Deck 13: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 13: Gases
1
Convert 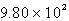 atm to torr.
atm to torr.
A)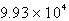 torr
torr
B) 1.289 torr
C) 68.1 torr
D)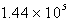 torr
torr
E)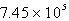 torr
torr
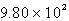 atm to torr.
atm to torr.A)
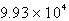 torr
torrB) 1.289 torr
C) 68.1 torr
D)
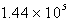 torr
torrE)
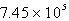 torr
torr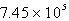 torr
torr 2
Convert 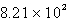 torr to psi.
torr to psi.
A) 1.08 psi
B) 15.9 psi
C)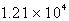 psi
psi
D)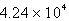 psi
psi
E)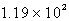 psi
psi
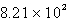 torr to psi.
torr to psi.A) 1.08 psi
B) 15.9 psi
C)
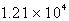 psi
psiD)
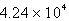 psi
psiE)
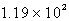 psi
psi15.9 psi
3
Consider a gas at 1.00 atm in a 5.00-L container at 20.°C. What pressure does the gas exert when transferred to a volume of 2.77 L at 43°C?
A) 3.88 atm
B) 1.67 atm
C) 0.371 atm
D) 1.95 atm
E) 0.597 atm
A) 3.88 atm
B) 1.67 atm
C) 0.371 atm
D) 1.95 atm
E) 0.597 atm
1.95 atm
4
You have a sample of argon gas at a certain pressure, volume, and temperature. You double the volume, double the number of moles of argon, and double the Kelvin temperature. How does the final pressure (Pf) compare to the original pressure (Po)? Pf =
A) (1/8)Po
B) (1/2)Po
C) 2Po
D) 4Po
E) 8P2
A) (1/8)Po
B) (1/2)Po
C) 2Po
D) 4Po
E) 8P2

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
What is the volume of a helium balloon that contains 1.95 mol helium at 27°C and 1.10 atm?
A) 3.93 L
B) 39.7 L
C) 48.0 L
D) 43.6 L
E) 4.32 L
A) 3.93 L
B) 39.7 L
C) 48.0 L
D) 43.6 L
E) 4.32 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Perform the following conversion of pressure units: 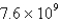 Pa = ____________ atm
Pa = ____________ atm
A)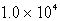
B)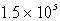
C)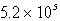
D)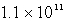
E)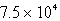
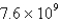 Pa = ____________ atm
Pa = ____________ atmA)
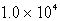
B)
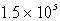
C)
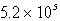
D)
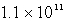
E)
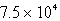

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
Consider a sample of gas in a container on a comfortable spring day in Chicago, IL. The Celsius temperature suddenly doubles, and you transfer the gas to a container with twice the volume of the first container. If the original pressure was 11 atm, what is a good estimate for the new pressure?
A) 12 atm
B) 2.8 atm
C) 5.9 atm
D) 11 atm
E) 22 atm
A) 12 atm
B) 2.8 atm
C) 5.9 atm
D) 11 atm
E) 22 atm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
Perform the following conversion of pressure units: 193 torr = ____________ atm
A) 1.91
B) 13.1
C) 0.254
D) 3.94
E) 953
A) 1.91
B) 13.1
C) 0.254
D) 3.94
E) 953

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
When analyzing ideal gases, the temperature must be measured on the Kelvin scale
A) because otherwise you could calculate a negative volume.
B) so that you are using an absolute scale.
C) to directly measure the average kinetic energy of the gas particles.
D) Both a and b are correct.
E) a, b, and c are correct.
A) because otherwise you could calculate a negative volume.
B) so that you are using an absolute scale.
C) to directly measure the average kinetic energy of the gas particles.
D) Both a and b are correct.
E) a, b, and c are correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
Perform the following conversion of pressure units: 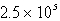 torr = ___________ atm
torr = ___________ atm
A)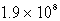
B)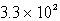
C)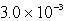
D)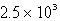
E)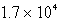
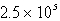 torr = ___________ atm
torr = ___________ atmA)
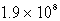
B)
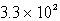
C)
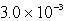
D)
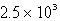
E)
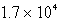

