Deck 38: Photons and Matter Waves
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 38: Photons and Matter Waves
1
In a photoelectric effect experiment no electrons are ejected if the frequency of the incident light is less than A/h, where h is the Planck constant and A is:
A) the maximum energy needed to eject the least energetic electron
B) the minimum energy needed to eject the least energetic electron
C) the maximum energy needed to eject the most energetic electron
D) the minimum energy needed to eject the most energetic electron
E) the intensity of the incident light
A) the maximum energy needed to eject the least energetic electron
B) the minimum energy needed to eject the least energetic electron
C) the maximum energy needed to eject the most energetic electron
D) the minimum energy needed to eject the most energetic electron
E) the intensity of the incident light
the minimum energy needed to eject the most energetic electron
2
Which of the following electromagnetic radiations has photons with the greatest momentum?
A) blue light
B) yellow light
C) x rays
D) radio waves
E) microwaves
A) blue light
B) yellow light
C) x rays
D) radio waves
E) microwaves
x rays
3
A photon in light beam A has twice the energy of a photon in light beam B. The ratio pA/pB of their momenta is:
A) 1/2
B) 1/4
C) 1
D) 2
E) 4
A) 1/2
B) 1/4
C) 1
D) 2
E) 4
2
4
In a photoelectric effect experiment at a frequency above cut off, the number of electrons ejected is proportional to:
A) their kinetic energy
B) their potential energy
C) the work function
D) the frequency of the incident light
E) the number of photons that hit the sample
A) their kinetic energy
B) their potential energy
C) the work function
D) the frequency of the incident light
E) the number of photons that hit the sample

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
Light beams A and B have the same intensity but the wavelength associated with beam A is longer than that associated with beam B. The photon flux (number crossing a unit area per unit time) is:
A) greater for A than for B
B) greater for B than for A
C) the same for A and B
D) greater for A than for B only if both have short wavelengths
E) greater for B than for A only if both have short wavelengths
A) greater for A than for B
B) greater for B than for A
C) the same for A and B
D) greater for A than for B only if both have short wavelengths
E) greater for B than for A only if both have short wavelengths

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
Rank following electromagnetic radiations according to the energies of their photons, from least to greatest. 
A) 1, 2, 3, 4
B) 4, 2, 1, 3
C) 4, 1, 2, 3
D) 3, 2, 1, 4
E) 3, 1, 2, 4

A) 1, 2, 3, 4
B) 4, 2, 1, 3
C) 4, 1, 2, 3
D) 3, 2, 1, 4
E) 3, 1, 2, 4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following electromagnetic radiations has photons with the greatest energy?
A) blue light
B) yellow light
C) x rays
D) radio waves
E) microwaves
A) blue light
B) yellow light
C) x rays
D) radio waves
E) microwaves

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
The wavelength of light beam B is twice the wavelength of light beam B. The energy of a photon in beam A is:
A) one-fourth the energy of a photon in beam B
B) half the energy of photon in beam B
C) equal to the energy of a photon in beam B
D) twice energy of a photon in beam B
E) four times the energy of a photon in beam B
A) one-fourth the energy of a photon in beam B
B) half the energy of photon in beam B
C) equal to the energy of a photon in beam B
D) twice energy of a photon in beam B
E) four times the energy of a photon in beam B

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
The main problem physicists had with understanding the photoelectric effect before Einstein explained it in terms of photons was:
A) the intensity of emitted electrons did not depend on the intensity of the source.
B) the maximum energy of the emitted electrons did not depend on the frequency of the source.
C) the maximum energy of the emitted electrons did not depend on the intensity of the source.
D) the cutoff frequency depended on the material used as a target.
E) the cutoff frequency did not depend on the material used as a target.
A) the intensity of emitted electrons did not depend on the intensity of the source.
B) the maximum energy of the emitted electrons did not depend on the frequency of the source.
C) the maximum energy of the emitted electrons did not depend on the intensity of the source.
D) the cutoff frequency depended on the material used as a target.
E) the cutoff frequency did not depend on the material used as a target.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
In a photoelectric effect experiment the stopping potential is:
A) the energy required to remove an electron from the sample
B) the kinetic energy of the most energetic electron ejected
C) the potential energy of the most energetic electron ejected
D) the photon energy
E) the electric potential that causes the electron current to vanish
A) the energy required to remove an electron from the sample
B) the kinetic energy of the most energetic electron ejected
C) the potential energy of the most energetic electron ejected
D) the photon energy
E) the electric potential that causes the electron current to vanish

