Deck 9: Chemical Change
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/51
Play
Full screen (f)
Deck 9: Chemical Change
1
In which pair are both strong acids?
A)HF and HCl
B)HC2H3O2 and HN3
C)HNO2 and H2SO3
D)H2CO3 and H3PO4
E)HClO4 and HI
A)HF and HCl
B)HC2H3O2 and HN3
C)HNO2 and H2SO3
D)H2CO3 and H3PO4
E)HClO4 and HI
HClO4 and HI
2
What happens when a strong acid is placed in water?
A)The solution cools as the hydronium ions form
B)Hydrogen gas is released by the reaction of the acid with the water
C)The acid remains in its molecular form
D)The water separates completely into ions
E)The acid dissociates completely into ions
A)The solution cools as the hydronium ions form
B)Hydrogen gas is released by the reaction of the acid with the water
C)The acid remains in its molecular form
D)The water separates completely into ions
E)The acid dissociates completely into ions
The acid dissociates completely into ions
3
If a solution conducts electricity,it is positive evidence that...
A)molecules are dissolved in the solution.
B)the solution is pure water.
C)the solute must be a nonelectrolyte.
D)the solute is an strong electrolyte.
E)mobile ions are present.
A)molecules are dissolved in the solution.
B)the solution is pure water.
C)the solute must be a nonelectrolyte.
D)the solute is an strong electrolyte.
E)mobile ions are present.
mobile ions are present.
4
Which of the following statements is incorrect?
A)It is the movement of ions that makes up an electric current in a solution
B)A sugar solution is a nonconductor because no ions are present
C)An electric current is a movement of electric charge
D)There are no free-to-move electrons in a solution
E)Cations in a solution move toward the positively charged electrode
A)It is the movement of ions that makes up an electric current in a solution
B)A sugar solution is a nonconductor because no ions are present
C)An electric current is a movement of electric charge
D)There are no free-to-move electrons in a solution
E)Cations in a solution move toward the positively charged electrode

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
5
What ions are present in an aqueous solution of hydrochloric acid?
A)H2+(aq)+ Cl2-(aq)
B)HCl(aq)
C)H+(aq)+ Cl-(aq)
D)H3O+(aq)+ ClO3-(aq)
E)H+(aq)+ ClO3-(aq)
A)H2+(aq)+ Cl2-(aq)
B)HCl(aq)
C)H+(aq)+ Cl-(aq)
D)H3O+(aq)+ ClO3-(aq)
E)H+(aq)+ ClO3-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
6
Consider a solution in which the solute exists only as the molecules shown below.  Which of the following describes this solute?
Which of the following describes this solute?
A)nonelectrolyte
B)strong electrolyte
C)weak electrolyte
D)ionic
 Which of the following describes this solute?
Which of the following describes this solute?A)nonelectrolyte
B)strong electrolyte
C)weak electrolyte
D)ionic

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
7
In an ionic solution,positively charged cations are attracted to the negatively charged electrode,called ____(i)____.Similarly,negatively charged anions move to the positively charged electrode,called ___(ii)___.
A)(i)a cathode (ii)an anode
B)(i)an anode (ii)a cathode
C)(i)a strong electrolyte (ii)a weak electrolyte
D)(i)a weak electrolyte (ii)a strong electrolyte
E)(i)a nonelectrolyte (ii)an electrolyte
A)(i)a cathode (ii)an anode
B)(i)an anode (ii)a cathode
C)(i)a strong electrolyte (ii)a weak electrolyte
D)(i)a weak electrolyte (ii)a strong electrolyte
E)(i)a nonelectrolyte (ii)an electrolyte

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following gives the correct formulas and ratio of ions present when sodium carbonate is dissolved in water?
A)S2-(aq)+ CO2+(aq)
B)2 Na+(aq)+ CO32-(aq)
C)2 Na+(aq)+ C4+(aq)+ 3 O2-(aq)
D)Na2+(aq)+ CO42-(aq)
E)2 Na+(aq)+ CO42-(aq)
A)S2-(aq)+ CO2+(aq)
B)2 Na+(aq)+ CO32-(aq)
C)2 Na+(aq)+ C4+(aq)+ 3 O2-(aq)
D)Na2+(aq)+ CO42-(aq)
E)2 Na+(aq)+ CO42-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the best description of what happens when a weak acid is placed in water?
A)The acid is only slightly dissociated
B)About half of the water molecules dissociate into ions
C)The acid almost completely ionizes
D)A chemical reaction results in the production of hydrogen gas
E)A large amount of electrical current is generated,although it is weak
A)The acid is only slightly dissociated
B)About half of the water molecules dissociate into ions
C)The acid almost completely ionizes
D)A chemical reaction results in the production of hydrogen gas
E)A large amount of electrical current is generated,although it is weak