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
Determine the pressure exerted by 2.26 mol of gas in a 2.92-L container at 32°C.
A) 2.03 atm
B) 56.6 atm
C) 19.4 atm
D) 5.93 atm
E) 17.3 atm
A) 2.03 atm
B) 56.6 atm
C) 19.4 atm
D) 5.93 atm
E) 17.3 atm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
You are playing with a helium balloon on a typically warm California day. Suddenly, the Celsius temperature doubles. Which of the following is true?
A) The volume of the balloon will double.
B) The volume of the balloon will slightly increase.
C) The pressure inside the balloon will double.
D) The volume of the balloon will decrease.
E) The actual temperatures are needed to answer this question.
A) The volume of the balloon will double.
B) The volume of the balloon will slightly increase.
C) The pressure inside the balloon will double.
D) The volume of the balloon will decrease.
E) The actual temperatures are needed to answer this question.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
Perform the following conversion of pressure units: 232 kPa = ____________ Pa
A) 0.232
B)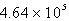
C) 2.32
D)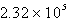
E)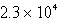
A) 0.232
B)
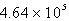
C) 2.32
D)
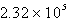
E)
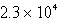

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
A 15.0-g sample of a hydrocarbon is placed in a balloon at 1.00 atm and 25°C, and the volume of the balloon is 12.2 L. The hydrocarbon is 79.89% carbon and 20.11% hydrogen by mass. Determine the molecular formula of the hydrocarbon.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
Perform the following conversion of pressure units: 0.875 atm = ____________ torr
A) 869
B) 88.6
C) 12.9
D) 665
E) 690
A) 869
B) 88.6
C) 12.9
D) 665
E) 690

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
A certain balloon will pop if it expands to a volume greater than 16.0 L. The balloon is currently filled with air at a volume of 8.0 L. You heat the balloon such that the temperature measured in degrees Celsius doubles. Which of the following best describes what happens?
A) The balloon will expand to a volume greater than 16.0 L and pop.
B) The balloon will expand to a volume less than 16.0 L and not pop.
C) The balloon will expand to a volume of 16.0 L and pop.
D) The balloon will expand to a volume of 16.0 L and not pop.
E) The volume of the balloon will remain the same but the pressure will increase.
A) The balloon will expand to a volume greater than 16.0 L and pop.
B) The balloon will expand to a volume less than 16.0 L and not pop.
C) The balloon will expand to a volume of 16.0 L and pop.
D) The balloon will expand to a volume of 16.0 L and not pop.
E) The volume of the balloon will remain the same but the pressure will increase.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
You are holding two helium balloons, a large and a small balloon. How do the pressures of the helium compare?
A) The pressure in the large balloon is greater than the pressure in the small balloon. This is best explained by the fact that there must be more moles of gas in the large balloon, thus greater pressure.
B) The pressure in the small balloon is greater than the pressure in the large balloon. This is best explained by the fact that as the volume of a container decreases, the pressure of the gas increases.
C) The pressure in the large balloon is greater than the pressure in the small balloon. The fact that there is a greater pressure in the balloon explains why the volume is larger; as the particles push more on the inside, the volume increases.
D) The pressures are essentially the same and less than atmospheric pressure.
E) The pressures are essentially the same and equal to atmospheric pressure.
A) The pressure in the large balloon is greater than the pressure in the small balloon. This is best explained by the fact that there must be more moles of gas in the large balloon, thus greater pressure.
B) The pressure in the small balloon is greater than the pressure in the large balloon. This is best explained by the fact that as the volume of a container decreases, the pressure of the gas increases.
C) The pressure in the large balloon is greater than the pressure in the small balloon. The fact that there is a greater pressure in the balloon explains why the volume is larger; as the particles push more on the inside, the volume increases.
D) The pressures are essentially the same and less than atmospheric pressure.
E) The pressures are essentially the same and equal to atmospheric pressure.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
The air in the inner tube of the tire of a racing bike has a pressure of 116.8 psi. Convert this pressure to atm.
A) 0.1537 atm
B) 7.946 atm
C) 1717 atm
D) 1.153 atm
E) 116.8 atm
A) 0.1537 atm
B) 7.946 atm
C) 1717 atm
D) 1.153 atm
E) 116.8 atm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
A gaseous compound containing carbon and hydrogen was analyzed and found to consist of between 80 and 90 percent carbon by mass. At 145ºC and 1.00 atm, the density of this compound is 2.45 g/L. What is the molar mass of the compound?
A) 84.2 g/mol
B) 29.2 g/mol
C) 55.0 g/mol
D) 99.1 g/mol
E) 42.1 g/mol
A) 84.2 g/mol
B) 29.2 g/mol
C) 55.0 g/mol
D) 99.1 g/mol
E) 42.1 g/mol

