stable nuclei generally:
A) have a greater number of protons than neutrons
B) have low mass numbers
C) have high mass numbers
D) are beta emitters
E) none of the above
A) have a greater number of protons than neutrons
B) have low mass numbers
C) have high mass numbers
D) are beta emitters
E) none of the above
none of the above
4
A nucleus with a mass number of 64 has a mean radius of about:
A) 4.8 fm
B) 9.6 fm
C) 77 fm
D) 260 fm
E) 2.6 * 105 fm
A) 4.8 fm
B) 9.6 fm
C) 77 fm
D) 260 fm
E) 2.6 * 105 fm

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Iron has atomic number 26. Naturally mined iron contains isotopes of mass numbers 54, 56, 57, and 58. Which of the following statements is FALSE?
A) every atom of iron has 26 protons
B) some iron atoms have 30 neutrons
C) some iron atoms have 54 neutrons
D) the isotopes may be separated in a mass spectrometer
E) there are four kinds of naturally occurring iron atoms with the same chemical properties
A) every atom of iron has 26 protons
B) some iron atoms have 30 neutrons
C) some iron atoms have 54 neutrons
D) the isotopes may be separated in a mass spectrometer
E) there are four kinds of naturally occurring iron atoms with the same chemical properties

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
The greatest binding energy per nucleon occurs for nuclides with masses near that of:
A) helium
B) sodium
C) iron
D) mercury
E) uranium
A) helium
B) sodium
C) iron
D) mercury
E) uranium

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
In the Rutherford scattering experiment, an alpha particle is aimed directly at a gold nucleus. The distance of closest approach of the alpha particle to the nucleus occurs when:
A) the alpha particle hits the nucleus
B) the alpha particle hits the electron cloud
C) the kinetic energy of the alpha particle is completely transformed to potential energy due to the nuclear force
D) the kinetic energy of the alpha particle is completely transformed to potential energy due to the electric field of the nucleus
E) the kinetic energy of the alpha particle is completely transformed to potential energy due to the electric field of the electrons
A) the alpha particle hits the nucleus
B) the alpha particle hits the electron cloud
C) the kinetic energy of the alpha particle is completely transformed to potential energy due to the nuclear force
D) the kinetic energy of the alpha particle is completely transformed to potential energy due to the electric field of the nucleus
E) the kinetic energy of the alpha particle is completely transformed to potential energy due to the electric field of the electrons

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
1 atomic mass unit is about:
A) 1.66 *10-31 kg
B) 9.11 * 10-31 kg
C) 1.66 *10-27 kg
D) 9.11 *10-27 kg
E) 1.66 * 10-25 kg
A) 1.66 *10-31 kg
B) 9.11 * 10-31 kg
C) 1.66 *10-27 kg
D) 9.11 *10-27 kg
E) 1.66 * 10-25 kg

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
A proton in a large nucleus:
A) has a net attractive force on all other protons in the nucleus
B) has a net repulsive force on all other protons in the nucleus
C) has a net repulsive force on all other neutrons in the nucleus
D) has a net attractive force on some protons in the nucleus and a net repulsive force on others
E) has a net attractive force on some neutrons in the nucleus and a net repulsive force on others
A) has a net attractive force on all other protons in the nucleus
B) has a net repulsive force on all other protons in the nucleus
C) has a net repulsive force on all other neutrons in the nucleus
D) has a net attractive force on some protons in the nucleus and a net repulsive force on others
E) has a net attractive force on some neutrons in the nucleus and a net repulsive force on others

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
Volumes of atomic nuclei are proportional to:
A) the mass number
B) the atomic number
C) the total nuclear spin
D) the number of neutrons
E) none of these
A) the mass number
B) the atomic number
C) the total nuclear spin
D) the number of neutrons
E) none of these

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
If a nucleus has mass M, Z protons (mass mp) and N neutrons (mass mn) its binding energy is equal to:
A) Mc2
B) (M - Zmp - Nmn)c2
C) (Zmp + Nmn - M)c2
D) (Zmp + Nmn)c2
E) (Zmp - M)c2
A) Mc2
B) (M - Zmp - Nmn)c2
C) (Zmp + Nmn - M)c2
D) (Zmp + Nmn)c2
E) (Zmp - M)c2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
The binding energy of a nucleus is the energy that must be supplied to:
A) remove a nucleon
B) remove an alpha particle
C) remove a beta particle
D) separate the nucleus into its constituent nucleons
E) separate the nucleus into a collection of alpha particles
A) remove a nucleon
B) remove an alpha particle
C) remove a beta particle
D) separate the nucleus into its constituent nucleons
E) separate the nucleus into a collection of alpha particles