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
10
Some solutes have solutions that conduct electricity,but poorly.Which of the following terms best describes these solutes?
A)Highly charged ions
B)Ions with fractional charges
C)Nonconductor
D)Weak electrolyte
E)Cation solutions
A)Highly charged ions
B)Ions with fractional charges
C)Nonconductor
D)Weak electrolyte
E)Cation solutions

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following correctly describes an electric current?
(i)Spontaneous flow of protons in gold,silver,copper,or aluminum wires
(ii)Exchange of protons between cations in a solution
(iii)The movement of ions in a solution
(iv)The movement of negatively charged electrons in a metal
A)i only
B)iv only
C)i and ii
D)iii and iv
E)i,ii,and iv
(i)Spontaneous flow of protons in gold,silver,copper,or aluminum wires
(ii)Exchange of protons between cations in a solution
(iii)The movement of ions in a solution
(iv)The movement of negatively charged electrons in a metal
A)i only
B)iv only
C)i and ii
D)iii and iv
E)i,ii,and iv

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a weak acid?
A)Hydrochloric acid,HCl
B)Hydrocyanic acid,HCN
C)Chloric acid,HClO3
D)Nitric acid,HNO3
E)Sulfuric acid,H2SO4
A)Hydrochloric acid,HCl
B)Hydrocyanic acid,HCN
C)Chloric acid,HClO3
D)Nitric acid,HNO3
E)Sulfuric acid,H2SO4

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
13
A solute whose solution is a good conductor of electricity is classified into which of the following categories?
A)a molecular substance
B)a weak electrolyte
C)a strong electrolyte
D)a nonelectrolyte
E)an insulator
A)a molecular substance
B)a weak electrolyte
C)a strong electrolyte
D)a nonelectrolyte
E)an insulator

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
14
How should pure water be classified with respect to its electrical conductivity properties?
A)Weak electrolyte
B)Nonelectrolyte
C)Strong electrolyte
D)Good conductor
E)Weak conductor
A)Weak electrolyte
B)Nonelectrolyte
C)Strong electrolyte
D)Good conductor
E)Weak conductor

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
15
What ions are present in what ratio in a solution of aqueous calcium chloride?
A)Ca2+(aq)+ Cl22-(aq)
B)Ca2+(aq)+ Cl2-(aq)
C)Ca2+(aq)+ 2 Cl-(aq)
D)CaCl+(aq)+ Cl-(aq)
E)Ca2+(aq)+ Cl2-(aq)
A)Ca2+(aq)+ Cl22-(aq)
B)Ca2+(aq)+ Cl2-(aq)
C)Ca2+(aq)+ 2 Cl-(aq)
D)CaCl+(aq)+ Cl-(aq)
E)Ca2+(aq)+ Cl2-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is the best explanation of why a weak acid is a poor conductor?
A)The weak acid molecules undergo a precipitation reaction in aqueous solution
B)The weak acid molecules move too slowly to conduct well
C)It is only slightly ionized in water
D)It can form only dilute solutions in water
E)It will not dissolve in water
A)The weak acid molecules undergo a precipitation reaction in aqueous solution
B)The weak acid molecules move too slowly to conduct well
C)It is only slightly ionized in water
D)It can form only dilute solutions in water
E)It will not dissolve in water

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
17
What are the formulas of the major species present in a mixture of HNO3 and HF in water?
A)HNO3(aq)+ HF(aq)
B)HNO3(aq)+ H+(aq)+ F-(aq)
C)H+(aq)+ NO3(aq)+ F-(aq)
D)H+(aq)+ NO3-(aq)+ HF(aq)
E)H2(g)+ NO3-(aq)+ F+(aq)
A)HNO3(aq)+ HF(aq)
B)HNO3(aq)+ H+(aq)+ F-(aq)
C)H+(aq)+ NO3(aq)+ F-(aq)
D)H+(aq)+ NO3-(aq)+ HF(aq)
E)H2(g)+ NO3-(aq)+ F+(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the best explanation of why a strong acid is an excellent conductor?
A)It is almost completely ionized in water
B)It undergoes a vigorous chemical reaction with the water molecules
C)It is immiscible in water
D)All acids are natural conductors
E)It reduces electronic vibration and thus allows greater conductivity
A)It is almost completely ionized in water
B)It undergoes a vigorous chemical reaction with the water molecules
C)It is immiscible in water
D)All acids are natural conductors
E)It reduces electronic vibration and thus allows greater conductivity

