Deck 39: More About Matter Waves
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/41
Play
Full screen (f)
Deck 39: More About Matter Waves
1
An electron in an atom drops from an energy level at -1.1 *10-18 J to an energy level at -2.4 * 10-18 J. The wave associated with the emitted photon has a frequency of:
A) 2.0 * 1017 Hz
B) 2.0* 1015 Hz
C) 2.0 * 1013 Hz
D) 2.0 * 1011 Hz
E) 2.0 * 109 Hz
A) 2.0 * 1017 Hz
B) 2.0* 1015 Hz
C) 2.0 * 1013 Hz
D) 2.0 * 1011 Hz
E) 2.0 * 109 Hz
2.0* 1015 Hz
2
A particle is trapped in a one-dimensional well with infinite potential energy at the walls. Three possible pairs of energy levels are 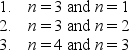 Order these pairs according to the difference in energy, least to greatest.
Order these pairs according to the difference in energy, least to greatest.
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 3, 1, 2
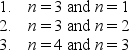 Order these pairs according to the difference in energy, least to greatest.
Order these pairs according to the difference in energy, least to greatest.A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 3, 1, 2
2, 3, 1
3
A particle is confined by finite potential energy walls to a one-dimensional trap from x = 0 to x = L. Its wave function in the region x > L has the form:
A) (x) = A sin(kx)
B) (x) = Aekx
C) (x) = Ae-kx
D) (x) = Aeikx
E) (x) = 0
A) (x) = A sin(kx)
B) (x) = Aekx
C) (x) = Ae-kx
D) (x) = Aeikx
E) (x) = 0
(x) = Ae-kx
4
If a wave function for a particle moving along the x axis is "normalized" then:
A) 2dt = 1
B) 2dx = 1
C) / x = 1
D) / t = 1
E) 2 = 1
A) 2dt = 1
B) 2dx = 1
C) / x = 1
D) / t = 1
E) 2 = 1

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
5
An electron in an atom initially has an energy 7.5 eV above the ground state energy. It drops to a state with an energy of 3.2 eV above the ground state energy and emits a photon in the process. The momentum of the photon is:
A) 1.7 * 10-27 kg . m/s
B) 2.3 *10-27 kg . m/s
C) 4.0 * 10-27 kg .m/s
D) 5.7* 10-27 kg .m/s
E) 8.0 *10-27 kg . m/s
A) 1.7 * 10-27 kg . m/s
B) 2.3 *10-27 kg . m/s
C) 4.0 * 10-27 kg .m/s
D) 5.7* 10-27 kg .m/s
E) 8.0 *10-27 kg . m/s

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
6
An electron in an atom initially has an energy 5.5 eV above the ground state energy. It drops to a state with energy 3.2 eV above the ground state energy and emits a photon in the process. The wave associated with the photon has a wavelength of:
A) 5.4 * 10 - 7 m
B) 3.0 *10 - 7 m
C) 1.7 * 10 - 7 m
D) 1.2 *10 - 7 m
E) 1.0 *10 - 7 m
A) 5.4 * 10 - 7 m
B) 3.0 *10 - 7 m
C) 1.7 * 10 - 7 m
D) 1.2 *10 - 7 m
E) 1.0 *10 - 7 m

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
7
A particle is trapped in an infinite potential energy well. It is in the state with quantum number n = 14. How many nodes does the probability density have (counting the nodes at the ends of the well)?
A) 0
B) 7
C) 13
D) 14
E) 15
A) 0
B) 7
C) 13
D) 14
E) 15

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
8
An electron is in a one-dimensional well with finite potential energy barriers at the walls. The matter wave:
A) is zero at the barriers
B) is zero everywhere within each barrier
C) is zero in the well
D) extends into the barriers
E) is discontinuous at the barriers
A) is zero at the barriers
B) is zero everywhere within each barrier
C) is zero in the well
D) extends into the barriers
E) is discontinuous at the barriers

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
9
An electron is trapped in a deep well with a width of 0.3 nm. If it is in the state with quantum number n = 3 its kinetic energy is:
A) 6.0 * 10-28 J
B) 1.8 * 10-27 J
C) 6.7* 10-19 J
D) 2.0 * 10-18 J
E) 6.0 * 10-18 J
A) 6.0 * 10-28 J
B) 1.8 * 10-27 J
C) 6.7* 10-19 J
D) 2.0 * 10-18 J
E) 6.0 * 10-18 J

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
10
Two one-dimensional traps have infinite potential energy at their walls. Trap A has width L and trap B has width 2L. For which value of the quantum number n does a particle in trap B have the same energy as a particle in the ground state of trap A?
A) n = 1
B) n = 2
C) n = 3
D) n = 4
E) n = 5
A) n = 1
B) n = 2
C) n = 3
D) n = 4
E) n = 5