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
Perform the following conversion of pressure units: 1.177 atm = ____________ torr
A) 894.5
B) 645.7
C)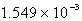
D) 17.30
E) 1.177
A) 894.5
B) 645.7
C)
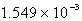
D) 17.30
E) 1.177

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
You are holding two balloons, an orange balloon and a blue balloon. The orange balloon is filled with neon (Ne) gas, and the blue balloon is filled with argon (Ar) gas. The orange balloon has twice the volume of the blue balloon. Which of the following best represents the mass ratio of Ne:Ar in the balloons?
A) 1:1
B) 1:2
C) 2:1
D) 1:3
E) 3:1
A) 1:1
B) 1:2
C) 2:1
D) 1:3
E) 3:1

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Suppose a balloon has a maximum volume of 7.00 L. Also suppose each time you blow into a balloon, you expel 0.045 mol of air. How many times can you blow into the balloon before the balloon pops? Assume atmospheric conditions of 1.0 atm and 22°C and that your breath is at room temperature.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
A sample of helium gas occupies 14.3 L at 23°C and 0.956 atm. What volume will it occupy at 40.°C and 0.956 atm?
A) 24.9 L
B) 0.0661 L
C) 13.5 L
D) 15.1 L
E) none of these
A) 24.9 L
B) 0.0661 L
C) 13.5 L
D) 15.1 L
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
A sample of an ideal gas containing 0.637 mol is collected at 742 torr pressure and 31°C. Calculate the volume.
A) 16.3 L
B) 1.66 L
C)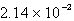 L
L
D)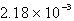 L
L
E) none of these
A) 16.3 L
B) 1.66 L
C)
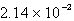 L
LD)
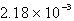 L
LE) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
You are holding four balloons, each containing 6.0 g of a different gas. The balloon containing which gas is the largest?
A) He
B) Ne
C) Kr
D) N2
E) All have the same volume.
A) He
B) Ne
C) Kr
D) N2
E) All have the same volume.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements is true of 19.0 g of F2(g) at STP?
A) It contains 6.02 * 1023 molecules.
B) It contains the same number of molecules as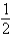 mol of O2(g) at STP.
mol of O2(g) at STP.
C) It occupies a volume of 22.4 L.
D) It exists only in the form of ions.
E) none of the above
A) It contains 6.02 * 1023 molecules.
B) It contains the same number of molecules as
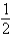 mol of O2(g) at STP.
mol of O2(g) at STP.C) It occupies a volume of 22.4 L.
D) It exists only in the form of ions.
E) none of the above

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
A gas originally occupying 10.1 L at 0.925 atm and 25°C is changed to 13.3 L at 625 torr. What is the new temperature?
A) 349 °C
B) 76 °C
C) -244 °C
D) 622 °C
E) 50 °C
A) 349 °C
B) 76 °C
C) -244 °C
D) 622 °C
E) 50 °C

