Deck 18: Oxidation-Reduction Reactions and Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/81
Play
Full screen (f)
Deck 18: Oxidation-Reduction Reactions and Electrochemistry
1
Reduction is a gain of electrons.
True
2
The oxidation state of sulfur in 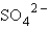 is
is
A) +6
B) +2
C) +4
D) 0
E) +8
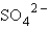 is
isA) +6
B) +2
C) +4
D) 0
E) +8
+6
3
In the reaction 2Ca(s) + O2(g) 2CaO(s), the calcium is ________.
A) oxidized
B) electrolyzed
C) synthesized
D) reduced
E) none of these
A) oxidized
B) electrolyzed
C) synthesized
D) reduced
E) none of these
oxidized
4
The oxidation state of chlorine in ClO- is
A) -2
B) -1
C) 0
D) +1
E) +2
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds contains oxygen with an oxidation state of -2?
A) Na2CO3
B) TiO
C) KOH
D) Li3PO4
E) All of the compounds contain oxygen with an oxidation state of -2.
A) Na2CO3
B) TiO
C) KOH
D) Li3PO4
E) All of the compounds contain oxygen with an oxidation state of -2.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
6
What is the oxidation state of copper in CuSO3 is
A) +2
B) -2
C) 0
D) +1
E) -1
A) +2
B) -2
C) 0
D) +1
E) -1

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the following reaction: Ba + Cl2 BaCl2
Which of the following statements is false?
A) The barium atom is losing electrons; therefore, it is oxidized.
B) The chlorine atom is gaining electrons; therefore, it is oxidized.
C) The barium atom is gaining electrons; therefore, it is oxidized.
D) The chlorine atom is losing electrons; therefore, it is reduced.
E) none of these
Which of the following statements is false?
A) The barium atom is losing electrons; therefore, it is oxidized.
B) The chlorine atom is gaining electrons; therefore, it is oxidized.
C) The barium atom is gaining electrons; therefore, it is oxidized.
D) The chlorine atom is losing electrons; therefore, it is reduced.
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
8
The oxidation state of phosphorus in Mg3(PO4)2 is
A) 0
B) +2
C) +4
D) +5
E) +6
A) 0
B) +2
C) +4
D) +5
E) +6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
9
The oxidation state of oxygen in O2 is
A) -2
B) -1
C) 0
D) -4
E) +4
A) -2
B) -1
C) 0
D) -4
E) +4

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
10
The oxidation state of titanium in TiO is
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
11
What is the oxidation state of Cr in Cr2O72-?
A) +12
B) +6
C) +3
D) 0
E) -2
A) +12
B) +6
C) +3
D) 0
E) -2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
12
In the reaction 2Sr(s) + F2(g) 2SrF(s), the fluorine is ________.
A) reduced
B) electrolyzed
C) galvanized
D) oxidized
E) none of these
A) reduced
B) electrolyzed
C) galvanized
D) oxidized
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
13
The oxidation state of silicon in SiO2 is
A) +4
B) +2
C) 0
D) -2
E) -4
A) +4
B) +2
C) 0
D) -2
E) -4

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
14
The oxidation state of Rb in any compound is
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
15
The oxidation state of Ba in any compound is
A) 0
B) +1
C) +2
D) +3
E) +4
A) 0
B) +1
C) +2
D) +3
E) +4

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
16
The oxidation state of chlorine in MgCl2 is
A) -1
B) +1
C) 0
D) -2
E) +2
A) -1
B) +1
C) 0
D) -2
E) +2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
17
The oxidation state of sulfur in H2SO4 is
A) +6
B) +4
C) +2
D) +8
E) 0
A) +6
B) +4
C) +2
D) +8
E) 0

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
18
The oxidation state of oxygen in Li2CO3 is
A) -2
B) 0
C) +2
D) +4
E) +6
A) -2
B) 0
C) +2
D) +4
E) +6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
19
The oxidation state of manganese in 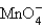 is
is
A) 0
B) +3
C) +4
D) +7
E) +8
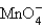 is
isA) 0
B) +3
C) +4
D) +7
E) +8