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
The smallest particle of any chemical element that can exist by itself and yet retain the qualities that distinguish it as that element is:
A) an electron
B) a proton
C) a neutron
D) an atom
E) a molecule
A) an electron
B) a proton
C) a neutron
D) an atom
E) a molecule

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following nuclides is least stable?
A) 52Fe (Z = 26)
B) 115Nd (Z = 60)
C) 175Lu (Z = 71)
D) 208Pb (Z = 82)
E) 238U (Z = 92)
A) 52Fe (Z = 26)
B) 115Nd (Z = 60)
C) 175Lu (Z = 71)
D) 208Pb (Z = 82)
E) 238U (Z = 92)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Let A be the mass number and Z be the atomic number of a nucleus. Which of the following is approximately correct for light nuclei?
A) Z = 2A
B) Z = A
C) Z = A/2
D) Z =
E) Z = A2
A) Z = 2A
B) Z = A
C) Z = A/2
D) Z =
E) Z = A2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
The mass density of an atomic nucleus:
A) is about 1015 kg/m3
B) is about 1012 kg/m3
C) increases with increasing nuclear mass
D) increases with decreasing nuclear radius
E) is roughly constant independent of atomic number
A) is about 1015 kg/m3
B) is about 1012 kg/m3
C) increases with increasing nuclear mass
D) increases with decreasing nuclear radius
E) is roughly constant independent of atomic number

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
The atomic number of an element is:
A) the whole number nearest to its mass
B) the number of protons in its nucleus
C) the nearest whole number of hydrogen atoms having the same mass as a single atom of the given element
D) the number of neutrons in its nucleus
E) its order of discovery
A) the whole number nearest to its mass
B) the number of protons in its nucleus
C) the nearest whole number of hydrogen atoms having the same mass as a single atom of the given element
D) the number of neutrons in its nucleus
E) its order of discovery

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
A femtometer is:
A) larger than 10-9 m
B) 10-9 m
C) 10-12 m
D) 10-15 m
E) 10-18 m
A) larger than 10-9 m
B) 10-9 m
C) 10-12 m
D) 10-15 m
E) 10-18 m

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Bromine, with atomic mass 79.942 u, is composed of nearly equal amounts of two isotopes, one of which contains 79 nucleons per atom. The mass number of the other isotope is:
A) 78
B) 79
C) 80
D) 81
E) 82
A) 78
B) 79
C) 80
D) 81
E) 82

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
The Rutherford scattering experiment showed that:
A) light has both particle-like and wavelike properties
B) atoms are electrically neutral
C) the positive and negative charges in the atom are uniformly distributed throughout its volume
D) the wavelength of scattered light depends on the scattering angle
E) the atom consists of a very tiny, massive, positively charged nucleus surrounded by almost empty space
A) light has both particle-like and wavelike properties
B) atoms are electrically neutral
C) the positive and negative charges in the atom are uniformly distributed throughout its volume
D) the wavelength of scattered light depends on the scattering angle
E) the atom consists of a very tiny, massive, positively charged nucleus surrounded by almost empty space

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Starting with a sample of pure 66Cu, 7/8 of it decays into Zn in 15 minutes. The corresponding half-life is:
A) 3.75 minutes
B) 5 minutes
C) 7 minutes
D) 10 minutes
E) 15 minutes
A) 3.75 minutes
B) 5 minutes
C) 7 minutes
D) 10 minutes
E) 15 minutes

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Two protons are about 10-10 m apart. Their relative motion is chiefly determined by:
A) gravitational forces
B) electrical forces
C) nuclear forces
D) magnetic forces
E) torque due to electric dipole moments
A) gravitational forces
B) electrical forces
C) nuclear forces
D) magnetic forces
E) torque due to electric dipole moments

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
At the end of 14 min, 1/16 of a sample of radioactive polonium remains. The corresponding half-life is:
A) (7/8) min
B) (8/7) min
C) (7/4) min
D) (7/2) min
E) (14/3) min
A) (7/8) min
B) (8/7) min
C) (7/4) min
D) (7/2) min
E) (14/3) min