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
A 12.0-g sample of helium gas occupies a volume of 14.4 L at a certain temperature and pressure. What volume does a 24.0-g sample of neon occupy at these conditions of temperature and pressure?
A) 5.71 L
B) 28.8 L
C) 22.4 L
D) 36.3 L
E) 11.4 L
A) 5.71 L
B) 28.8 L
C) 22.4 L
D) 36.3 L
E) 11.4 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
You fill a balloon with 10.0 g of N2 gas. You wish to add 10.0 g of another gas to make the balloon more than twice as large as it is with only the N2 (that is, more than twice the original volume). Which gas should you add (assume constant temperature)?
A) O2
B) CO2
C) CO
D) He
E) You cannot make the balloon more than twice as large with 10.0 g of any gas.
A) O2
B) CO2
C) CO
D) He
E) You cannot make the balloon more than twice as large with 10.0 g of any gas.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
Two moles of gas A spontaneously convert to 3 moles of gas B in a container where the temperature and pressure are held constant. The sample originally took up 36.2 L of volume. What is the new volume of the products?
A) 24.1 L
B) 109 L
C) 72.4 L
D) 7.24 L
E) 54.3 L
A) 24.1 L
B) 109 L
C) 72.4 L
D) 7.24 L
E) 54.3 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
A specified quantity of an unknown gas has the volume of 14.1 mL at 22°C and 659 torr. Calculate the volume at STP.
A) 0.912 mL
B) 13.2 mL
C) 11.3 mL
D) 15.0 mL
E) none of these
A) 0.912 mL
B) 13.2 mL
C) 11.3 mL
D) 15.0 mL
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
You are holding two balloons of the same volume. One balloon contains 1.05 g helium. The other balloon contains neon. What is the mass of neon in the balloon?
A) 0.262 g
B) 1.05 g
C) 76.9 g
D) 5.29 g
E) 20.0 g
A) 0.262 g
B) 1.05 g
C) 76.9 g
D) 5.29 g
E) 20.0 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following will give a graph with a straight line and a y-intercept of 0?
A) volume vs. 1/temperature (°C)
B) volume vs. temperature (°C)
C) volume vs. temperature (K)
D) volume vs. 1/temperature (K)
E) none of these
A) volume vs. 1/temperature (°C)
B) volume vs. temperature (°C)
C) volume vs. temperature (K)
D) volume vs. 1/temperature (K)
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
A sample of a gas in a container fitted with a piston has a temperature above 0°C. The Celsius temperature is doubled. What is true about the ratio of final volume to initial volume for the gas?
A) It is 1:1.
B) It is 2:1.
C) It is 1:2.
D) It is greater than 2:1.
E) It is less than 2:1.
A) It is 1:1.
B) It is 2:1.
C) It is 1:2.
D) It is greater than 2:1.
E) It is less than 2:1.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements are true of real gases?
A) A real gas behaves more like an ideal gas at high pressures and low temperatures.
B) The individual gas particles have no volume.
C) The individual gas particles are not attracted to one another.
D) The particles collide with the walls of its container and exert pressure.
E) The kinetic energy of the gas particles is directly proportional to the temperature of the gas in degrees Celsius.
A) A real gas behaves more like an ideal gas at high pressures and low temperatures.
B) The individual gas particles have no volume.
C) The individual gas particles are not attracted to one another.
D) The particles collide with the walls of its container and exert pressure.
E) The kinetic energy of the gas particles is directly proportional to the temperature of the gas in degrees Celsius.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
If temperature and pressure are held constant, the volume and number of moles of a gas are
A) independent of each other
B) directly proportional
C) inversely proportional
D) equal
E) not enough information given
A) independent of each other
B) directly proportional
C) inversely proportional
D) equal
E) not enough information given

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
You have a partially filled party balloon with 2.00 g of helium gas. You then add 3.00 g of hydrogen gas to the balloon. Assuming constant temperature and pressure, how many times bigger is the party balloon - comparing before and after the hydrogen gas has been added. Choose the best formula to use to solve this problem. (V = volume of the gas, n = moles of the gas, m = mass of the gas, P = pressure of the gas.)
A)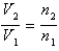
B)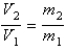
C)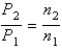
D)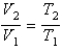
E)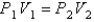
A)
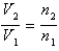
B)
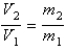
C)
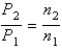
D)
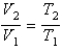
E)
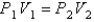

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
You transfer a sample of gas at 17°C from a volume of 5.68 L and 1.10 atm to a container at 37°C that has a pressure of 1.10 atm. What is the new volume of the gas?
A) 12.4 L
B) 6.07 L
C) 5.31 L
D) 0.165 L
E) none of these
A) 12.4 L
B) 6.07 L
C) 5.31 L
D) 0.165 L
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
You have a certain mass of helium gas in a rigid steel container. You add the same mass of neon gas to this container. Which of the following best describes what happens? Assume temperature is constant.
A) The pressure in the container doubles.
B) The pressure in the container increases but does not double.
C) The pressure in the container more than doubles.
D) The volume of the container doubles.
E) The volume of the container more than doubles.
A) The pressure in the container doubles.
B) The pressure in the container increases but does not double.
C) The pressure in the container more than doubles.
D) The volume of the container doubles.
E) The volume of the container more than doubles.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
Gaseous chlorine is held in two separate containers at identical temperature and pressure. The volume of container 1 is 1.30 L, and it contains 6.70 mol of the gas. The volume of container 2 is 2.77 L. How many moles of the gas are in container 2?
A) 14.3 mol
B)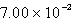 mol
mol
C) 3.14 mol
D) 4.07 mol
E) none of these
A) 14.3 mol
B)
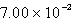 mol
molC) 3.14 mol
D) 4.07 mol
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid whose vapor pressure is the same as that of pure water at 20.0°C (18 torr). The volume of the mixture is 1.7 L, and its total pressure is 0.508 atm. Determine the partial pressure of the hydrogen gas in this mixture.
A) 404 torr
B) 656 torr
C) 368 torr
D) 626 torr
E) 386 torr
A) 404 torr
B) 656 torr
C) 368 torr
D) 626 torr
E) 386 torr