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
20
The oxidation state of sulfur in Li2SO3 is
A) +4
B) +6
C) +2
D) -2
E) 0
A) +4
B) +6
C) +2
D) -2
E) 0

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
21
The oxidation state of nitrogen in NH4Cl is
A) +1
B) +3
C) -3
D) -4
E) -2
A) +1
B) +3
C) -3
D) -4
E) -2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
22
What is the oxidation state of oxygen in Fe2(CO3)3?
A) +4
B) +3
C) +2
D) 0
E) -2
A) +4
B) +3
C) +2
D) 0
E) -2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
23
In the reaction P4(s) + 10Cl2(g) 4PCl5(s), phosphorus is ______________.
A) reduced
B) electrolyzed
C) synthesized
D) oxidized
E) none of these
A) reduced
B) electrolyzed
C) synthesized
D) oxidized
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement is true of the following reaction? 2K + CuCl2 2KCl + Cu
A) Copper is reduced.
B) Chlorine is reduced.
C) Potassium is reduced.
D) Copper is oxidized.
E) Chlorine is oxidized.
A) Copper is reduced.
B) Chlorine is reduced.
C) Potassium is reduced.
D) Copper is oxidized.
E) Chlorine is oxidized.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
25
The following reaction occurs in aqueous acid solution: NO3- + I- IO3- + NO2
The oxidation state of iodine in IO3- is
A) 0
B) +3
C) -3
D) +5
E) -5
The oxidation state of iodine in IO3- is
A) 0
B) +3
C) -3
D) +5
E) -5

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
26
The oxidation state of chlorine in Cl2 is
A) 0
B) +1
C) -1
D) -2
E) none of these
A) 0
B) +1
C) -1
D) -2
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
27
Answer the questions that refer to the following reaction:
TiCl4(l) + O2(g) TiO2(s) + 2Cl2(g)
-Which species is oxidized?
A) Ti
B) Cl
C) O
D) TiO2
E) O2
TiCl4(l) + O2(g) TiO2(s) + 2Cl2(g)
-Which species is oxidized?
A) Ti
B) Cl
C) O
D) TiO2
E) O2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
28
What is the oxidation state of carbon in Fe2(CO3)3?
A) +4
B) +3
C) +2
D) 0
E) -2
A) +4
B) +3
C) +2
D) 0
E) -2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
29
In the reaction P4(s) + 10Cl2(g) 4PCl5(s), chlorine is ______________.
A) oxidized
B) reduced
C) synthesized
D) electrolyzed
E) none of these
A) oxidized
B) reduced
C) synthesized
D) electrolyzed
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
30
Answer the questions that refer to the following reaction:
SiO2(s) + 2C(s) Si(s) + 2CO(g)
-Which species is reduced?
A) Si
B) C
C) O
D) CO
E) SiO2
SiO2(s) + 2C(s) Si(s) + 2CO(g)
-Which species is reduced?
A) Si
B) C
C) O
D) CO
E) SiO2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
31
In which of the following does nitrogen have an oxidation state of +4?
A) HNO3
B) NO2
C) N2O
D) NH4Cl
E) NaNO2
A) HNO3
B) NO2
C) N2O
D) NH4Cl
E) NaNO2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
32
In reacting, metal atoms characteristically
A) become anions
B) lose electrons
C) decrease in oxidation state
D) form covalent bonds
A) become anions
B) lose electrons
C) decrease in oxidation state
D) form covalent bonds

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
33
What is the oxidation state of bromine in NH4Br?
A) -4
B) -2
C) -1
D) 0
E) +1
A) -4
B) -2
C) -1
D) 0
E) +1

