Deck 10: Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 10: Energy
1
Perform the indicated conversion: 38.86 kJ =____ kcal
A)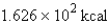
B) 9.288 kcal
C)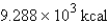
D)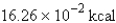
E) 0.1077 kcal
A)
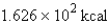
B) 9.288 kcal
C)
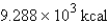
D)
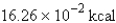
E) 0.1077 kcal
9.288 kcal
2
Which of the following is a valid unit for specific heat (or specific heat capacity)?
A) cal/g·°C
B) cal
C) cal/g
D) °C
E) g·°C/cal
A) cal/g·°C
B) cal
C) cal/g
D) °C
E) g·°C/cal
cal/g·°C
3
The amount of energy needed to heat 2.00 g mercury from 50.0°C to 85.0°C is 9.87 J. The specific heat capacity of this sample of mercury is
A) 0.141 J/g·°C
B) 0.058 J/g·°C
C) 0.282 J/g·°C
D) 345 J/g·°C
E) 691 J/g·°C
A) 0.141 J/g·°C
B) 0.058 J/g·°C
C) 0.282 J/g·°C
D) 345 J/g·°C
E) 691 J/g·°C
0.141 J/g·°C
4
The specific heat capacity of aluminum is 0.89 J/g·°C. Calculate the amount of energy needed to warm 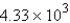 g of aluminum from 73.0°C to 155.0°C.
g of aluminum from 73.0°C to 155.0°C.
A)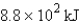
B)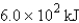
C)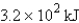
D)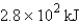
E) none of these
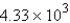 g of aluminum from 73.0°C to 155.0°C.
g of aluminum from 73.0°C to 155.0°C.A)
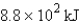
B)
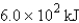
C)
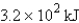
D)
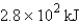
E) none of these

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
Heat is typically measured in
A) °C
B) °F
C) joules
D) grams
A) °C
B) °F
C) joules
D) grams

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
A gas absorbs 75 kJ of heat and does 21 kJ of work. Calculate 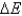 .
.
A) 54 kJ
B) 96 kJ
C) 3.6 kJ
D) -54 kJ
E) -96 kJ
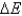 .
.A) 54 kJ
B) 96 kJ
C) 3.6 kJ
D) -54 kJ
E) -96 kJ

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
The specific heat capacity of iron is 0.45 J/g·°C. How many joules of energy are needed to warm 1.31 g of iron from 20.00°C to 29.00°C?
A) 17 J
B) 12 J
C) 26 J
D) 11 J
E) 5.3 J
A) 17 J
B) 12 J
C) 26 J
D) 11 J
E) 5.3 J

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
Calculate the heat given off when 159.7 g of copper cools from 155.0°C to 23.0°C. The specific heat capacity of copper is 0.385 J/g·°C.
A)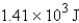
B)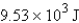
C)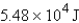
D)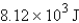
E) none of these
A)
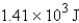
B)
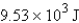
C)
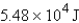
D)
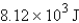
E) none of these

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
Assume that 491.8 J of heat is added to 5.00 g of water originally at 23.0°C. What would be the final temperature of the water? (Specific heat capacity of water = 4.184 J/g·°C.)
A) 23.5 °C
B) 61.5 °C
C) 74.5 °C
D) 46.5 °C
E) none of these
A) 23.5 °C
B) 61.5 °C
C) 74.5 °C
D) 46.5 °C
E) none of these

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
Which is larger, one calorie or one joule?

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
A system absorbs 159 kJ of heat, and performs 89 kJ of work on the surroundings. What is 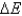 of the system?
of the system?
A) 248 kJ
B) -248 kJ
C) 70 kJ
D) -70 kJ
E) 1.8 kJ
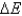 of the system?
of the system?A) 248 kJ
B) -248 kJ
C) 70 kJ
D) -70 kJ
E) 1.8 kJ

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
How many joules of energy would be required to heat 15.9 g of carbon from 23.6°C to 54.2°C? (Specific heat capacity of carbon = 0.71 J/g·°C.)
A)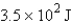
B)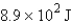
C)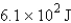
D)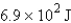
E) none of these
A)
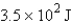
B)
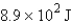
C)
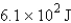
D)
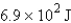
E) none of these

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
How much energy will be needed to heat 69.7 gal of water from 22.0°C to 110.0°C? (Note that 1.00 gal weighs 3.77 kg and that water has a specific heat capacity of 4.184 J/g·°C.)
A)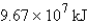
B)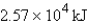
C)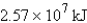
D)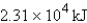
E)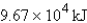
A)
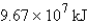
B)
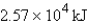
C)
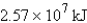
D)
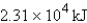
E)
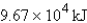