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
11
A particle is confined to a one-dimensional trap by infinite potential energy walls. Of the following states, designed by the quantum number n, for which one is the probability density greatest near the center of the well?
A) n = 2
B) n = 3
C) n = 4
D) n = 5
E) n = 6
A) n = 2
B) n = 3
C) n = 4
D) n = 5
E) n = 6

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
12
An electron is in a one-dimensional trap with zero potential energy in the interior and infinite potential energy at the walls. The ratio E3/E1 of the energy for n = 3 to that for n = 1 is:
A) 1/3
B) 1/9
C) 3/1
D) 9/1
E) 1/1
A) 1/3
B) 1/9
C) 3/1
D) 9/1
E) 1/1

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
13
The ground state energy of an electron in a one-dimensional trap with zero potential energy in the interior and infinite potential energy at the walls:
A) is zero
B) decreases with temperature
C) increases with temperature
D) is independent of temperature
E) oscillates with time
A) is zero
B) decreases with temperature
C) increases with temperature
D) is independent of temperature
E) oscillates with time

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
14
An electron is in a one-dimensional trap with zero potential energy in the interior and infinite potential energy at the walls. A graph of its probability density P(x) versus x is shown. The value of the quantum number n is: 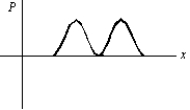
A) 0
B) 1
C) 2
D) 3
E) 4

A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
15
Four different particles are trapped in one-dimensional wells with infinite potential energy at their walls. The masses of the particles and the width of the wells are 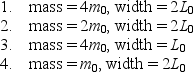 Rank them according to the kinetic energies of the particles when they are in their ground states, lowest to highest.
Rank them according to the kinetic energies of the particles when they are in their ground states, lowest to highest.
A) 3 and 4 tied, then 2, then 1
B) 1, 2, then 3 and 4 tied
C) 1 and 2 tied, then 3, then 4
D) 4, 3, 2, 1
E) 3, 1, 2, 4
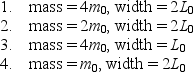 Rank them according to the kinetic energies of the particles when they are in their ground states, lowest to highest.
Rank them according to the kinetic energies of the particles when they are in their ground states, lowest to highest.A) 3 and 4 tied, then 2, then 1
B) 1, 2, then 3 and 4 tied
C) 1 and 2 tied, then 3, then 4
D) 4, 3, 2, 1
E) 3, 1, 2, 4

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
16
Identical particles are trapped in one-dimensional wells with infinite potential energy at the walls. The widths L of the traps and the quantum numbers n of the particles are 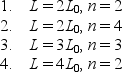 Rank them according to the kinetic energies of the particles, least to greatest.
Rank them according to the kinetic energies of the particles, least to greatest.
A) 1, 2, 3, 4
B) 4, 3, 2, 1
C) 1 and 3 tied, then 2, then 4
D) 4, then 1 and 3 tied, then 2
E) 2, then 1 and 3 tied, then 4
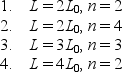 Rank them according to the kinetic energies of the particles, least to greatest.
Rank them according to the kinetic energies of the particles, least to greatest.A) 1, 2, 3, 4
B) 4, 3, 2, 1
C) 1 and 3 tied, then 2, then 4
D) 4, then 1 and 3 tied, then 2
E) 2, then 1 and 3 tied, then 4

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
17
An electron confined in a one-dimensional infinite potential well has an energy of 180 eV. What is its wavelength?
A) 65 pm
B) 91 pm
C) 130 pm
D) 370 pm
E) 520 pm
A) 65 pm
B) 91 pm
C) 130 pm
D) 370 pm
E) 520 pm

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
18
A particle is trapped in an infinite potential energy well. It is in the state with quantum number n = 14. How many maxima does the probability density have?
A) 0
B) 7
C) 13
D) 14
E) 15
A) 0
B) 7
C) 13
D) 14
E) 15

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
19
The ground state energy of an electron in a one-dimensional trap with zero potential energy in the interior and infinite potential energy at the walls is 2.0 eV. If the width of the well is doubled, the ground state energy will be:
A) 0.5 eV
B) 1.0 eV
C) 2.0 eV
D) 4.0 eV
E) 8.0 eV
A) 0.5 eV
B) 1.0 eV
C) 2.0 eV
D) 4.0 eV
E) 8.0 eV

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
20
An electron is in a one-dimensional trap with zero potential energy in the interior and infinite potential energy at the walls. A graph of its wave function (x) versus x is shown. The value of quantum number n is: 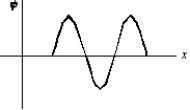
A) 0
B) 1
C) 2
D) 4
E) 8