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
34
What is the oxidation state of iron in Fe2(CO3)3?
A) +4
B) +3
C) +2
D) 0
E) -2
A) +4
B) +3
C) +2
D) 0
E) -2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
35
Answer the questions that refer to the following reaction:
SiO2(s) + 2C(s) Si(s) + 2CO(g)
-Which species is the oxidizing agent?
A) Si
B) C
C) O
D) SiO2
E) CO
SiO2(s) + 2C(s) Si(s) + 2CO(g)
-Which species is the oxidizing agent?
A) Si
B) C
C) O
D) SiO2
E) CO

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
36
The oxidation state of oxygen in Na2CrO4 is
A) +1
B) +3
C) +7
D) +5
E) none of these
A) +1
B) +3
C) +7
D) +5
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
37
In the reaction N2(g) + 3H2(g) 2NH3(g), nitrogen is ______________.
A) oxidized
B) reduced
C) synthesized
D) electrolyzed
E) none of these
A) oxidized
B) reduced
C) synthesized
D) electrolyzed
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
38
The oxidation state of carbon in CH4 is
A) +4
B) -4
C) +2
D) -2
E) none of these
A) +4
B) -4
C) +2
D) -2
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
39
The oxidation state of carbon in CO2 is
A) +4
B) -4
C) +2
D) -2
E) none of these
A) +4
B) -4
C) +2
D) -2
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
40
Answer the questions that refer to the following reaction:
TiCl4(l) + O2(g) TiO2(s) + 2Cl2(g)
-Which species is the reducing agent?
A) Ti
B) Cl
C) O
D) TiCl4
E) O2
TiCl4(l) + O2(g) TiO2(s) + 2Cl2(g)
-Which species is the reducing agent?
A) Ti
B) Cl
C) O
D) TiCl4
E) O2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
41
Balance the following half-reaction that occurs in acidic solution:
NO3-(aq) NO2(g)
NO3-(aq) NO2(g)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
42
Balance the following half-reaction that occurs in acidic solution:
MnO4-(aq) Mn2+(aq)
MnO4-(aq) Mn2+(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
43
In the reaction C(s) + O2(g) CO2(g), carbon is ______________.
A) reduced
B) electrolyzed
C) synthesized
D) oxidized
E) none of these
A) reduced
B) electrolyzed
C) synthesized
D) oxidized
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
44
Balance the following reaction that takes place in acidic solution:
Mn(s) + NO3-(aq) Mn2+(aq) + NO2(g)
Mn(s) + NO3-(aq) Mn2+(aq) + NO2(g)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
45
Answer the following questions that refer to the unbalanced reaction shown below as it occurs in acidic solution:
_______ Cr2O72- (aq) + _______ I-(aq) _______ Cr3+(aq) + _______ I2(s)
-Determine the coefficient for the Cr3+ ions.
A) 1
B) 2
C) 3
D) 6
E) 7
_______ Cr2O72- (aq) + _______ I-(aq) _______ Cr3+(aq) + _______ I2(s)
-Determine the coefficient for the Cr3+ ions.
A) 1
B) 2
C) 3
D) 6
E) 7

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
46
Of the following four reactions, how many are oxidation-reduction reactions? NaOH + HCl NaCl + H2O
Cu + 2AgNO3 2Ag + Cu(NO3)2
Mg(OH)2 MgO + H2O
N2 + 3H2 2NH3
A) 0
B) 1
C) 2
D) 3
E) 4
Cu + 2AgNO3 2Ag + Cu(NO3)2
Mg(OH)2 MgO + H2O
N2 + 3H2 2NH3
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following are oxidation-reduction reactions? I. PCl3 + Cl2 PCl5
II) Cu + 2AgNO3 Cu(NO3)2 + 2Ag
III) CO2 + 2LiOH Li2CO3 + H2O
IV) FeCl2 + 2NaOH Fe(OH)2 + 2NaCl
A) III
B) IV
C) I and II
D) I, II, and III
E) I, II, III, and IV
II) Cu + 2AgNO3 Cu(NO3)2 + 2Ag
III) CO2 + 2LiOH Li2CO3 + H2O
IV) FeCl2 + 2NaOH Fe(OH)2 + 2NaCl
A) III
B) IV
C) I and II
D) I, II, and III
E) I, II, III, and IV