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
The specific heat capacity of silver is 0.24 J/g·°C. How many joules of energy are needed to warm 0.960 g of silver from 25.0°C to 27.5°C?
A) 6.3 J
B) 0.58 J
C) 12.1 J
D) 10.0 J
E) none of these
A) 6.3 J
B) 0.58 J
C) 12.1 J
D) 10.0 J
E) none of these

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
SI units for specific heat capacity are
A) cal
B) cal/g
C) J/g·°C
D) g/mL
E) g·°C/cal
A) cal
B) cal/g
C) J/g·°C
D) g/mL
E) g·°C/cal

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
The quantity of heat required to change the temperature of 1 g of a substance by 1°C is defined as
A) a joule
B) specific heat capacity
C) a calorie
D) density
A) a joule
B) specific heat capacity
C) a calorie
D) density

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
A 6.75-g sample of gold (specific heat capacity = 0.130 J/g °C) is heated using 55.8 J of energy. If the original temperature of the gold is 25.0°C, what is its final temperature?
A) 88.6 °C
B) 38.6 °C
C) 76.9 °C
D) 73.6 °C
E) 63.6 °C
A) 88.6 °C
B) 38.6 °C
C) 76.9 °C
D) 73.6 °C
E) 63.6 °C

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
Express 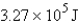 in kilocalories.
in kilocalories.
A)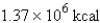
B)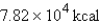
C) 78.2 kcal
D) 327 kcal
E)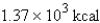
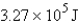 in kilocalories.
in kilocalories.A)
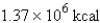
B)
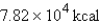
C) 78.2 kcal
D) 327 kcal
E)
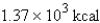

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate 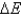 given the following information:
given the following information: 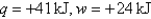
A) 17 kJ
B) 65 kJ
C) -65 kJ
D) -17 kJ
E) 1.7 kJ
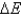 given the following information:
given the following information: 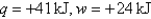
A) 17 kJ
B) 65 kJ
C) -65 kJ
D) -17 kJ
E) 1.7 kJ

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
A 4.60-g sample of iron is heated from 47.0°C to 75.0°C. The amount of energy required is 57.96 J. The specific heat capacity of this sample of iron is
A) 0.450 J/g·°C
B) 1.233 J/g·°C
C) 2.07 J/g·°C
D) 1623 J/g·°C
E) 7465 J/g·°C
A) 0.450 J/g·°C
B) 1.233 J/g·°C
C) 2.07 J/g·°C
D) 1623 J/g·°C
E) 7465 J/g·°C

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
Energy can be classified as either potential or kinetic energy.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
The energy gained by the surroundings must be equal to the energy lost by the system.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
____ is a measure of the random motions of the components of a substance.
A) Heat
B) Temperature
C) Energy
D) Radiation
E) Matter
A) Heat
B) Temperature
C) Energy
D) Radiation
E) Matter

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
For the reaction 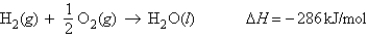 Calculate the enthalpy change when 2.80 g of water is produced.
Calculate the enthalpy change when 2.80 g of water is produced.
A) 102 kJ
B) 801 kJ
C) -801 kJ
D) 44.4 kJ
E) -44.4 kJ
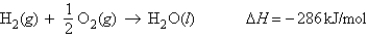 Calculate the enthalpy change when 2.80 g of water is produced.
Calculate the enthalpy change when 2.80 g of water is produced.A) 102 kJ
B) 801 kJ
C) -801 kJ
D) 44.4 kJ
E) -44.4 kJ

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
Determine the enthalpy change when 19.4 g of carbon is reacted with oxygen according to the reaction 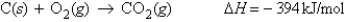
A) 636 kJ
B)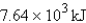
C) -636 kJ
D) -7.64 kJ
E) 6.76 kJ
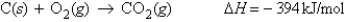
A) 636 kJ
B)
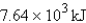
C) -636 kJ
D) -7.64 kJ
E) 6.76 kJ

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
Perform the indicated conversion: 1.345 kcal =____ J
A)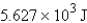
B) 5.627 J
C)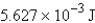
D)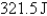
E) 3.111 J
A)
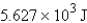
B) 5.627 J
C)
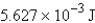
D)
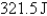
E) 3.111 J

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following processes is endothermic?
A) water droplets condensing on a soda can on a hot summer day
B) an ice pack getting cold (due to ammonium nitrate dissolving in water inside the pack)
C) thermite reaction between iron(III) oxide and aluminum (spectacular flames are observed)
D) freezing water to make ice cubes
E) none of the above are endothermic processes
A) water droplets condensing on a soda can on a hot summer day
B) an ice pack getting cold (due to ammonium nitrate dissolving in water inside the pack)
C) thermite reaction between iron(III) oxide and aluminum (spectacular flames are observed)
D) freezing water to make ice cubes
E) none of the above are endothermic processes