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
In a photoelectric effect experiment at a frequency above cut off, the stopping potential is proportional to:
A) the energy of the least energetic electron before it is ejected
B) the energy of the least energetic electron after it is ejected
C) the energy of the most energetic electron before it is ejected
D) the energy of the most energetic electron after it is ejected
E) the electron potential energy at the surface of the sample
A) the energy of the least energetic electron before it is ejected
B) the energy of the least energetic electron after it is ejected
C) the energy of the most energetic electron before it is ejected
D) the energy of the most energetic electron after it is ejected
E) the electron potential energy at the surface of the sample

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
The frequency of light beam A is twice that of light beam B. The ratio EA/EB of photon energies is:
A) 1/2
B) 1/4
C) 1
D) 2
E) 4
A) 1/2
B) 1/4
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
In Compton scattering from stationary electrons the largest change in wavelength occurs when the photon is scattered through:
A) 0
B) 22.5
C) 45
D) 90
E) 180
A) 0
B) 22.5
C) 45
D) 90
E) 180

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
The quantization of energy, E = nhf, is not important for an ordinary pendulum because:
A) the formula applies only to mass-spring oscillators
B) the allowed energy levels are too closely spaced
C) the allowed energy levels are too widely spaced
D) the formula applies only to atoms
E) the value of h for a pendulum is too large
A) the formula applies only to mass-spring oscillators
B) the allowed energy levels are too closely spaced
C) the allowed energy levels are too widely spaced
D) the formula applies only to atoms
E) the value of h for a pendulum is too large

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
The work function for a certain sample is 2.3 eV. The stopping potential for electrons ejected from the sample by 7.0 * 1014-Hz electromagnetic radiation is:
A) 0 V
B) 0.60 V
C) 2.3 V
D) 2.9 V
E) 5.2 V
A) 0 V
B) 0.60 V
C) 2.3 V
D) 2.9 V
E) 5.2 V

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
The concentration of photons in a uniform light beam with a wavelength of 500 nm is 1.7 *1013 m - 3. The intensity of the beam is:
A) 6.8 *10 - 6 W/m2
B) 3.2 * 102 W/m2
C) 1.0 * 103 W/m2
D) 2.0 *103 W/m2
E) 4.0 * 103 W/m2
A) 6.8 *10 - 6 W/m2
B) 3.2 * 102 W/m2
C) 1.0 * 103 W/m2
D) 2.0 *103 W/m2
E) 4.0 * 103 W/m2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
The diagram shows the graphs of the stopping potential as a function of the frequency of the incident light for photoelectric experiments performed on three different materials. Rank the materials according to the values of their work functions, from least to greatest. 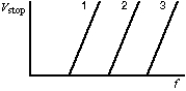
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 2, 1, 3
E) 1, 3, 2

A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 2, 1, 3
E) 1, 3, 2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
The stopping potential for electrons ejected by 6.8 * 1014-Hz electromagnetic radiation incident on a certain sample is 1.8 V. The kinetic energy of the most energetic electrons ejected and the work function of the sample, respectively, are:
A) 1.8 eV, 2.8 eV
B) 1.8 eV, 1.0 eV
C) 1.8 eV, 4.6 eV
D) 2.8 eV, 1.0 eV
E) 1.0 eV, 4.6 eV
A) 1.8 eV, 2.8 eV
B) 1.8 eV, 1.0 eV
C) 1.8 eV, 4.6 eV
D) 2.8 eV, 1.0 eV
E) 1.0 eV, 4.6 eV

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
The units of the Planck constant h are those of:
A) energy
B) power
C) momentum
D) angular momentum
E) frequency
A) energy
B) power
C) momentum
D) angular momentum
E) frequency

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
The intensity of a light beam with a wavelength of 500 nm is 2000 W/m2. The photon flux is about:
A) 5* 1017 /m2.s
B) 5 *1019 /m2.s
C) 5 * 1021 /m2.s
D) 5 * 1023 /m2.s
E) 5 *1025 /m2.s
A) 5* 1017 /m2.s
B) 5 *1019 /m2.s
C) 5 * 1021 /m2.s
D) 5 * 1023 /m2.s
E) 5 *1025 /m2.s