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
48
Balance the following reaction that takes place in acidic solution:
I-(aq) + IO3- (aq) I3-(aq)
I-(aq) + IO3- (aq) I3-(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
49
Balance the following reaction that takes place in acidic solution:
Cu(s) + NO3-(aq) Cu2+(aq) + NO(g)
Cu(s) + NO3-(aq) Cu2+(aq) + NO(g)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
50
Answer the following questions that refer to the unbalanced reaction shown below as it occurs in acidic solution:
_______ Cr2O72- (aq) + _______ I-(aq) _______ Cr3+(aq) + _______ I2(s)
-Determine the coefficient for the iodide ions.
A) 1
B) 2
C) 3
D) 6
E) 7
_______ Cr2O72- (aq) + _______ I-(aq) _______ Cr3+(aq) + _______ I2(s)
-Determine the coefficient for the iodide ions.
A) 1
B) 2
C) 3
D) 6
E) 7

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
51
In the following reaction, which element is oxidized? 8NaI + 5H2SO4 4I2 + H2S + 4Na2SO4 + 4H2O
A) sodium
B) iodine
C) sulfur
D) hydrogen
E) oxygen
A) sodium
B) iodine
C) sulfur
D) hydrogen
E) oxygen

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
52
Balance the following half-reaction that occurs in acidic solution:
SO32-(aq) SO42-(aq)
SO32-(aq) SO42-(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
53
In the following reaction, which species is the reducing agent? 3Cu + 6H+ + 2HNO3 3Cu2+ + 2NO + 4 H2O
A) H+
B) Cu
C) N in NO
D) Cu2+
E) N in HNO3
A) H+
B) Cu
C) N in NO
D) Cu2+
E) N in HNO3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements is/are true? Oxidation and reduction
A) cannot occur independently of each other
B) accompany all chemical changes
C) describe the loss and gain of electron(s), respectively
D) result in a change in the oxidation states of the species involved
E) a, c, and d are true.
A) cannot occur independently of each other
B) accompany all chemical changes
C) describe the loss and gain of electron(s), respectively
D) result in a change in the oxidation states of the species involved
E) a, c, and d are true.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
55
Answer the following questions that refer to the unbalanced reaction shown below as it occurs in acidic solution:
_______ Cr2O72- (aq) + _______ I-(aq) _______ Cr3+(aq) + _______ I2(s)
-Determine the coefficient for water in the balanced equation for the reaction.
A) 1
B) 2
C) 3
D) 6
E) 7
_______ Cr2O72- (aq) + _______ I-(aq) _______ Cr3+(aq) + _______ I2(s)
-Determine the coefficient for water in the balanced equation for the reaction.
A) 1
B) 2
C) 3
D) 6
E) 7

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
56
In the reaction shown below, which species is oxidized? 2KI + F2 2KF + I2
A) I-
B) K+
C) F2
D) F-
E) I2
A) I-
B) K+
C) F2
D) F-
E) I2