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
19
What ions are present in what ratio in an aqueous solution of iron(III)sulfate?
A)Fe22+(aq)+ SO42-(aq)
B)Fe22+(aq)+ SO32-(aq)
C)2 Fe3+(aq)+ 12 SO2-(aq)
D)2 Fe3+(aq)+ 3 SO42-(aq)
E)Fe3+(aq)+ S2-(aq)+ O2-(aq)
A)Fe22+(aq)+ SO42-(aq)
B)Fe22+(aq)+ SO32-(aq)
C)2 Fe3+(aq)+ 12 SO2-(aq)
D)2 Fe3+(aq)+ 3 SO42-(aq)
E)Fe3+(aq)+ S2-(aq)+ O2-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
20
What ions are present in what ratio in a solution of aqueous (NH4)2SO4?
A)(NH4)22+(aq)+ SO42-(aq)
B)2 NH4+(aq)+ SO42-(aq)
C)NH4+(aq)+ SO4-(aq)
D)2 N3-(aq)+ 8 H+(aq)+ 4 SO2-(aq)
E)2 N3-(aq)+ 8 H+(aq)+S2-(aq)+ 4 O2-(aq)
A)(NH4)22+(aq)+ SO42-(aq)
B)2 NH4+(aq)+ SO42-(aq)
C)NH4+(aq)+ SO4-(aq)
D)2 N3-(aq)+ 8 H+(aq)+ 4 SO2-(aq)
E)2 N3-(aq)+ 8 H+(aq)+S2-(aq)+ 4 O2-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
21
Write the net ionic equation for the reaction that produces solid silver sulfide.What are the reactants?
A)2 Ag+(aq)+ S2-(aq)
B)Ag+(aq)+ S-(aq)
C)Ag2+(aq)+ 2 S-(aq)
D)Ag2+(aq)+ S2-(aq)
E)2 Ag+(aq)+ SO42-(aq)
A)2 Ag+(aq)+ S2-(aq)
B)Ag+(aq)+ S-(aq)
C)Ag2+(aq)+ 2 S-(aq)
D)Ag2+(aq)+ S2-(aq)
E)2 Ag+(aq)+ SO42-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
22
In the net ionic equation for the following,what are the spectator ions? NH4NO3(aq)+ KCl(aq) KNO3(aq)+ NH4Cl(aq)
A)None of the ions are spectator ions
B)The cations are spectators; the anions are not
C)All the ions are spectators
D)The anions are spectators; the cations are not
E)The potassium ions are spectators
A)None of the ions are spectator ions
B)The cations are spectators; the anions are not
C)All the ions are spectators
D)The anions are spectators; the cations are not
E)The potassium ions are spectators

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
23
Write the net ionic equation for the reaction that occurs,if any,when solutions of aluminum nitrate and sodium hydroxide are combined,producing an insoluble precipitate.What is/are the products?
A)NaNO3(s)+ Al(OH)3(s)
B)NaNO3(s)
C)3 Na+(aq)+ 3 NO3-(s)+ Al(OH)3(s)
D)Al(OH)3(s)
E)There is no reaction
A)NaNO3(s)+ Al(OH)3(s)
B)NaNO3(s)
C)3 Na+(aq)+ 3 NO3-(s)+ Al(OH)3(s)
D)Al(OH)3(s)
E)There is no reaction