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following three particles:  Rank them according to the wavelengths of their matter waves, least to greatest.
Rank them according to the wavelengths of their matter waves, least to greatest.
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied
 Rank them according to the wavelengths of their matter waves, least to greatest.
Rank them according to the wavelengths of their matter waves, least to greatest.A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
The frequency and wavelength of the matter wave associated with a 10-eV free electron are:
A) 1.5 * 1034 Hz, 3.9 * 10-10 m
B) 1.5*1034 Hz, 1.3 *10-34 m
C) 2.4 * 1015 Hz, 1.2 *10-9 m
D) 2.4 * 1015 Hz, 3.9 *10-10 m
E) 4.8* 1015 Hz, 1.9*10-10 m
A) 1.5 * 1034 Hz, 3.9 * 10-10 m
B) 1.5*1034 Hz, 1.3 *10-34 m
C) 2.4 * 1015 Hz, 1.2 *10-9 m
D) 2.4 * 1015 Hz, 3.9 *10-10 m
E) 4.8* 1015 Hz, 1.9*10-10 m

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
Electromagnetic radiation with a wavelength of 3.5 * 10-12 m is scattered from stationary electrons, and photons that have been scattered through 50 are detected. After a scattering event the magnitude of the photon's momentum is:
A) 0 kg.m/s
B) 8.7 *10-23 kg.m/s
C) 1.5 * 10-22 kg.m/s
D) 2.0 *10-22 kg.m/s
E) 2.2 * 10-22 kg.m/s
A) 0 kg.m/s
B) 8.7 *10-23 kg.m/s
C) 1.5 * 10-22 kg.m/s
D) 2.0 *10-22 kg.m/s
E) 2.2 * 10-22 kg.m/s

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
In Compton scattering from stationary particles the maximum change in wavelength can be made smaller by using:
A) higher frequency radiation
B) lower frequency radiation
C) more massive particles
D) less massive particles
E) particles with greater charge
A) higher frequency radiation
B) lower frequency radiation
C) more massive particles
D) less massive particles
E) particles with greater charge

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
The surface of the Sun is at a temperature of approximately 5800 K, and radiates a peak wavelength of 500 nm. According to the Planck radiation law, what is its emitted intensity per unit wavelength at the peak?
A) 8.4 W/cm2∙nm
B) 42 W/cm2∙nm
C) 84 W/cm2∙nm
D) 8.4 x 103 W/cm2∙nm
E) 4.2 x 107 W/cm2∙nm
A) 8.4 W/cm2∙nm
B) 42 W/cm2∙nm
C) 84 W/cm2∙nm
D) 8.4 x 103 W/cm2∙nm
E) 4.2 x 107 W/cm2∙nm

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
Electromagnetic radiation with a wavelength of 3.5 *10-12 m is scattered from stationary electrons and photons that have been scattered through 50 are detected. An electron from which one of these photons was scattered receives an energy of:
A) 0 J
B) 1.1 * 10-14 J
C) 1.9 *10-14 J
D) 2.3 * 10-14 J
E) 1.3 *10-13 J
A) 0 J
B) 1.1 * 10-14 J
C) 1.9 *10-14 J
D) 2.3 * 10-14 J
E) 1.3 *10-13 J

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is NOT evidence for the wave nature of matter?
A) The photoelectric effect
B) The diffraction pattern obtained when electrons pass through a slit
C) Electron tunneling
D) The validity of the Heisenberg uncertainty principle
E) The interference pattern obtained when electrons pass through a two-slit system
A) The photoelectric effect
B) The diffraction pattern obtained when electrons pass through a slit
C) Electron tunneling
D) The validity of the Heisenberg uncertainty principle
E) The interference pattern obtained when electrons pass through a two-slit system

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
Separate Compton effect experiments are carried out using visible light and x rays. The scattered radiation is observed at the same scattering angle. For these experiments:
A) the x rays have the greater shift in wavelength and the greater change in photon energy
B) the two radiations have the same shift in wavelength and the x rays have the greater change in photon energy
C) the two radiations have the same shift in wavelength and the visible light has the greater change in photon energy
D) the two radiations have the same shift in wavelength and the same change in photon energy
E) the visible light has the greater shift in wavelength and the greater shift in photon energy
A) the x rays have the greater shift in wavelength and the greater change in photon energy
B) the two radiations have the same shift in wavelength and the x rays have the greater change in photon energy
C) the two radiations have the same shift in wavelength and the visible light has the greater change in photon energy
D) the two radiations have the same shift in wavelength and the same change in photon energy
E) the visible light has the greater shift in wavelength and the greater shift in photon energy