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
A gas occupies 42.6 L at 2.00 atm pressure and 27°C. Calculate its volume if the pressure is decreased to 1.00 atm at constant temperature.
A) 21.3 L
B) 85.2 L
C) 128 L
D) 28.5 L
E) 179 L
A) 21.3 L
B) 85.2 L
C) 128 L
D) 28.5 L
E) 179 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
A vessel with an internal volume of 15.3 L contains 2.80 g of nitrogen gas, 0.403 g of hydrogen gas, and 79.9 g of argon gas. At 25oC, what is the pressure (in atm) inside the vessel?
A) 860 atm
B) 0.308 atm
C) 3.68 atm
D) 0.272 atm
E) 133 atm
A) 860 atm
B) 0.308 atm
C) 3.68 atm
D) 0.272 atm
E) 133 atm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
A 6.08-g piece of solid CO2 (dry ice) is allowed to vaporize (change to CO2(g)) in a balloon. The final volume of the balloon is 1.00 L at 300. K. What is the pressure of the gas?
A) 3.40 atm
B) 6.50 atm
C) 0.306 atm
D) 153 atm
E) none of these
A) 3.40 atm
B) 6.50 atm
C) 0.306 atm
D) 153 atm
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
A gas occupies 32.6 L at 2.00 atm pressure and 27°C. Calculate its volume if the pressure remains at 2.0 atm but the temperature is raised to 54°C.
A) 29.9 L
B) 65.2 L
C) 0.0281 L
D) 35.5 L
E) 23.8 L
A) 29.9 L
B) 65.2 L
C) 0.0281 L
D) 35.5 L
E) 23.8 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
An oxygen sample has a volume of 7.01 L at 27°C and 800.0 torr. How many oxygen molecules does it contain?
A)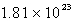
B)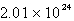
C)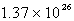
D)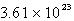
E) none of these
A)
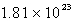
B)
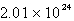
C)
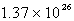
D)
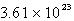
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
A 251-mL sample of a gas at STP is heated to 45°C. The final pressure is 1.86 atm. Calculate the volume of this gas under the new conditions.
A) 22.2 mL
B) 116 mL
C) 8630 mL
D) 157 mL
E) 105 mL
A) 22.2 mL
B) 116 mL
C) 8630 mL
D) 157 mL
E) 105 mL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
A sample of oxygen gas at 70oC is twice as hot as a sample of oxygen gas at 35oC.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
A gas is collected over water at a certain temperature. The total pressure is 758 torr. The vapor pressure of water at this temperature is 17 torr. The partial pressure of the gas collected is
A) 758 torr
B) 17 torr
C) 775 torr
D) 741 torr
E) 733 torr
A) 758 torr
B) 17 torr
C) 775 torr
D) 741 torr
E) 733 torr

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
A 16.4-L sample of gas at STP is heated to 55°C at 605 torr. What is the new volume?
A) 24.8 L
B) 4.15 L
C) 17.1 L
D) 15.7 L
E) 16.6 L
A) 24.8 L
B) 4.15 L
C) 17.1 L
D) 15.7 L
E) 16.6 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
A gas occupies 18.5 L at STP. What volume will it occupy at 735 torr and 57°C?
A) 3.99 L
B) 21.6 L
C) 15.8 L
D) 15.5 L
E) 23.1 L
A) 3.99 L
B) 21.6 L
C) 15.8 L
D) 15.5 L
E) 23.1 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
The volume of a sample of gas is 561.5 mL at STP. What volume will the sample occupy at 0.0°C and 950.0 torr?
A) 701.9 mL
B) 449.2 mL
C) 411.5 mL
D) 561.2 mL
E) none of these
A) 701.9 mL
B) 449.2 mL
C) 411.5 mL
D) 561.2 mL
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
What volume will 55.6 g of N2 occupy at STP?
A) 48.5 L
B)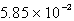 L
L
C) 44.5 L
D) 4.07 L
E) none of these
A) 48.5 L
B)
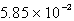 L
LC) 44.5 L
D) 4.07 L
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
A gas occupies 33.5 L at 2.00 atm pressure and 27°C. Calculate its volume at STP.
A) 73.6 L
B) 677 L
C) 30.5 L
D) 74.0 L
E) 61.0 L
A) 73.6 L
B) 677 L
C) 30.5 L
D) 74.0 L
E) 61.0 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
Zinc metal is added to hydrochloric acid to generate hydrogen gas and is collected over a liquid whose vapor pressure is the same as that of pure water at 20.0°C (18 torr). The volume of the mixture is 1.7 L, and its total pressure is 0.788 atm. Determine the number of moles of hydrogen gas present in the sample.
A) 0.792 mol
B) 0.111 mol
C) 0.216 mol
D) 0.0540 mol
E) 0.0318 mol
A) 0.792 mol
B) 0.111 mol
C) 0.216 mol
D) 0.0540 mol
E) 0.0318 mol