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
24
Write the net ionic equation for the reaction that occurs,if any,when cesium metal (Z = 55)is placed in hydrochloric acid.Cesium metal is above hydrogen gas in the activity series.
A)Cs(s)+ H+(aq) H(g)+ Cs+(aq)
B)2 Cs(s)+ 2 H+(aq) H2(g)+ 2 Cs+(aq)
C)2 Cs(s)+ 2 H+(aq)+ 2 Cl-(aq) H2(g)+ 2 Cs+(aq)+ 2 Cl-(aq)
D)2 Cs(s)+ 2 HCl(aq) H2(g)+ 2 CsCl(aq)
E)There is no reaction
A)Cs(s)+ H+(aq) H(g)+ Cs+(aq)
B)2 Cs(s)+ 2 H+(aq) H2(g)+ 2 Cs+(aq)
C)2 Cs(s)+ 2 H+(aq)+ 2 Cl-(aq) H2(g)+ 2 Cs+(aq)+ 2 Cl-(aq)
D)2 Cs(s)+ 2 HCl(aq) H2(g)+ 2 CsCl(aq)
E)There is no reaction

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
25
Write the net ionic equation for the reaction that occurs,if any,when solutions of silver chlorate and sodium sulfide are combined.What are the reactants (with appropriate coefficients)?
A)Na+(aq)+ Cl-(aq)
B)Na+(aq)+ ClO3-(aq)
C)Ag2+(aq)+ S2-(aq)
D)2 Ag+(aq)+ SO42-(aq)
E)2 Ag+(aq)+ S2-(aq)
A)Na+(aq)+ Cl-(aq)
B)Na+(aq)+ ClO3-(aq)
C)Ag2+(aq)+ S2-(aq)
D)2 Ag+(aq)+ SO42-(aq)
E)2 Ag+(aq)+ S2-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
26
Ethanol,C2H5OH,is burned.Write the balanced chemical equation for this reaction.What is the coefficient for H2O?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
27
Write the equation for the complete oxidation of C3H7COC2H5.What is the sum of the coefficients?
A)45
B)43
C)31
D)30
E)none of the above
A)45
B)43
C)31
D)30
E)none of the above

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
28
Write the equation for the complete oxidation of HC2H3O2.What is the sum of the coefficients?
A)4 or less
B)5
C)6
D)7
E)8 or more
A)4 or less
B)5
C)6
D)7
E)8 or more

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following substances will precipitate from an aqueous solution?
A)NaF
B)CaCO3
C)NH4Cl
D)K2SO4
E)Cu(NO3)2
A)NaF
B)CaCO3
C)NH4Cl
D)K2SO4
E)Cu(NO3)2

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
30
What are the reactants in the net ionic equation for the reaction that has magnesium phosphate as the only solid product?
A)3 Mg2+(aq)+ 2 P3-(aq)
B)Mg2+(aq)+ PO32-(aq)
C)Mg2+(aq)+ PO42-(aq)
D)3 Mg2+(aq)+ 2 PO33-(aq)
E)3 Mg2+(aq)+ 2 PO43-(aq)
A)3 Mg2+(aq)+ 2 P3-(aq)
B)Mg2+(aq)+ PO32-(aq)
C)Mg2+(aq)+ PO42-(aq)
D)3 Mg2+(aq)+ 2 PO33-(aq)
E)3 Mg2+(aq)+ 2 PO43-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
31
Write the equation for the complete oxidation of C4H8O.What is the coefficient for O2?
A)2
B)4
C)8
D)11
E)12
A)2
B)4
C)8
D)11
E)12

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
32
Write the net ionic equation for the reaction that occurs,if any,when gold is placed in a solution of hydrobromic acid.Hydrogen gas is above gold in the activity series.What are the products?
A)H2(g)+ 2 AuBr(aq)
B)H2(g)+ 2 Au+(aq)+ 2 Br-(aq)
C)H2(g)+ 2 Au+(aq)
D)H2(g)+ Au2+(aq)
E)There is no reaction
A)H2(g)+ 2 AuBr(aq)
B)H2(g)+ 2 Au+(aq)+ 2 Br-(aq)
C)H2(g)+ 2 Au+(aq)
D)H2(g)+ Au2+(aq)
E)There is no reaction