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
The specific heat capacity of gold is 0.13 J/g°C. How many calories of energy are needed to warm 0.570 g of gold from 30.0°C to 39.5°C?
A) 0.70 cal
B) 0.17 cal
C) 1.2 cal
D) 2.9 cal
E) 23 cal
A) 0.70 cal
B) 0.17 cal
C) 1.2 cal
D) 2.9 cal
E) 23 cal

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
For the reaction 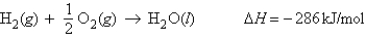 Calculate the enthalpy change when 4.73 g of hydrogen gas is reacted with excess oxygen.
Calculate the enthalpy change when 4.73 g of hydrogen gas is reacted with excess oxygen.
A) -60.5 kJ
B) 671 kJ
C) -671 kJ
D)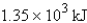
E) -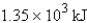
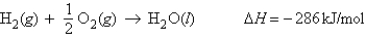 Calculate the enthalpy change when 4.73 g of hydrogen gas is reacted with excess oxygen.
Calculate the enthalpy change when 4.73 g of hydrogen gas is reacted with excess oxygen.A) -60.5 kJ
B) 671 kJ
C) -671 kJ
D)
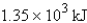
E) -

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
____ is a flow of energy due to a temperature difference.
A) Heat
B) Radiation
C) Matter
D) Gravity
E) Work
A) Heat
B) Radiation
C) Matter
D) Gravity
E) Work

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
A 100.0 g sample of water at 27.0°C is poured into a 77.4 g sample of water at 89.0°C. What will be the final temperature of the water?
A) 88.7°C
B) 424°C
C) 23.6°C
D) 185°C
E) 54.1°C
A) 88.7°C
B) 424°C
C) 23.6°C
D) 185°C
E) 54.1°C

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
Perform the indicated conversion: 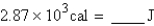
A) 686 J
B)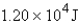
C) 12.0 J
D)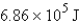
E)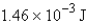
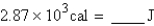
A) 686 J
B)
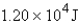
C) 12.0 J
D)
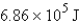
E)
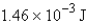

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
For the reaction 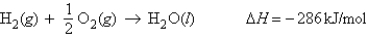 What is the enthalpy change when 15.0 mol of hydrogen gas reacts with excess oxygen.
What is the enthalpy change when 15.0 mol of hydrogen gas reacts with excess oxygen.
A) 19.1 kJ
B) -19.1 kJ
C)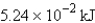
D) -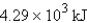
E) -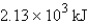
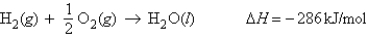 What is the enthalpy change when 15.0 mol of hydrogen gas reacts with excess oxygen.
What is the enthalpy change when 15.0 mol of hydrogen gas reacts with excess oxygen.A) 19.1 kJ
B) -19.1 kJ
C)
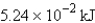
D) -
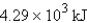
E) -
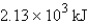

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
The law of conservation of energy states that energy can be converted from one form to another but can be neither created nor destroyed.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements about energy is false?
A) A reaction cannot be exothermic overall if activation energy is required.
B) A system is the most stable when it is at its lowest energy state.
C) Energy can be defined as whatever is required to oppose a natural tendency.
D) Energy transferred into a system can also be transferred out of the system.
E) An atom in an excited state can return to its ground state by releasing visible light.
A) A reaction cannot be exothermic overall if activation energy is required.
B) A system is the most stable when it is at its lowest energy state.
C) Energy can be defined as whatever is required to oppose a natural tendency.
D) Energy transferred into a system can also be transferred out of the system.
E) An atom in an excited state can return to its ground state by releasing visible light.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
The distance traveled from New York to Los Angeles is an example of a state function.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following processes is exothermic?
A) rolling a ball up a hill
B) boiling water in a beaker to make steam
C) allowing meat to thaw after taking it out of the freezer
D) reacting hydrogen and oxygen gases to make water
E) a popsicle melting on a warm summer day
A) rolling a ball up a hill
B) boiling water in a beaker to make steam
C) allowing meat to thaw after taking it out of the freezer
D) reacting hydrogen and oxygen gases to make water
E) a popsicle melting on a warm summer day

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
Perform the indicated conversion: 7.079 kcal =____ kJ
A) 29.62 kJ
B) 1.692 kJ
C) 0.5910 kJ
D)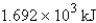
E)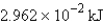
A) 29.62 kJ
B) 1.692 kJ
C) 0.5910 kJ
D)
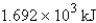
E)
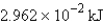