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
A gas occupies 30.3 L at 2.00 atm pressure and 27°C. How many moles of gas are present in the sample?
A) 27.4 mol
B) 1.23 mol
C) 2.46 mol
D) 3.96 mol
E) 4.86 mol
A) 27.4 mol
B) 1.23 mol
C) 2.46 mol
D) 3.96 mol
E) 4.86 mol

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
Mercury vapor contains Hg atoms. What is the volume of 200. g of mercury vapor at 822 K and 0.415 atm?
A) 162 L
B) 27.9 L
C) 216 L
D) 108 L
E) 324 L
A) 162 L
B) 27.9 L
C) 216 L
D) 108 L
E) 324 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
A 4.06-L sample of carbon monoxide is collected at 55°C and 0.892 atm. What volume will the gas occupy at 1.05 atm and 20.°C?
A) 1.25 L
B) 3.86 L
C) 3.08 L
D) 4.27 L
E) none of these
A) 1.25 L
B) 3.86 L
C) 3.08 L
D) 4.27 L
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
What volume is occupied by 22.6 g of methane, CH4, at 27°C and 1.59 atm?
A) 34.7 L
B) 21.8 L
C) 1.96 L
D) 31.5 L
E) not enough data to calculate
A) 34.7 L
B) 21.8 L
C) 1.96 L
D) 31.5 L
E) not enough data to calculate

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
A 41.1-mL sample of H2 at STP would contain how many grams of hydrogen?
A)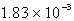 g
g
B) 3.70 g
C) 1.83 g
D)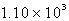 g
g
E)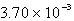 g
g
A)
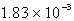 g
gB) 3.70 g
C) 1.83 g
D)
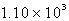 g
gE)
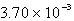 g
g
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
You have a partially filled party balloon with 2.00 g of helium gas. You then add 3.45 g of hydrogen gas to the balloon. Assuming constant temperature and pressure, how many times bigger is the party balloon - comparing before and after the hydrogen gas has been added?
A) 3.42 times bigger
B) 7.85 times bigger
C) 4.42 times bigger
D) 6.85 times bigger
E) 5.45 times bigger
A) 3.42 times bigger
B) 7.85 times bigger
C) 4.42 times bigger
D) 6.85 times bigger
E) 5.45 times bigger