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
33
What is the net ionic equation for the preparation of Ca3(PO4)2(s)from aqueous solution?
A)3 Ca2+(aq)+ 2 PO43-(aq) Ca3(PO4)2(s)
B)3 Ca(s)+ 2 PO4(aq) Ca3(PO4)2(s)
C)3 CaO(aq)+ 2 P2(s)+ 3 O2(g) Ca3PO4(aq)+ 2 PO4(g)
D)3 Ca2+(aq)+ 2 PO3-(aq)+ 2 O2-(g) Ca3(PO4)2(aq)
E)3 Ca+(aq)+ 2 PO4-(aq) Ca3(PO4)2(s)
A)3 Ca2+(aq)+ 2 PO43-(aq) Ca3(PO4)2(s)
B)3 Ca(s)+ 2 PO4(aq) Ca3(PO4)2(s)
C)3 CaO(aq)+ 2 P2(s)+ 3 O2(g) Ca3PO4(aq)+ 2 PO4(g)
D)3 Ca2+(aq)+ 2 PO3-(aq)+ 2 O2-(g) Ca3(PO4)2(aq)
E)3 Ca+(aq)+ 2 PO4-(aq) Ca3(PO4)2(s)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
34
What is the net ionic equation for the single replacement reaction Ca(s)+ 2 HNO3(aq) H2(g)+ Ca(NO3)2(aq)Calcium is above hydrogen in the activity series.
A)Ca(s)+ NO3-(aq) CaNO3-(aq)
B)2 H+(aq) H2(g)
C)Ca(s)+ 2 H+(aq)+ 2 NO3-(aq) Ca2+(aq)+ H2(g)+ N2O(g)
D)Ca(s)+ 2 H+(aq) Ca2+(aq)+ H2(g)
E)Ca(s)+ 2 H+(aq)+ 2 NO3-(aq) H2(g)+ Ca2+(aq)+ 2 NO3-(aq)
A)Ca(s)+ NO3-(aq) CaNO3-(aq)
B)2 H+(aq) H2(g)
C)Ca(s)+ 2 H+(aq)+ 2 NO3-(aq) Ca2+(aq)+ H2(g)+ N2O(g)
D)Ca(s)+ 2 H+(aq) Ca2+(aq)+ H2(g)
E)Ca(s)+ 2 H+(aq)+ 2 NO3-(aq) H2(g)+ Ca2+(aq)+ 2 NO3-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
35
Propane,C3H8,is burned as a fuel in heating homes.Write the balanced chemical equation for this reaction.What is the coefficient for CO2?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
36
What are the spectator ions in the reaction between HCl and AgNO3 in dilute aqueous solution? The reaction is shown below.  Given that AgCl is insoluble in water and that AgNO3 is soluble,
Given that AgCl is insoluble in water and that AgNO3 is soluble,
A)Ag+(aq)+ Cl-(aq)
B)Cl-(aq)+ NO3-(aq)
C)NO3-(aq)+ H+(aq)
D)Ag+(aq)+ H+(aq)
E)There are no spectator ions in this reaction
 Given that AgCl is insoluble in water and that AgNO3 is soluble,
Given that AgCl is insoluble in water and that AgNO3 is soluble,A)Ag+(aq)+ Cl-(aq)
B)Cl-(aq)+ NO3-(aq)
C)NO3-(aq)+ H+(aq)
D)Ag+(aq)+ H+(aq)
E)There are no spectator ions in this reaction

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
37
Write the net ionic equation for the reaction between copper and AgC2H3O2.The apparatus for the reaction is shown below.  Regard all salts containing acetate ion as soluble in water.Copper is above silver in the activity series.What will appear on the product side of the balanced net ionic equation?
Regard all salts containing acetate ion as soluble in water.Copper is above silver in the activity series.What will appear on the product side of the balanced net ionic equation?
A)Cu(s)+ 2 Ag+(aq)
B)Cu2+(aq)+ 2 Ag(s)
C)C2H3O2-(aq)+ Ag+(aq)
D)Cu(aq)+ 2 Ag+(s)
E)2 Cu(s)+ AgC2H3O2(aq)
 Regard all salts containing acetate ion as soluble in water.Copper is above silver in the activity series.What will appear on the product side of the balanced net ionic equation?
Regard all salts containing acetate ion as soluble in water.Copper is above silver in the activity series.What will appear on the product side of the balanced net ionic equation?A)Cu(s)+ 2 Ag+(aq)
B)Cu2+(aq)+ 2 Ag(s)
C)C2H3O2-(aq)+ Ag+(aq)
D)Cu(aq)+ 2 Ag+(s)
E)2 Cu(s)+ AgC2H3O2(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
38
Write the net ionic equation for the reaction that occurs,if any,when a calcium chip is dropped into a hydrochloric acid solution.Calcium is above hydrogen in the activity series.What are the reactants (with appropriate coefficients)?
A)Ca(s)+ 2 HCl(aq)
B)Ca(s)+ 2 H+(aq)
C)Ca2+(aq)+ 2 H+(aq)
D)Ca(s)+ H2+(aq)
E)There is no net reaction
A)Ca(s)+ 2 HCl(aq)
B)Ca(s)+ 2 H+(aq)
C)Ca2+(aq)+ 2 H+(aq)
D)Ca(s)+ H2+(aq)
E)There is no net reaction