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
The half-life of a radioactive isotope is 6.5 h. If there are initially 48 * 1032 atoms of this isotope, the number of atoms of this isotope remaining after 26 h is:
A) 12 * 1032
B) 6 * 1032
C) 3 * 1032
D) 6 *104
E) 3 * 102
A) 12 * 1032
B) 6 * 1032
C) 3 * 1032
D) 6 *104
E) 3 * 102

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
A large collection of nuclei are undergoing alpha decay. The rate of decay at any instant is proportional to:
A) the number of undecayed nuclei present at that instant
B) the time since the decays started
C) the time remaining before all have decayed
D) the half-life of the decay
E) the average time between decays
A) the number of undecayed nuclei present at that instant
B) the time since the decays started
C) the time remaining before all have decayed
D) the half-life of the decay
E) the average time between decays

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
The relationship between the activity R, the disintegration constant λ, and the remaining number N of radioactive nuclei is:
A) N = λR
B) R = λN
C) R = λ/N
D) R = N/ λ
E) R = λ ln N
A) N = λR
B) R = λN
C) R = λ/N
D) R = N/ λ
E) R = λ ln N

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
210Bi (an isotope of bismuth) has a half-life of 5.0 days. The time for three-quarters of a sample of 210Bi to decay is:
A) 2.5 days
B) 3.75 days
C) 10 days
D) 15 days
E) 20 days
A) 2.5 days
B) 3.75 days
C) 10 days
D) 15 days
E) 20 days

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
The half-life of a radioactive substance is:
A) half the time it takes for the entire substance to decay
B) usually about 50 years
C) the time for radium to change into lead
D) calculated from E = mc2
E) the time for half the substance to decay
A) half the time it takes for the entire substance to decay
B) usually about 50 years
C) the time for radium to change into lead
D) calculated from E = mc2
E) the time for half the substance to decay

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Possible units for the disintegration constant are:
A) kg/s
B) s/kg
C) hour
D) day-1
E) cm-1
A) kg/s
B) s/kg
C) hour
D) day-1
E) cm-1

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
One curie is equivalent to:
A) one Becquerel
B) one decay per second
C) 3.0 x 108 decays per second
D) 3.7 x 1010 decays per second
E) 106 Becquerel
A) one Becquerel
B) one decay per second
C) 3.0 x 108 decays per second
D) 3.7 x 1010 decays per second
E) 106 Becquerel

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Which expression correctly describes the radioactive decay of a substance whose half-life is T?
A) N(t) = N0e-(t ln2)/T
B) N(t) = N0e-t/T
C) N(t) = N0e-tT
D) N(t) = N0e-tT ln2
E) N(t) = N0e-t/T ln2
A) N(t) = N0e-(t ln2)/T
B) N(t) = N0e-t/T
C) N(t) = N0e-tT
D) N(t) = N0e-tT ln2
E) N(t) = N0e-t/T ln2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
The half-life of a radioactive isotope is 140 days. In how many days does the decay rate of a sample of this isotope decrease to one fourth its initial decay rate?
A) 35 days
B) 70 days
C) 105 days
D) 210 days
E) 280 days
A) 35 days
B) 70 days
C) 105 days
D) 210 days
E) 280 days

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
The half-life of radium is about 1600 years. If a rock initially contains 1 g of radium, the amount left after 8000 years will be about:
A) 200 mg
B) 63 mg
C) 31 mg
D) 16 mg
E) less than 1 mg
A) 200 mg
B) 63 mg
C) 31 mg
D) 16 mg
E) less than 1 mg

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
The half-life of a given nuclear disintegration A B:
A) depends on the initial number of A atoms
B) depends on the initial number of B atoms
C) is an exponentially increasing function of time
D) is an exponentially decreasing function of time
E) none of the above
A) depends on the initial number of A atoms
B) depends on the initial number of B atoms
C) is an exponentially increasing function of time
D) is an exponentially decreasing function of time
E) none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
The graph shows the decay rate R as a function of the time t for three radioactive samples. Rank the samples according to their half-lives, shortest to longest. 
A) 1, 2 ,3
B) 1, 3, 2
C) 2, 1, 3
D) 2, 3, 1
E) 3, 1, 2