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
____ is the ability to do work or produce heat.
A) Gravity
B) Temperature
C) Radiation
D) Matter
E) Energy
A) Gravity
B) Temperature
C) Radiation
D) Matter
E) Energy

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
A 51.1-g sample of aluminum at 95.0°C is dropped into 35.0 g of water at 40.0°C. What is the final temperature of the mixture? (specific heat capacity of aluminum = 0.89 J/g°C; specific heat capacity of water = 4.184 J/g°C)
A) 53°C
B) -8.0°C
C) 101°C
D) 24°C
E) -15°C
A) 53°C
B) -8.0°C
C) 101°C
D) 24°C
E) -15°C

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
Enthalpy is not a state function.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
The calorie is defined as the amount of energy (heat) required to raise the temperature of one gram of water by one degree Celsius.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
The first law of ____ states that the energy of the universe is constant.
A) matter
B) mass
C) thermodynamics
D) motion
E) energy
A) matter
B) mass
C) thermodynamics
D) motion
E) energy

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
On a cold winter day, a steel metal fence post feels colder than a wooden fence post of identical size because
A) the specific heat capacity of steel is higher than the specific heat capacity of wood.
B) the specific heat capacity of steel is lower than the specific heat capacity of wood.
C) steel has the ability to resist a temperature change better than wood.
D) the mass of steel is less than wood, so it loses heat faster.
E) Two of the above statements are true.
A) the specific heat capacity of steel is higher than the specific heat capacity of wood.
B) the specific heat capacity of steel is lower than the specific heat capacity of wood.
C) steel has the ability to resist a temperature change better than wood.
D) the mass of steel is less than wood, so it loses heat faster.
E) Two of the above statements are true.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following processes: 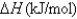
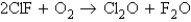 167.4
167.4 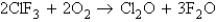 341.4
341.4 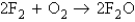 -43.4 Calculate
-43.4 Calculate 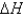 for the reaction
for the reaction 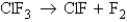
A) +217.5 kJ/mol
B) +130.2 kJ/mol
C) +108.7 kJ/mol
D) -217.5 kJ/mol
E) none of these
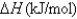
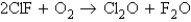 167.4
167.4 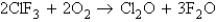 341.4
341.4 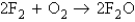 -43.4 Calculate
-43.4 Calculate 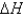 for the reaction
for the reaction 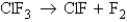
A) +217.5 kJ/mol
B) +130.2 kJ/mol
C) +108.7 kJ/mol
D) -217.5 kJ/mol
E) none of these

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the reaction: 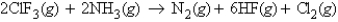 When calculating the
When calculating the 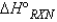 , why is the
, why is the 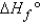 for
for 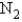 not important?
not important?
A) Because nitrogen is in its standard elemental state, and no energy is needed for this product to exist.
B) Because any element or compound in the gaseous state requires a negligible amount of energy to exist.
C) Because the products are not included when calculating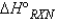 .
.
D) Because nitrogen is in its elemental state and does not contribute to the reaction itself.
E) Two of the above statements explain why N2 is not important when calculating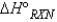 .
.
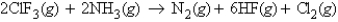 When calculating the
When calculating the 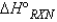 , why is the
, why is the 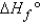 for
for 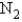 not important?
not important?A) Because nitrogen is in its standard elemental state, and no energy is needed for this product to exist.
B) Because any element or compound in the gaseous state requires a negligible amount of energy to exist.
C) Because the products are not included when calculating
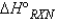 .
.D) Because nitrogen is in its elemental state and does not contribute to the reaction itself.
E) Two of the above statements explain why N2 is not important when calculating
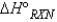 .
.
Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
____ was formed from the remains of plants that were buried and subjected to high pressure and heat over long periods of time.
A) Natural gas
B) Asphalt
C) Kerosene
D) Coal
E) Wood
A) Natural gas
B) Asphalt
C) Kerosene
D) Coal
E) Wood