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
Electromagnetic radiation with a wavelength of 5.7 * 10-12 m is incident on stationary electrons. Radiation that has a wavelength of 6.6 * 10-12 m is detected at a scattering angle of:
A) 10
B) 40
C) 50
D) 69
E) 111
A) 10
B) 40
C) 50
D) 69
E) 111

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
Evidence for the wave nature of matter is:
A) electron diffraction experiments of Davisson and Germer
B) Thompson's measurement of e/m
C) Young's double slit experiment
D) the Compton effect
E) Lenz's law
A) electron diffraction experiments of Davisson and Germer
B) Thompson's measurement of e/m
C) Young's double slit experiment
D) the Compton effect
E) Lenz's law

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
Monoenergetic electrons are incident on a single slit barrier. If the energy of each incident electron is increased the central maximum of the diffraction pattern:
A) widens
B) narrows
C) stays the same width
D) widens for slow electrons and narrows for fast electrons
E) narrows for slow electrons and widens for fast electrons
A) widens
B) narrows
C) stays the same width
D) widens for slow electrons and narrows for fast electrons
E) narrows for slow electrons and widens for fast electrons

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
Of the following, Compton scattering from electrons is most easily observed for:
A) microwaves
B) infrared light
C) visible light
D) ultraviolet light
E) x rays
A) microwaves
B) infrared light
C) visible light
D) ultraviolet light
E) x rays

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
In Compton scattering from stationary electrons the largest change in wavelength that can occur is:
A) 2.43 * 10-15 m
B) 2.43 * 10-12 m
C) 2.43 * 10-9 m
D) dependent on the frequency of the incident light
E) dependent on the work function
A) 2.43 * 10-15 m
B) 2.43 * 10-12 m
C) 2.43 * 10-9 m
D) dependent on the frequency of the incident light
E) dependent on the work function

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
The main problem that physicists had in understanding blackbody radiation before Planck's work was:
A) Blackbody radiation came from objects that were not actually black.
B) The classically predicted frequency spectrum showed an infinitely large peak at low frequencies.
C) The classically predicted frequency spectrum showed an infinitely large peak at high frequencies.
D) The classically predicted frequency spectrum had a minimum intensity rather than a maximum as observed.
E) The classically predicted frequency spectrum had a maximum intensity that decreased with temperature, rather than increasing as observed.
A) Blackbody radiation came from objects that were not actually black.
B) The classically predicted frequency spectrum showed an infinitely large peak at low frequencies.
C) The classically predicted frequency spectrum showed an infinitely large peak at high frequencies.
D) The classically predicted frequency spectrum had a minimum intensity rather than a maximum as observed.
E) The classically predicted frequency spectrum had a maximum intensity that decreased with temperature, rather than increasing as observed.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
A free electron has a momentum of 5.0 * 10-24 kg . m/s. Its wavelength, as given by its wave function, is:
A) 1.3 * 10-8 m
B) 1.3 * 10-10 m
C) 2.3 * 10-11 m
D) 2.3 *10-13 m
E) none of these
A) 1.3 * 10-8 m
B) 1.3 * 10-10 m
C) 2.3 * 10-11 m
D) 2.3 *10-13 m
E) none of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the following: I. A photoelectric process in which all emitted electrons have energy less than hf, where f is the frequency of the incident light.
II) A photoelectric process in which some emitted electrons have kinetic energy greater than hf.
III) Compton scattering from stationary electrons for which the emitted light has a frequency that is greater than that of the incident light.
IV) Compton scattering from stationary electrons for which the emitted light has a frequency that is less than that of the incident light.
The only possible processes are:
A) I
B) III
C) I and III
D) I and IV
E) II and IV
II) A photoelectric process in which some emitted electrons have kinetic energy greater than hf.
III) Compton scattering from stationary electrons for which the emitted light has a frequency that is greater than that of the incident light.
IV) Compton scattering from stationary electrons for which the emitted light has a frequency that is less than that of the incident light.
The only possible processes are:
A) I
B) III
C) I and III
D) I and IV
E) II and IV

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
Of the following which is the best evidence for the wave nature of matter?
A) The photoelectric effect
B) The Compton effect
C) The spectral radiancy of cavity radiation
D) The relationship between momentum and energy for an electron
E) The reflection of electrons by crystals
A) The photoelectric effect
B) The Compton effect
C) The spectral radiancy of cavity radiation
D) The relationship between momentum and energy for an electron
E) The reflection of electrons by crystals