A) 1, 2 ,3
B) 1, 3, 2
C) 2, 1, 3
D) 2, 3, 1
E) 3, 1, 2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
In an alpha decay the disintegration energy appears as:
A) photon energies
B) the kinetic energies of the alpha and the daughter nucleus
C) the excitation energy of the daughter nucleus
D) the excitation energy of the alpha particle
E) heat
A) photon energies
B) the kinetic energies of the alpha and the daughter nucleus
C) the excitation energy of the daughter nucleus
D) the excitation energy of the alpha particle
E) heat

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Radioactive 90Sr has a half-life of 30 years. What percent of a sample of 90Sr will remain after 60 years?
A) 0%
B) 14%
C) 25%
D) 50%
E) 75%
A) 0%
B) 14%
C) 25%
D) 50%
E) 75%

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Radioactive element A decays to the stable element B with a half-life T. Starting with a sample of pure A and no B, which graph below most correctly shows the number of A atoms, NA, as a function of time t? 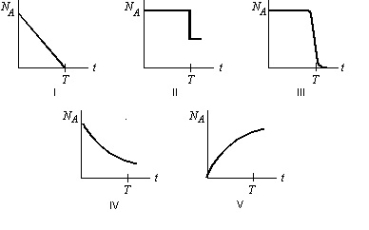
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Two protons are separated by 10-16 m. The nuclear (N), electrostatic (E), and gravitational (G) forces between these protons when written in order of increasing strength are:
A) N, E, G
B) N, G, E
C) G, E, N
D) G, N, E
E) E, G, N
A) N, E, G
B) N, G, E
C) G, E, N
D) G, N, E
E) E, G, N

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
The relation between the disintegration constant and the half-life T of a radioactive substance is:
A) = 2T
B) = 1/T
C) = 2/T
D) T = ln 2
E) T = ln(1/2)
A) = 2T
B) = 1/T
C) = 2/T
D) T = ln 2
E) T = ln(1/2)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
An alpha particle is:
A) a helium atom with two electrons removed
B) an aggregate of two or more electrons
C) a hydrogen atom
D) the ultimate unit of positive charge
E) sometimes negatively charged
A) a helium atom with two electrons removed
B) an aggregate of two or more electrons
C) a hydrogen atom
D) the ultimate unit of positive charge
E) sometimes negatively charged

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Aluminum has atomic number 13, helium has atomic number 2, and silicon has atomic number 14. In the nuclear reaction 27Al + 4He 30Si + ( ) the missing particle is:
A) an particle
B) a positron
C) an electron
D) a proton
E) a neutron
A) an particle
B) a positron
C) an electron
D) a proton
E) a neutron

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
A radium atom, 226Ra (Z = 86), emits an alpha particle. The number of protons in the resulting atom is:
A) 84
B) 85
C) 86
D) 88
E) some other number
A) 84
B) 85
C) 86
D) 88
E) some other number

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Radioactive polonium, 214Po (Z = 84), decays by alpha emission to:
A) 214Po (Z = 84)
B) 210Pb (Z = 82)
C) 214At (Z = 85)
D) 218Po (Z = 84)
E) 210Bi (Z = 83)
A) 214Po (Z = 84)
B) 210Pb (Z = 82)
C) 214At (Z = 85)
D) 218Po (Z = 84)
E) 210Bi (Z = 83)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
The energies of electrons emitted in - decays have a continuous spectrum because:
A) the original neutron has a continuous spectrum
B) the neutrino can carry off energy
C) the emitted electron is free
D) energy is not conserved
E) the daughter nucleus may have any energy
A) the original neutron has a continuous spectrum
B) the neutrino can carry off energy
C) the emitted electron is free
D) energy is not conserved
E) the daughter nucleus may have any energy

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
A certain nucleus, after absorbing a neutron, emits a - and then splits into two alpha particles. The (A, Z) of the original nucleus must have been:
A) 6, 2
B) 6, 3
C) 7, 2
D) 7, 3
E) 8, 4
A) 6, 2
B) 6, 3
C) 7, 2
D) 7, 3
E) 8, 4

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
A beta particle is:
A) a helium nucleus
B) an electron or a positron
C) a radioactive element
D) any negative particle
E) a hydrogen atom
A) a helium nucleus
B) an electron or a positron
C) a radioactive element
D) any negative particle
E) a hydrogen atom