A) 0
B) 1
C) 2
D) 4
E) 8

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
21
The binding energy of an electron in the ground state in a hydrogen atom is about:
A) 1.0 eV
B) 3.4 eV
C) 10.2 eV
D) 13.6 eV
E) 27.2 eV
A) 1.0 eV
B) 3.4 eV
C) 10.2 eV
D) 13.6 eV
E) 27.2 eV

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
22
The figure shows the energy levels for an electron in a finite potential energy well. If the electron makes a transition from the n = 3 state to the ground state, what is the wavelength of the emitted photon? 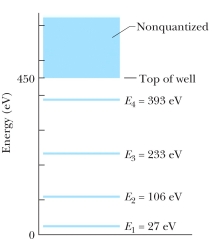
A) 6.0 nm
B) 5.7 nm
C) 5.3 nm
D) 3.0 nm
E) 2.3 nm

A) 6.0 nm
B) 5.7 nm
C) 5.3 nm
D) 3.0 nm
E) 2.3 nm

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
23
If P(r) is the radial probability density for a hydrogen atom then the probability that the separation of the electron and proton is between r and r + dr is:
A) P dr
B) P 2 dr
C) 4 r2P dr
D) 4 r2 P dr
E) 4 P dr
A) P dr
B) P 2 dr
C) 4 r2P dr
D) 4 r2 P dr
E) 4 P dr

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
24
If the wave function is spherically symmetric then the radial probability density is given by:
A) 4 r2
B) 2
C) 4 r2 2
D) 4 2
E) 4 r 2
A) 4 r2
B) 2
C) 4 r2 2
D) 4 2
E) 4 r 2

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
25
The Balmer series of hydrogen is important because it:
A) is the only one for which the Bohr theory can be used
B) is the only series which occurs for hydrogen
C) is in the visible region
D) involves the lowest possible quantum number n
E) involves the highest possible quantum number n
A) is the only one for which the Bohr theory can be used
B) is the only series which occurs for hydrogen
C) is in the visible region
D) involves the lowest possible quantum number n
E) involves the highest possible quantum number n

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following sets of quantum numbers is possible for an electron in a hydrogen atom?
A) n = 4, ℓ = 3, mℓ = −3
B) n = 4, ℓ = 4, mℓ = −2
C) n = 5, ℓ = −1, mℓ = 2
D) n = 3, ℓ = 1, mℓ = −2
E) n = 2, ℓ = 3, mℓ = −2
A) n = 4, ℓ = 3, mℓ = −3
B) n = 4, ℓ = 4, mℓ = −2
C) n = 5, ℓ = −1, mℓ = 2
D) n = 3, ℓ = 1, mℓ = −2
E) n = 2, ℓ = 3, mℓ = −2

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
27
The quantum number n is most closely associated with what property of the electron in a hydrogen atom?
A) Energy
B) Orbital angular momentum
C) Spin angular momentum
D) Magnetic moment
E) z component of angular momentum
A) Energy
B) Orbital angular momentum
C) Spin angular momentum
D) Magnetic moment
E) z component of angular momentum

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
28
The wave function for an electron in a state with zero angular momentum:
A) is zero everywhere
B) is spherically symmetric
C) depends on the angle from the z axis
D) depends on the angle from the x axis
E) is spherically symmetric for some shells and depends on the angle from the z axis for others
A) is zero everywhere
B) is spherically symmetric
C) depends on the angle from the z axis
D) depends on the angle from the x axis
E) is spherically symmetric for some shells and depends on the angle from the z axis for others

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
29
The series limit for the Balmer series represents a transition m n, where (m, n) is
A) (2,1)
B) (3,2)
C) ( ,0)
D) ( ,1)
E) ( ,2)
A) (2,1)
B) (3,2)
C) ( ,0)
D) ( ,1)
E) ( ,2)

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following: 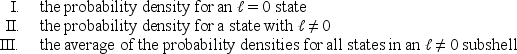 Of these which are spherically symmetric?
Of these which are spherically symmetric?
A) only I
B) only II
C) only I and II
D) only I and III
E) I, II, and III
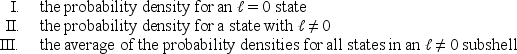 Of these which are spherically symmetric?
Of these which are spherically symmetric?A) only I
B) only II
C) only I and II
D) only I and III
E) I, II, and III

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
31
The radial probability density for the electron in the ground state of a hydrogen atom has a peak at about:
A) 0.5 pm
B) 5 pm
C) 50 pm
D) 500 pm
E) 5000 pm
A) 0.5 pm
B) 5 pm
C) 50 pm
D) 500 pm
E) 5000 pm