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
What is the temperature of a burner on an electric stove when its glow is barely visible, at a wavelength of 700 nm? Assume the burner radiates as an ideal blackbody and that 700 nm represents the peak of its emission spectrum.
A) 41 K
B) 240 K
C) 410 K
D) 2400 K
E) 4100 K
A) 41 K
B) 240 K
C) 410 K
D) 2400 K
E) 4100 K

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
In Compton scattering from stationary electrons the frequency of the emitted light is independent of:
A) the frequency of the incident light
B) the recoil speed of the electron
C) the scattering angle
D) the electron recoil energy
E) none of the above
A) the frequency of the incident light
B) the recoil speed of the electron
C) the scattering angle
D) the electron recoil energy
E) none of the above

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the following three particles:  Rank them according to the wavelengths of their waves, least to greatest.
Rank them according to the wavelengths of their waves, least to greatest.
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied
 Rank them according to the wavelengths of their waves, least to greatest.
Rank them according to the wavelengths of their waves, least to greatest.A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
The uncertainty in position of an electron in a certain state is 5 * 10-10 m. The uncertainty in its momentum could be
A) 5.0 * 10-24 kg.m/s
B) 4.0 *10-24 kg.m/s
C) 3.0 *10-24 kg.m/s
D) any of the above
E) none of the above
A) 5.0 * 10-24 kg.m/s
B) 4.0 *10-24 kg.m/s
C) 3.0 *10-24 kg.m/s
D) any of the above
E) none of the above

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
A free electron in motion along the x axis has a localized wave function. The uncertainty in its momentum is decreased if:
A) the wave function is made more narrow
B) the wave function is made less narrow
C) the wave function remains the same but the energy of the electron is increased
D) the wave function remains the same but the energy of the electron is decreased
E) none of the above
A) the wave function is made more narrow
B) the wave function is made less narrow
C) the wave function remains the same but the energy of the electron is increased
D) the wave function remains the same but the energy of the electron is decreased
E) none of the above

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
An electron with energy E is incident upon a potential energy barrier of height Epot < E and thickness L. The reflection coefficient R:
A) is always equal to zero
B) is always equal to 1
C) does not depend on E - Epot
D) is, in general, not equal to zero
E) is equal to T + 1 where T is the transmission coefficient
A) is always equal to zero
B) is always equal to 1
C) does not depend on E - Epot
D) is, in general, not equal to zero
E) is equal to T + 1 where T is the transmission coefficient

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
The probability that a particle is in a given small region of space is proportional to:
A) its energy
B) its momentum
C) the magnitude of its wave function
D) the wavelength of its wave function
E) the square of the magnitude of its wave function
A) its energy
B) its momentum
C) the magnitude of its wave function
D) the wavelength of its wave function
E) the square of the magnitude of its wave function

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
Identical particles, each with energy E, are incident on the following four potential energy barriers: 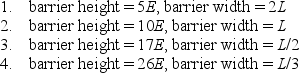 Rank the barriers in terms of the probability that the particles tunnel through them, from least probability to greatest probability.
Rank the barriers in terms of the probability that the particles tunnel through them, from least probability to greatest probability.
A) 1, 2, 3, 4
B) 4, 3, 2, 1
C) 1 and 2 tied, then 3, then4
D) 1, then 2 and 3 tied, then 4
E) 3, 2, 1, 4
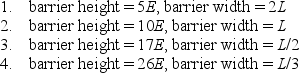 Rank the barriers in terms of the probability that the particles tunnel through them, from least probability to greatest probability.
Rank the barriers in terms of the probability that the particles tunnel through them, from least probability to greatest probability.A) 1, 2, 3, 4
B) 4, 3, 2, 1
C) 1 and 2 tied, then 3, then4
D) 1, then 2 and 3 tied, then 4
E) 3, 2, 1, 4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
The significance of 2 is:
A) probability
B) energy
C) probability density
D) energy density
E) wavelength
A) probability
B) energy
C) probability density
D) energy density
E) wavelength

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
An electron with energy E is incident on a potential energy barrier of height Epot and thickness L. The probability of tunneling increases if:
A) E decreases without any other changes
B) Epot increases without any other changes
C) L decreases without any other changes
D) E and Epot increase by the same amount
E) E and Epot decrease by the same amount
A) E decreases without any other changes
B) Epot increases without any other changes
C) L decreases without any other changes
D) E and Epot increase by the same amount
E) E and Epot decrease by the same amount