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
A nucleus with mass number A and atomic number Z undergoes - decay. The mass number and atomic number, respectively, of the daughter nucleus are:
A) A, Z - 1
B) A - 1, Z
C) A + 1, Z - 1
D) A, Z + 1
E) A, Z
A) A, Z - 1
B) A - 1, Z
C) A + 1, Z - 1
D) A, Z + 1
E) A, Z

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Some alpha emitters have longer half-lives than others because:
A) their alpha particles have greater mass
B) their alpha particles have less mass
C) their barriers to decay are higher and wider
D) their barriers to decay are lower and narrower
E) their decays include the emission of a photon
A) their alpha particles have greater mass
B) their alpha particles have less mass
C) their barriers to decay are higher and wider
D) their barriers to decay are lower and narrower
E) their decays include the emission of a photon

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
An atom of 235U (Z = 92) disintegrates to 207Pb (Z = 82) with a half-life of about a billion years by emitting seven alpha particles and ______ - particles:
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
In addition to the daughter nucleus and an electron or positron, the products of a beta decay include:
A) a neutron
B) a neutrino
C) a proton
D) an alpha particle
E) no other particle
A) a neutron
B) a neutrino
C) a proton
D) an alpha particle
E) no other particle

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
A nucleus with mass number A and atomic number Z emits an alpha particle. The mass number and atomic number, respectively, of the daughter nucleus are:
A) A, Z -2
B) A - 2, Z - 2
C) A - 2, Z
D) A - 4, Z
E) A - 4, Z - 2
A) A, Z -2
B) A - 2, Z - 2
C) A - 2, Z
D) A - 4, Z
E) A - 4, Z - 2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
A nucleus with mass number A and atomic number Z undergoes + decay. The mass number and atomic number, respectively, of the daughter nucleus are:
A) A - 1, Z - 1
B) A - 1, Z + 1
C) A + 1, Z - 1
D) A, Z + 1
E) A, Z - 1
A) A - 1, Z - 1
B) A - 1, Z + 1
C) A + 1, Z - 1
D) A, Z + 1
E) A, Z - 1

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Magnesium has atomic number 12, hydrogen has atomic number 1, and helium has atomic number 2. In the nuclear reaction 24Mg + 2H ( ) + 4He the missing quantity is:
A) 23Na (Z = 11)
B) 22Ne (Z = 10)
C) 21Na (Z = 11)
D) 21Ne (Z = 10)
E) 22Na (Z = 11)
A) 23Na (Z = 11)
B) 22Ne (Z = 10)
C) 21Na (Z = 11)
D) 21Ne (Z = 10)
E) 22Na (Z = 11)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Rank the following collections of particles according to the total binding energy of all the particles in each collection, least to greatest. 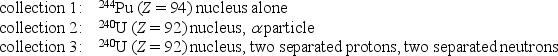
A) 1,2,3
B) 3,2,1
C) 2,1,3
D) 1,3,2
E) 2,3,1
A) 1,2,3
B) 3,2,1
C) 2,1,3
D) 1,3,2
E) 2,3,1

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
The 66Cu (Z = 29) produced in a nuclear bombardment is unstable, changing to 66Zn (Z = 30) by the emission of:
A) a proton
B) a gamma ray photon
C) a positron
D) an electron
E) an alpha particle
A) a proton
B) a gamma ray photon
C) a positron
D) an electron
E) an alpha particle

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
Bombardment of 28Si (Z = 14) with alpha particles may produce:
A) a proton and 31P (Z = 15)
B) hydrogen and 32S (Z = 16)
C) a deuteron and 27Al (Z = 13)
D) helium and 31P (Z = 15)
E) 35Cl (Z = 17)
A) a proton and 31P (Z = 15)
B) hydrogen and 32S (Z = 16)
C) a deuteron and 27Al (Z = 13)
D) helium and 31P (Z = 15)
E) 35Cl (Z = 17)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
A radioactive atom X emits a - particle. The resulting atom:
A) must be very reactive chemically
B) has an atomic number that is one more than that of X
C) has a mass number that is one less than that of X
D) has an atomic number that is one less than that of X
E) is the same chemical element as X
A) must be very reactive chemically
B) has an atomic number that is one more than that of X
C) has a mass number that is one less than that of X
D) has an atomic number that is one less than that of X
E) is the same chemical element as X

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Beta particles from various radioactive sources all have:
A) the same mass
B) the same speed
C) the same charge
D) the same deflection
E) the same energy in a magnetic field
A) the same mass
B) the same speed
C) the same charge
D) the same deflection
E) the same energy in a magnetic field