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
How many liters of HCl(g) measured at STP can be produced from 5.44 g of Cl2 and excess H2 according to the following equation? 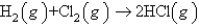
A) 1.72 L
B) 121.9 L
C) 3.44 L
D) 5.16 L
E) none of these
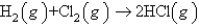
A) 1.72 L
B) 121.9 L
C) 3.44 L
D) 5.16 L
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
You place 15.0 g of nitrogen gas and 15.0 g of hydrogen gas in a container fitted with a massless, frictionless piston. If the original volume of the container is 11.4 L, what is the volume after the reaction has run to completion? Assume constant temperature. N2(g) + 3H2(g) 2NH3(g)
A) 13.17 L
B) 1.53 L
C) 7.09 L
D) 9.87 L
E) 1.32 L
A) 13.17 L
B) 1.53 L
C) 7.09 L
D) 9.87 L
E) 1.32 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
You transfer a gas at 25oC from a volume of 2.49 L and 0.950 atm to a vessel at 32oC which has a volume of 8.75 L. What is the new pressure of the gas?
A) 0.346 atm
B) 0.264 atm
C) 3.42 atm
D) 0.291 atm
E) 0.277 atm
A) 0.346 atm
B) 0.264 atm
C) 3.42 atm
D) 0.291 atm
E) 0.277 atm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements is true about the kinetic molecular theory?
A) The volume of a gas particle is considered to be small: about 0.10 mL.
B) Pressure is due to the collisions of the gas particles with the walls of the container.
C) Gas particles repel each other but do not attract one another.
D) Adding an ideal gas to a closed container will cause an increase in temperature.
E) At least two of these statements are correct.
A) The volume of a gas particle is considered to be small: about 0.10 mL.
B) Pressure is due to the collisions of the gas particles with the walls of the container.
C) Gas particles repel each other but do not attract one another.
D) Adding an ideal gas to a closed container will cause an increase in temperature.
E) At least two of these statements are correct.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
How many moles of O2(g) are needed to react completely with 51.2 L of CH4(g) at STP to produce CO2(g) and H2O(g) according to the following reaction? 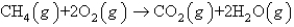
A) 4.57
B)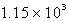
C) 1.14
D) 2.29
E) none of these
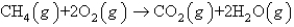
A) 4.57
B)
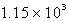
C) 1.14
D) 2.29
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
A compressed gas cylinder, at 137 atm and 23oC, is in a room where a fire occurs. The fire raises the temperature of the gas to 475oC. What is the new pressure in the cylinder?
A)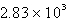 atm
atm
B) 3.30 atm
C) 85.4 atm
D)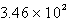 atm
atm
E) 2.16 atm
A)
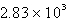 atm
atmB) 3.30 atm
C) 85.4 atm
D)
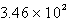 atm
atmE) 2.16 atm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
When acetylene gas, C2H2, burns, how many liters of O2 at STP are used for every 9.31 mol of acetylene burned? (Hint: Write the balanced equation for the reaction first.)
A) 209 L
B) 417 L
C) 83.4 L
D) 23.3 L
E) 521 L
A) 209 L
B) 417 L
C) 83.4 L
D) 23.3 L
E) 521 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
A sample of argon gas occupies a certain volume at 15oC. At what temperature would the volume of the gas be three times as big? Assume the pressure remains constant.
A) 864oC
B) 45oC
C) 318oC
D) 591oC
E) 1137oC
A) 864oC
B) 45oC
C) 318oC
D) 591oC
E) 1137oC

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
A sample of oxygen gas (O2) has a volume of 7.08 L at a temperature of 19oC and a pressure of 1.38 atm. Calculate the moles of O2 molecules present in this gas sample.
A) 6.27 mol
B) 0.408 mol
C) 0.816 mol
D) 0.204 mol
E) none of these
A) 6.27 mol
B) 0.408 mol
C) 0.816 mol
D) 0.204 mol
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Solid carbon dioxide is placed into a Ziploc bag. Solid carbon dioxide sublimes (turns directly from a solid to a gas) at a rate of 0.726 g/minute. If the pressure builds to 3.75 atm at a temperature of 21.1oC, what is the final volume of the bag after 4.97 minutes? (Assume the volume of the solid carbon dioxide is negligible.)
A) 37.9 mL
B) 0.379 mL
C) 1.98 mL
D) 0.528 mL
E) 528 mL
A) 37.9 mL
B) 0.379 mL
C) 1.98 mL
D) 0.528 mL
E) 528 mL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
Hydrogen peroxide decomposes to form water and oxygen gas according to the following equation: 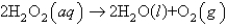 Suppose 145.9 g of hydrogen peroxide decomposes and all of the oxygen gas is collected in a balloon at 1.00 atm and 25oC. Determine the volume of the balloon.
Suppose 145.9 g of hydrogen peroxide decomposes and all of the oxygen gas is collected in a balloon at 1.00 atm and 25oC. Determine the volume of the balloon.
A) 4.40 L
B) 104.9 L
C) 52.4 L
D) 8.80 L
E) none of these
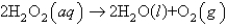 Suppose 145.9 g of hydrogen peroxide decomposes and all of the oxygen gas is collected in a balloon at 1.00 atm and 25oC. Determine the volume of the balloon.
Suppose 145.9 g of hydrogen peroxide decomposes and all of the oxygen gas is collected in a balloon at 1.00 atm and 25oC. Determine the volume of the balloon.A) 4.40 L
B) 104.9 L
C) 52.4 L
D) 8.80 L
E) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
The largest a party balloon can get before bursting is 8.55 L at 25oC and 1.00 atm. Supposing you fill the balloon only with oxygen gas, how many grams of oxygen can be added to the balloon before it pops? Assume you have not yet added any gas to the balloon at all (so essentially the balloon is flat).
A) 11.2 g O2
B) 0.350 g O2
C) 4.17 g O2
D) 133 g O2
E) 2.86 g O2
A) 11.2 g O2
B) 0.350 g O2
C) 4.17 g O2
D) 133 g O2
E) 2.86 g O2