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
An electron with energy E is incident upon a potential energy barrier of height Epot < E and thickness L. If the reflection coefficient R = 0.05,
A) the electron has a 0.05% chance of being reflected
B) the electron has a 5% chance of being reflected
C) the electron has a 95% chance of being reflected
D) the electron will be partially reflected and partially transmitted
E) the electron has no chance of being reflected
A) the electron has a 0.05% chance of being reflected
B) the electron has a 5% chance of being reflected
C) the electron has a 95% chance of being reflected
D) the electron will be partially reflected and partially transmitted
E) the electron has no chance of being reflected

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
A free electron and a free proton have the same momentum. This means that, compared to the matter wave associated with the proton, the matter wave associated with the electron has:
A) a shorter wavelength and a greater frequency
B) a longer wavelength and a greater frequency
C) the same wavelength and the same frequency
D) the same wavelength and a greater frequency
E) the same wavelength and a smaller frequency
A) a shorter wavelength and a greater frequency
B) a longer wavelength and a greater frequency
C) the same wavelength and the same frequency
D) the same wavelength and a greater frequency
E) the same wavelength and a smaller frequency

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
The reflection coefficient R for a certain barrier tunneling problem is 0.80. The corresponding transmission coefficient T is:
A) 0.80
B) 0.60
C) 0.50
D) 0.20
E) 0
A) 0.80
B) 0.60
C) 0.50
D) 0.20
E) 0

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
A free electron in motion along the x axis has a localized wave function. If it enters a region of space where its potential energy increases,
A) its total energy will decrease.
B) its momentum will increase.
C) its wave number will increase.
D) its wavelength will increase.
E) its kinetic energy will increase.
A) its total energy will decrease.
B) its momentum will increase.
C) its wave number will increase.
D) its wavelength will increase.
E) its kinetic energy will increase.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
A free electron and a free proton have the same speed. This means that, compared to the matter wave associated with the proton, the matter wave associated with the electron has:
A) a shorter wavelength and a greater frequency
B) a longer wavelength and a greater frequency
C) a shorter wavelength and a smaller frequency
D) the same wavelength and a greater frequency
E) a longer wavelength and a smaller frequency
A) a shorter wavelength and a greater frequency
B) a longer wavelength and a greater frequency
C) a shorter wavelength and a smaller frequency
D) the same wavelength and a greater frequency
E) a longer wavelength and a smaller frequency

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
In order to tunnel through a potential barrier a particle must:
A) have energy greater than the barrier height
B) have spin
C) be massive
D) have a wavelength longer than the barrier width
E) none of the above
A) have energy greater than the barrier height
B) have spin
C) be massive
D) have a wavelength longer than the barrier width
E) none of the above

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
A non-relativistic free electron has kinetic energy K. If its wavelength doubles, its kinetic energy is:
A) 4 K
B) 2 K
C) K
D) K/2
E) K/4
A) 4 K
B) 2 K
C) K
D) K/2
E) K/4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
Maxwell's equations are to electric and magnetic fields as __________ equation is to the wave function of the particle.
A) Einstein's
B) Fermi's
C) Newton's
D) Schrödinger's
E) Bohr's
A) Einstein's
B) Fermi's
C) Newton's
D) Schrödinger's
E) Bohr's

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
A free electron and a free proton have the same kinetic energy. This means that, compared to the matter wave associated with the proton, the matter wave associated with the electron has:
A) a shorter wavelength and a greater frequency
B) a longer wavelength and a greater frequency
C) a shorter wavelength and the same frequency
D) a longer wavelength and the same frequency
E) a shorter wavelength and a smaller frequency
A) a shorter wavelength and a greater frequency
B) a longer wavelength and a greater frequency
C) a shorter wavelength and the same frequency
D) a longer wavelength and the same frequency
E) a shorter wavelength and a smaller frequency

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
An electron with energy E is incident upon a potential energy barrier of height Epot > E and thickness L. The transmission coefficient T:
A) is zero
B) decreases exponentially with L
C) is proportional to 1/L
D) is proportional to 1/L2
E) is non-zero and independent of L
A) is zero
B) decreases exponentially with L
C) is proportional to 1/L
D) is proportional to 1/L2
E) is non-zero and independent of L

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck