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
If 204Tl (Z = 81) emits a - particle from its nucleus:
A) stable Tl is formed
B) 202Hg (Z = 80) is formed
C) 204Pb (Z = 82) is formed
D) radioactive Tl is formed
E) 197Au (Z = 79) is formed
A) stable Tl is formed
B) 202Hg (Z = 80) is formed
C) 204Pb (Z = 82) is formed
D) radioactive Tl is formed
E) 197Au (Z = 79) is formed

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
A typical mammogram X-ray results in a radiation dose equivalent of 70 mrem. What absorbed dose does this correspond to?
A) 70 mGy
B) 70 Sv
C) 7.0 Gy
D) 0.70 Sv
E) 0.70 Gy
A) 70 mGy
B) 70 Sv
C) 7.0 Gy
D) 0.70 Sv
E) 0.70 Gy

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
The gray is the correct unit to use in reporting the measurement of:
A) the rate of decay of a radioactive source
B) the ability of a beam of gamma ray photons to produce ions in a target
C) the energy delivered by radiation to a target
D) the biological effect of radiation
E) none of the above
A) the rate of decay of a radioactive source
B) the ability of a beam of gamma ray photons to produce ions in a target
C) the energy delivered by radiation to a target
D) the biological effect of radiation
E) none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
The sievert is the correct unit to use in reporting the measurement of:
A) the rate of decay of a radioactive source
B) the ability of a beam of gamma ray photons to produce ions in a target
C) the energy delivered by radiation to a target
D) the biological effect of radiation
E) none of the above
A) the rate of decay of a radioactive source
B) the ability of a beam of gamma ray photons to produce ions in a target
C) the energy delivered by radiation to a target
D) the biological effect of radiation
E) none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
The becquerel is the correct unit to use in reporting the measurement of:
A) the rate of decay of a radioactive source
B) the ability of a beam of gamma ray photons to produce ions in a target
C) the energy delivered by radiation to a target
D) the biological effect of radiation
E) none of the above
A) the rate of decay of a radioactive source
B) the ability of a beam of gamma ray photons to produce ions in a target
C) the energy delivered by radiation to a target
D) the biological effect of radiation
E) none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
The model of the nucleus in which each nucleon has its own well-defined quantum numbers is called the:
A) collective model
B) quantum model
C) liquid drop model
D) independent model
E) atomic model
A) collective model
B) quantum model
C) liquid drop model
D) independent model
E) atomic model

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Magic nucleon numbers are associated with:
A) nuclei that decay via alpha decay
B) nuclei that decay via beta decay
C) nuclei that have equal numbers of protons and neutrons
D) nuclei that are relatively stable
E) nuclei that fission readily
A) nuclei that decay via alpha decay
B) nuclei that decay via beta decay
C) nuclei that have equal numbers of protons and neutrons
D) nuclei that are relatively stable
E) nuclei that fission readily

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
A fly caught in amber is thought to be 3 million years old. Why would it not be useful to confirm its age using radiocarbon dating?
A) The fly contains no carbon.
B) The fly is too small to contain a measurable amount of carbon.
C) The fly is too old to contain a measureable amount of carbon-14.
D) The carbon-14 doesn't begin to decay until the fly is removed from the amber.
E) Radiocarbon dating is too inaccurate to be useful.
A) The fly contains no carbon.
B) The fly is too small to contain a measurable amount of carbon.
C) The fly is too old to contain a measureable amount of carbon-14.
D) The carbon-14 doesn't begin to decay until the fly is removed from the amber.
E) Radiocarbon dating is too inaccurate to be useful.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
40K decays to 40Ar with a half-life of 1.25 x 109 yr. Assume that rocks contain no 40Ar when they form, and that the only way 40Ar can be present is through the decay of 40K. If the ratio of 40K to 40Ar in a particular rock is found to be 1:3, what is the age of the rock?
A) 1.25 x 109 yr
B) 2.50 x 109 yr
C) 3.75 x 109 yr
D) 5.00 x 109 yr
E) cannot be determined without knowing how much 40K was in the rock to begin with
A) 1.25 x 109 yr
B) 2.50 x 109 yr
C) 3.75 x 109 yr
D) 5.00 x 109 yr
E) cannot be determined without knowing how much 40K was in the rock to begin with

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