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
32
A particle is trapped in a finite potential energy well that is deep enough so that the electron can be in the state with n = 4. For this state how many nodes does the probability density have?
A) 0
B) 1
C) 3
D) 4
E) 5
A) 0
B) 1
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
33
Take the potential energy of a hydrogen atom to be zero for infinite separation of the electron and proton. Then the ground state energy of a hydrogen atom is -13.6 eV. The energy of the first excited state is:
A) 0 eV
B) -3.4 eV
C) -6.8 eV
D) -10.2 eV
E) -27 eV
A) 0 eV
B) -3.4 eV
C) -6.8 eV
D) -10.2 eV
E) -27 eV

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
34
The figure shows the energy levels for an electron in a finite potential energy well. If an electron in the n = 2 state absorbs a photon of wavelength 2.0 nm, what happens to the electron? 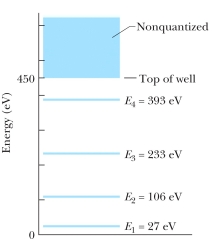
A) It makes a transition to the n = 3 state.
B) It makes a transition to the n = 4 state.
C) It escapes the well with a kinetic energy of 280 eV.
D) It escapes the well with a kinetic energy of 730 eV.
E) Nothing; this photon does not have an energy corresponding to an allowed transition so it is not absorbed.

A) It makes a transition to the n = 3 state.
B) It makes a transition to the n = 4 state.
C) It escapes the well with a kinetic energy of 280 eV.
D) It escapes the well with a kinetic energy of 730 eV.
E) Nothing; this photon does not have an energy corresponding to an allowed transition so it is not absorbed.

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
35
A particle in a certain finite potential energy well can have any of five quantized energy values and no more. Which of the following would allow it to have any of six quantized energy levels?
A) Increase the momentum of the particle
B) Decrease the momentum of the particle
C) Decrease the well width
D) Increase the well depth
E) Decrease the well depth
A) Increase the momentum of the particle
B) Decrease the momentum of the particle
C) Decrease the well width
D) Increase the well depth
E) Decrease the well depth

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
36
The diagram shows the energy levels for an electron in a certain atom. Of the transitions shown, which represents the emission of a photon with the most energy? 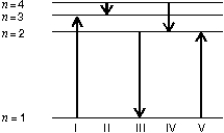
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
37
Take the potential energy of a hydrogen atom to be zero for infinite separation of the electron and proton. Then the ground state energy of a hydrogen atom is -13.6 eV. When the electron is in the first excited state its excitation energy (the difference between the energy of the state and that of the ground state) is:
A) 0
B) 3.4 eV
C) 6.8 eV
D) 10.2 eV
E) 13.6 eV
A) 0
B) 3.4 eV
C) 6.8 eV
D) 10.2 eV
E) 13.6 eV

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
38
Take the potential energy of a hydrogen atom to be zero for infinite separation of the electron and proton. Then the ground state energy of a hydrogen atom is -13.6 eV. The minus sign indicates:
A) the kinetic energy is negative
B) the potential energy is positive
C) the electron might escape from the atom
D) the electron and proton are bound together
E) none of the above
A) the kinetic energy is negative
B) the potential energy is positive
C) the electron might escape from the atom
D) the electron and proton are bound together
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
39
When a hydrogen atom makes the transition from the second excited state to the ground state (at -13.6 eV) the energy of the photon emitted is:
A) 0 eV
B) 1.5 eV
C) 9.1 eV
D) 12.1 eV
E) 13.6 eV
A) 0 eV
B) 1.5 eV
C) 9.1 eV
D) 12.1 eV
E) 13.6 eV

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
40
Take the potential energy of a hydrogen atom to be zero for infinite separation of the electron and proton. Then, according to the quantum theory the energy En of a state with principal quantum number n is proportional to:
A) n
B) n2
C) 1/n
D) 1/n2
E) none of the above
A) n
B) n2
C) 1/n
D) 1/n2
E) none of the above

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck
41
The following image is a dot plot of the ground state of the hydrogen atom. The dots represent: 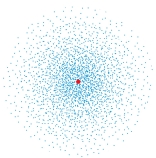
A) all the possible positions of the electron
B) every electron in the atom
C) the electron wave function
D) the volume probability density for the electron
E) the density of the electron cloud

A) all the possible positions of the electron
B) every electron in the atom
C) the electron wave function
D) the volume probability density for the electron
E) the density of the electron cloud

Unlock Deck
Unlock for access to all 41 flashcards in this deck.
Unlock Deck
k this deck