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
According to the first law of thermodynamics, the energy of the universe is constant. Does this mean that 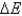 is always equal to zero?
is always equal to zero?
A) Yes,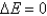 at all times, which is why
at all times, which is why 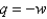 .
.
B) No,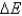 does not always equal zero, but this is due only to factors such as friction and heat.
does not always equal zero, but this is due only to factors such as friction and heat.
C) No,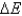 does not always equal zero because it refers to the system's internal energy, which is affected by heat and work.
does not always equal zero because it refers to the system's internal energy, which is affected by heat and work.
D) No,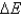 never equals zero because work is always being done on the system or by the system.
never equals zero because work is always being done on the system or by the system.
E) No,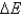 never equals zero because energy is always flowing between the system and surroundings.
never equals zero because energy is always flowing between the system and surroundings.
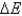 is always equal to zero?
is always equal to zero?A) Yes,
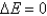 at all times, which is why
at all times, which is why 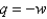 .
.B) No,
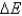 does not always equal zero, but this is due only to factors such as friction and heat.
does not always equal zero, but this is due only to factors such as friction and heat.C) No,
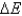 does not always equal zero because it refers to the system's internal energy, which is affected by heat and work.
does not always equal zero because it refers to the system's internal energy, which is affected by heat and work.D) No,
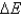 never equals zero because work is always being done on the system or by the system.
never equals zero because work is always being done on the system or by the system.E) No,
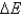 never equals zero because energy is always flowing between the system and surroundings.
never equals zero because energy is always flowing between the system and surroundings.
Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
The greenhouse effect occurs only on the planet Earth.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
____ is a measure of disorder or randomness.
A) Entropy
B) Enthalpy
C) Calorimetry
D) Internal energy
E) Hess's law
A) Entropy
B) Enthalpy
C) Calorimetry
D) Internal energy
E) Hess's law

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following standard heats of formation: 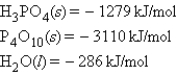 Calculate the change in enthalpy for the following process:
Calculate the change in enthalpy for the following process: 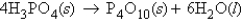
A) +290 kJ
B) -290 kJ
C) -2117 kJ
D) +2117 kJ
E) -9942 kJ
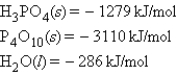 Calculate the change in enthalpy for the following process:
Calculate the change in enthalpy for the following process: 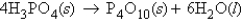
A) +290 kJ
B) -290 kJ
C) -2117 kJ
D) +2117 kJ
E) -9942 kJ

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the following reaction:  Is the reaction exothermic or endothermic?
Is the reaction exothermic or endothermic?
A) exothermic
B) endothermic
 Is the reaction exothermic or endothermic?
Is the reaction exothermic or endothermic?A) exothermic
B) endothermic

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
In the equation 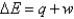 , the q represents
, the q represents
A) work
B) change in energy
C) moles of a substance
D) mass of a substance
E) heat
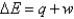 , the q represents
, the q representsA) work
B) change in energy
C) moles of a substance
D) mass of a substance
E) heat

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the following standard heats of formation: 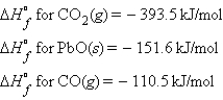 Calculate the change in enthalpy for the following process:
Calculate the change in enthalpy for the following process: 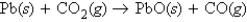
A) 0 kJ
B) -655.6 kJ
C) +655.6 kJ
D) -131.4 kJ
E) +131.4 kJ
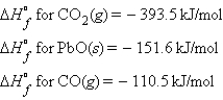 Calculate the change in enthalpy for the following process:
Calculate the change in enthalpy for the following process: 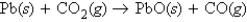
A) 0 kJ
B) -655.6 kJ
C) +655.6 kJ
D) -131.4 kJ
E) +131.4 kJ

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following statements is/are true?
(I) q (heat) is a state function because ΔH is a state function and q=Δ .
(II) When 50.0 g of aluminum at 20.0°C is placed in 50.0 mL of water at 30.0°C, the H2O will undergo a smaller temperature change than the aluminum. (density of H2O = 1.0 g/ml , specific heat capacity of H2O = 4.18 J/g.°C , specific heat capacity of aluminum = 0.89 J/g. °C)
III) When a gas is compressed, the work is negative since the surroundings is doing work on the system and energy flows out of the system.
(IV) For the reaction (at constant pressure) 2N2(g)+5O2(g) → 2N2O5(g) , the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps.
A) I, II, IV
B) II, III
C) II, III, IV
D) II, IV
E) All of the above statements are true.
(I) q (heat) is a state function because ΔH is a state function and q=Δ .
(II) When 50.0 g of aluminum at 20.0°C is placed in 50.0 mL of water at 30.0°C, the H2O will undergo a smaller temperature change than the aluminum. (density of H2O = 1.0 g/ml , specific heat capacity of H2O = 4.18 J/g.°C , specific heat capacity of aluminum = 0.89 J/g. °C)
III) When a gas is compressed, the work is negative since the surroundings is doing work on the system and energy flows out of the system.
(IV) For the reaction (at constant pressure) 2N2(g)+5O2(g) → 2N2O5(g) , the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps.
A) I, II, IV
B) II, III
C) II, III, IV
D) II, IV
E) All of the above statements are true.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