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
39
What is the net ionic equation corresponding to the formation of Cu(OH)2(s)from aqueous solution?
A)C2+(aq)+ U2+(aq)+ 2 OH2-(aq) Cu(OH)2(s)
B)Cu(s)+ O2(g)+ H2(g) Cu(OH)2(s)
C)Cu+(aq)+ 2 OH-(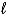 ) Cu(OH)2(s)
) Cu(OH)2(s)
D)Cu2+(aq)+ (OH)22-(aq) Cu(OH)2(s)
E)Cu2+(aq)+ 2 OH-(aq) Cu(OH)2(s)
A)C2+(aq)+ U2+(aq)+ 2 OH2-(aq) Cu(OH)2(s)
B)Cu(s)+ O2(g)+ H2(g) Cu(OH)2(s)
C)Cu+(aq)+ 2 OH-(
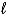 ) Cu(OH)2(s)
) Cu(OH)2(s)D)Cu2+(aq)+ (OH)22-(aq) Cu(OH)2(s)
E)Cu2+(aq)+ 2 OH-(aq) Cu(OH)2(s)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
40
Ethane,C2H6 ,is a component of natural gas and is used in home gas ranges.  In the balanced equation for this reaction what is the coefficient of water?
In the balanced equation for this reaction what is the coefficient of water?
A)4 or less
B)5
C)6
D)7
E)8 or more
 In the balanced equation for this reaction what is the coefficient of water?
In the balanced equation for this reaction what is the coefficient of water?A)4 or less
B)5
C)6
D)7
E)8 or more

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following combinations of reactant does not produce a net change as shown by a net ionic equation?
A)K2CO3(aq)+ CaCl2(aq)
B)HCl(aq)+ NaOH(aq)
C)NaNO3(aq)+ NH4Cl(aq)
D)Na2CO3(aq)+ H2SO4(aq)
E)Li2SO3(aq)+ HCl(aq)
A)K2CO3(aq)+ CaCl2(aq)
B)HCl(aq)+ NaOH(aq)
C)NaNO3(aq)+ NH4Cl(aq)
D)Na2CO3(aq)+ H2SO4(aq)
E)Li2SO3(aq)+ HCl(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
42
What is the net ionic equation for the reaction of any strong acid with a soluble,strong hydroxide base,such as NaOH?
A)H+(aq)+ Na+(aq) H2O(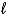 )+ Na(s)
)+ Na(s)
B)H2(g)+ 2 OH-(aq) 2 H2O(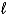 )
)
C)2 H+(aq)+ O(g) H2O(aq)
D)H+(aq)+ OH-(aq) H2O(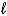 )
)
E)3 H+(aq)+ OH-(aq) H2(g)+ H2O(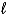 )
)
A)H+(aq)+ Na+(aq) H2O(
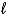 )+ Na(s)
)+ Na(s)B)H2(g)+ 2 OH-(aq) 2 H2O(
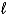 )
)C)2 H+(aq)+ O(g) H2O(aq)
D)H+(aq)+ OH-(aq) H2O(
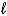 )
)E)3 H+(aq)+ OH-(aq) H2(g)+ H2O(
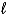 )
)
Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
43
In the balanced net ionic equation for the reaction of solid CaCO3 and aqueous hydrochloric acid,which produces aqueous chloride and an acid that decomposes into the other two products,what appears on the left side of the net ionic equation?
A)CaCO3(s)+ 2 H+(aq)+ 2 Cl-(aq)
B)CaCO3(s)+ 2 Cl-(aq)
C)CaCO3(aq)+ HCl(aq)
D)CaCO3(s)+ 2 H+(aq)
E)Ca2+(aq)+ 2 Cl-(aq)
A)CaCO3(s)+ 2 H+(aq)+ 2 Cl-(aq)
B)CaCO3(s)+ 2 Cl-(aq)
C)CaCO3(aq)+ HCl(aq)
D)CaCO3(s)+ 2 H+(aq)
E)Ca2+(aq)+ 2 Cl-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is a product of a reaction that will always be included in the net ionic equation for the reaction?
A)NH3
B)CO2
C)SO3
D)H2O
E)all of the above
A)NH3
B)CO2
C)SO3
D)H2O
E)all of the above