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
You carry out the reaction represented by the following equation:
N2(g) + 3H2(g) 2NH3(g)
You add an equal number of moles of nitrogen gas and hydrogen gas in a balloon. The volume of the balloon is 2.65 L before any reaction occurs. Determine the volume of the balloon after the reaction is complete.
a.
0.883 L
b.
1.77 L
c.
2.65 L
d.
0.442 L
e.
3.53 L
N2(g) + 3H2(g) 2NH3(g)
You add an equal number of moles of nitrogen gas and hydrogen gas in a balloon. The volume of the balloon is 2.65 L before any reaction occurs. Determine the volume of the balloon after the reaction is complete.
a.
0.883 L
b.
1.77 L
c.
2.65 L
d.
0.442 L
e.
3.53 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
There are two balloons, both the same size and temperature. One balloon contains hydrogen and the other balloon contains carbon dioxide. The hydrogen balloon floats, and the carbon dioxide balloon sinks in the air. How do the pressures inside of the two balloons compare?
A) The carbon dioxide balloon has a greater pressure than the hydrogen balloon.
B) The hydrogen balloon has a greater pressure than the carbon dioxide balloon.
C) The pressures in both balloons are essentially the same.
D) This problem cannot be solved without more information given.
A) The carbon dioxide balloon has a greater pressure than the hydrogen balloon.
B) The hydrogen balloon has a greater pressure than the carbon dioxide balloon.
C) The pressures in both balloons are essentially the same.
D) This problem cannot be solved without more information given.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Solid carbon dioxide is placed into a Ziploc bag. At a certain temperature, solid carbon dioxide sublimes (turns directly from a solid to a gas) at a rate of 0.726 g/minute. How many gaseous carbon dioxide molecules are present after 4.84 minutes?
A)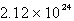
B)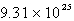
C)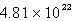
D)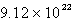
E)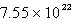
A)
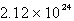
B)
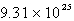
C)
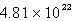
D)
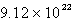
E)
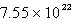

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
A flexible weather balloon contains helium gas at a volume of 855 L. Initially, the balloon is at sea level, where the temperature is 25oC and the barometric pressure is 0.947 atm. The balloon then rises to an altitude of 5500 ft, where the pressure is 0.785 atm and the temperature is 15oC. What is the change in the volume of the balloon as it ascends from sea level to 5500 ft?
A)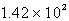 L
L
B)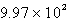 L
L
C)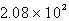 L
L
D)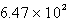 L
L
E)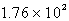 L
L
A)
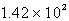 L
LB)
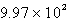 L
LC)
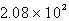 L
LD)
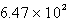 L
LE)
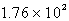 L
L
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
Which conditions of P and T are most ideal for a gas?
A) high P, high T
B) high P, low T
C) low P, high T
D) low P, low T
E) depends on the gas
A) high P, high T
B) high P, low T
C) low P, high T
D) low P, low T
E) depends on the gas

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
There are two balloons, both the same size and temperature. One balloon contains hydrogen and the other balloon contains carbon dioxide. The hydrogen balloon floats, and the carbon dioxide balloon sinks in the air. Which balloon contains a greater number of molecules?
A) The carbon dioxide balloon contains a greater number of molecules.
B) The hydrogen balloon contains a greater number of molecules.
C) Both balloons contain the same number of molecules.
D) This problem cannot be solved without more information given.
A) The carbon dioxide balloon contains a greater number of molecules.
B) The hydrogen balloon contains a greater number of molecules.
C) Both balloons contain the same number of molecules.
D) This problem cannot be solved without more information given.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
What volume of HCl(g) measured at STP can be produced from 1.91 g of H2 and excess Cl2 according to the following equation? 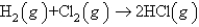
A) 21.2 L
B) 42.4 L
C) 86 L
D) 173 L
E) 64.2 L
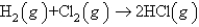
A) 21.2 L
B) 42.4 L
C) 86 L
D) 173 L
E) 64.2 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