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
57
In the reaction Mg + H2SO4 MgSO4 + H2, which element, if any, is oxidized?
A) magnesium
B) hydrogen
C) sulfur
D) oxygen
E) None is oxidized.
A) magnesium
B) hydrogen
C) sulfur
D) oxygen
E) None is oxidized.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
58
Balance the following reaction that takes place in acidic solution:
Mg(s) + HCl(aq) MgCl2(aq) + H2(g)
Mg(s) + HCl(aq) MgCl2(aq) + H2(g)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following reactions does not involve oxidation-reduction?
A) CH4 + 3O2 2H2O + CO4
B) Zn + 2HCl ZnCl2 + H2
C) 2Na + 2H2O 2NaOH + H2
D) MnO2 + 4HCl Cl2 + 2H2O + MnCl2
E) All are oxidation-reduction reactions.
A) CH4 + 3O2 2H2O + CO4
B) Zn + 2HCl ZnCl2 + H2
C) 2Na + 2H2O 2NaOH + H2
D) MnO2 + 4HCl Cl2 + 2H2O + MnCl2
E) All are oxidation-reduction reactions.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
60
Balance the following half-reaction that occurs in acidic solution:
IO3- (aq) I3-(aq)
IO3- (aq) I3-(aq)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is true for a galvanic cell based on the following reaction? Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s)
A) The zinc is being reduced.
B) The zinc serves as the anode.
C) The Cu2+ ion is being reduced.
D) The Cu serves as the anode.
E) two of these
A) The zinc is being reduced.
B) The zinc serves as the anode.
C) The Cu2+ ion is being reduced.
D) The Cu serves as the anode.
E) two of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
62
In a car battery, which of the following is the electrolyte?
A) Pb(s)
B) PbO2(s)
C) PbSO4(s)
D) H2SO4(aq)
E) none of these
A) Pb(s)
B) PbO2(s)
C) PbSO4(s)
D) H2SO4(aq)
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following statements is not true of electrolysis?
A) Electrolysis is a process where electrical energy is used to produce a chemical change.
B) Electrolysis involves forcing a current through a cell to produce a change that would otherwise not occur.
C) The electrolysis of water produces hydrogen gas and oxygen gas.
D) Electrolysis is used to recharge a lead storage battery.
E) None of these; that is, all these statements are true.
A) Electrolysis is a process where electrical energy is used to produce a chemical change.
B) Electrolysis involves forcing a current through a cell to produce a change that would otherwise not occur.
C) The electrolysis of water produces hydrogen gas and oxygen gas.
D) Electrolysis is used to recharge a lead storage battery.
E) None of these; that is, all these statements are true.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
64
Most metals oxidize in air more slowly than expected because
A) a thin layer of the metal oxide forms on the metal surface, thus inhibiting corrosion
B) cathodic protection naturally occurs
C) corrosion occurs only under water
D) the concentration of oxygen in the atmosphere is not high enough
E) none of these
A) a thin layer of the metal oxide forms on the metal surface, thus inhibiting corrosion
B) cathodic protection naturally occurs
C) corrosion occurs only under water
D) the concentration of oxygen in the atmosphere is not high enough
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
65
The following reaction occurs in aqueous acid solution: NO3- + I- IO3- + NO2
In the balanced equation, the coefficient of water is
A) 1
B) 2
C) 3
D) 4
E) 5
In the balanced equation, the coefficient of water is
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
66
For the redox reaction 2Fe2+ + Cl2 2Fe3+ + 6Cl-, which of the following are the correct half-reactions?
(I) Cl2 + 2e- 2Cl-
(II) Cl Cl- + e-
(III) Cl2 2Cl- + 2e-
(IV) Fe2+ Fe3+ + e-
(V) Fe2+ + e- Fe3+
A) I and IV
B) I and V
C) II and IV
D) II and V
E) III and IV
(I) Cl2 + 2e- 2Cl-
(II) Cl Cl- + e-
(III) Cl2 2Cl- + 2e-
(IV) Fe2+ Fe3+ + e-
(V) Fe2+ + e- Fe3+
A) I and IV
B) I and V
C) II and IV
D) II and V
E) III and IV

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following battery types is commonly used in an automobile?
A) dry cell
B) alkaline dry cell
C) lead storage
D) mercury cell
E) nickel-cadmium
A) dry cell
B) alkaline dry cell
C) lead storage
D) mercury cell
E) nickel-cadmium

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
68
In the balanced equation for the following redox equation, what is the sum of the coefficients? Fe3+ + I- Fe2+ + I2
A) 4
B) 5
C) 6
D) 7
E) 8
A) 4
B) 5
C) 6
D) 7
E) 8