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
45
What is the preferred way to write H2CO3 when it appears as a product in a net ionic equation?
A)H2(g)+ C(s)+ 1.5 O2(g)
B)H2(g)+ CO3(aq)
C)H2O2(aq)+ CO(g)
D)HCO3-(aq)+ H+(aq)
E)H2O(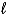 )+ CO2(g)
)+ CO2(g)
A)H2(g)+ C(s)+ 1.5 O2(g)
B)H2(g)+ CO3(aq)
C)H2O2(aq)+ CO(g)
D)HCO3-(aq)+ H+(aq)
E)H2O(
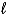 )+ CO2(g)
)+ CO2(g)
Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
46
What appears on the right side of the equation in the net ionic equation for the reaction between Ca(C2H3O2)2 and HCl,where all salts are soluble in water?
A)H+(aq)+ C2H3O2-(aq)
B)No reaction
C)HC2H3O2(aq)
D)Ca2+(aq)+ 2 Cl-(aq)
E)Ca(C2H3O2)2(aq)+ HCl(aq)
A)H+(aq)+ C2H3O2-(aq)
B)No reaction
C)HC2H3O2(aq)
D)Ca2+(aq)+ 2 Cl-(aq)
E)Ca(C2H3O2)2(aq)+ HCl(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following could be a product that would appear in the net ionic equation for a reaction that yields a molecular product?
A)H+(aq)
B)AgCl(s)
C)Br-(aq)
D)H2O(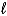 )
)
E)Any of the seven common strong acids
A)H+(aq)
B)AgCl(s)
C)Br-(aq)
D)H2O(
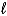 )
)E)Any of the seven common strong acids

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
48
In the net ionic equation for the reaction between a weak acid and strong base,what are the major species on the reactants side?
A)Molecules of acid; ions of the base
B)Molecules of acid; molecules of base
C)Ions of the acid; ions of the base
D)Ions of the acid; molecules of base
E)All species are spectator ions,thus there are no major species on the reactants side
A)Molecules of acid; ions of the base
B)Molecules of acid; molecules of base
C)Ions of the acid; ions of the base
D)Ions of the acid; molecules of base
E)All species are spectator ions,thus there are no major species on the reactants side

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
49
Write the net ionic equation for the reaction that produces solid aluminum phosphate.What are the reactants?
A)Al3+(aq)+ PO43-(aq)
B)Al3+(aq)+ P3-(aq)
C)Al(s)+ PO4(s)
D)Al(aq)+ PO4(aq)
E)Al3+(aq)+ HPO42-(aq)
A)Al3+(aq)+ PO43-(aq)
B)Al3+(aq)+ P3-(aq)
C)Al(s)+ PO4(s)
D)Al(aq)+ PO4(aq)
E)Al3+(aq)+ HPO42-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
50
What appears on the product side in the net ionic equation for the reaction of HNO3(aq)with KOH(aq).KNO3 is water soluble.
A)NO3-(aq)+ K+(aq)
B)H2O(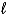 )
)
C)H+(aq)+ K+(aq)
D)H2(g)+ O2(g)
E)K+(aq)+ NO3-(aq)
A)NO3-(aq)+ K+(aq)
B)H2O(
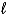 )
)C)H+(aq)+ K+(aq)
D)H2(g)+ O2(g)
E)K+(aq)+ NO3-(aq)

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
51
When it appears on the right side of a net ionic equation,ammonium hydroxide must be rewritten in another form.How should "ammonium hydroxide" appear on the net ionic equation as a product?
A)NH4+(aq)+ OH-(aq)
B)NH3(aq)+ H2O(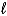 )
)
C)NH3(aq)+ OH-(g)
D)NH3(g)+ H2O(aq)
E)NH3OH(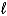 )+ H+(aq)
)+ H+(aq)
A)NH4+(aq)+ OH-(aq)
B)NH3(aq)+ H2O(
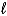 )
)C)NH3(aq)+ OH-(g)
D)NH3(g)+ H2O(aq)
E)NH3OH(
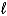 )+ H+(aq)
)+ H+(aq)
Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck