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
69
Answer the following questions for a galvanic cell that employs the reaction
2Na(l) + S(l) Na2S(s)
-What element serves as the cathode?
2Na(l) + S(l) Na2S(s)
-What element serves as the cathode?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
70
For the reaction of sodium bromide with chlorine gas to form sodium chloride and bromine, the appropriation half-reactions are (ox = oxidation and re = reduction)
A) ox: Cl2 + 2e- 2Cl- re: 2Br- Br2 + 2e-
B) ox: 2Br- Br2 + 2e- re: Cl2 + 2e- 2Cl-
C) ox: Cl + e- Cl- re: Br Br- + e-
D) ox: Br + 2e- Br- re: 2Cl- Cl2 + 2e-
E) ox: 2Na+ + 2e- 2Na re: 2Cl- Cl2 + 2e-
A) ox: Cl2 + 2e- 2Cl- re: 2Br- Br2 + 2e-
B) ox: 2Br- Br2 + 2e- re: Cl2 + 2e- 2Cl-
C) ox: Cl + e- Cl- re: Br Br- + e-
D) ox: Br + 2e- Br- re: 2Cl- Cl2 + 2e-
E) ox: 2Na+ + 2e- 2Na re: 2Cl- Cl2 + 2e-

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
71
Answer the following questions for a galvanic cell that employs the reaction
2Na(l) + S(l) Na2S(s)
-What element is reduced?
2Na(l) + S(l) Na2S(s)
-What element is reduced?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
72
When a metal corrodes, what is happening chemically?
A) The metal atoms lose electrons.
B) The metal atoms gain electrons.
C) Electrons are not involved.
D) The metal is combining with nitrogen gas.
E) none of these
A) The metal atoms lose electrons.
B) The metal atoms gain electrons.
C) Electrons are not involved.
D) The metal is combining with nitrogen gas.
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
73
In the balanced equation for the following reaction (in acidic solution) ClO3- + Fe2+ Cl- + Fe3+
The coefficient of Fe2+ is
A) 1
B) 6
C) 2
D) 8
E) 3
The coefficient of Fe2+ is
A) 1
B) 6
C) 2
D) 8
E) 3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
74
In a galvanic cell that employs the reaction
Cu(s) + 2Ag+(aq) Cu2+(aq) + 2Ag(s)
copper metal serves as the anode.
Cu(s) + 2Ag+(aq) Cu2+(aq) + 2Ag(s)
copper metal serves as the anode.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
In a galvanic cell that employs the reaction
Pb(s) + 2H2SO4(aq) + PbO2(s) 2PbSO4(s) + 2H2O(l)
lead metal serves as the cathode.
Pb(s) + 2H2SO4(aq) + PbO2(s) 2PbSO4(s) + 2H2O(l)
lead metal serves as the cathode.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
In a lead storage battery, PbO2 is found at the ______________.
A) cathode
B) anode
C) dry cell
D) galvanic cell
E) none of these
A) cathode
B) anode
C) dry cell
D) galvanic cell
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
Answer the following questions for a galvanic cell that employs the reaction
2Na(l) + S(l) Na2S(s)
-What element is oxidized?
2Na(l) + S(l) Na2S(s)
-What element is oxidized?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
78
Aluminum resists corrosion in air because aluminum metal gains electrons rather than losing electrons.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
79
When the following reaction is balanced in acidic solution, what is the coefficient of water? Zn(s) + NO3-(aq) Zn2+(aq) + NH4+(aq)
A) 1
B) 2
C) 3
D) 4
E) none of these
A) 1
B) 2
C) 3
D) 4
E) none of these

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
80
Answer the following questions for a galvanic cell that employs the reaction
2Na(l) + S(l) Na2S(s)
-What element serves as the anode?
2Na(l) + S(l) Na2S(s)
-What element serves as the anode?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck


