Deck 8: Chemical Composition
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/120
Play
Full screen (f)
Deck 8: Chemical Composition
1
Which represents the greatest number of atoms?
A) 15.0 g S
B) 15.0 g Cu
C) 15.0 g Zr
D) 15.0 g Sc
E) all the same
A) 15.0 g S
B) 15.0 g Cu
C) 15.0 g Zr
D) 15.0 g Sc
E) all the same
15.0 g S
2
You have 12.6 g of an unknown substance A and 35.0 g of chlorine gas. Substance A contains 1.5 times as many molecules as the chlorine gas. What is the identity of A?
A) NH3
B) BF3
C) O2
D) CO
E) none of these
A) NH3
B) BF3
C) O2
D) CO
E) none of these
NH3
3
Calculate the mass of 23.7 moles of He.
A) 94.9
B) 27.7
C) 1.43 * 1025
D) 5.92
E) 6.02 *1023
A) 94.9
B) 27.7
C) 1.43 * 1025
D) 5.92
E) 6.02 *1023
94.9
4
One atomic mass unit (amu) is the mass (in grams) of one mole of the substance.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
5
A 1.50-mol sample of Zr represents how many atoms?
A) 9.03 * 1023 atoms
B) 2.49 *10-24 atoms
C) 4.01 *1023 atoms
D) 8.24 * 1025 atoms
E) 1.64 *10-2 atoms
A) 9.03 * 1023 atoms
B) 2.49 *10-24 atoms
C) 4.01 *1023 atoms
D) 8.24 * 1025 atoms
E) 1.64 *10-2 atoms

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
6
A 3.37-mol sample of aluminum represents how many atoms?
A) 2.03 *1024 atoms
B) 5.60 *10-24 atoms
C) 1.25 * 1023 atoms
D) 5.48 * 1025 atoms
E) none of these
A) 2.03 *1024 atoms
B) 5.60 *10-24 atoms
C) 1.25 * 1023 atoms
D) 5.48 * 1025 atoms
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
7
One mole of something consists of ____________ units of that substance.
A) 12.01
B) 1.008
C)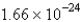
D)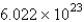
E) 32.00
A) 12.01
B) 1.008
C)
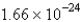
D)
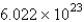
E) 32.00

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
8
A 30.5-g sample of Ca contains how many calcium atoms?
A) 4.58 *1023 atoms
B) 61.0 atoms
C) 7.61 * 10-1 atoms
D) 1.84* 1025 atoms
E) 30.5 atoms
A) 4.58 *1023 atoms
B) 61.0 atoms
C) 7.61 * 10-1 atoms
D) 1.84* 1025 atoms
E) 30.5 atoms

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
9
How many moles of Ca atoms are in 613.3 g Ca?
A) 15.30 mol
B) 6.535 * 10-2 mol
C) 2.458 * 104 mol
D) 30.60 mol
E) 613.3 mol
A) 15.30 mol
B) 6.535 * 10-2 mol
C) 2.458 * 104 mol
D) 30.60 mol
E) 613.3 mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
10
How many atoms of calcium are present in 58.2 g of calcium?
A) 8.74 *1023
B) 2.41 *10-24
C) 3.50 * 1025
D) 6.02 *1023
E) none of these
A) 8.74 *1023
B) 2.41 *10-24
C) 3.50 * 1025
D) 6.02 *1023
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
11
A 2.35-mole sample of H2O2 weighs
A) 79.9 g
B) 2.35 g
C) 36.4 g
D) 42.3 g
E) 42.3 amu
A) 79.9 g
B) 2.35 g
C) 36.4 g
D) 42.3 g
E) 42.3 amu

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
12
The formula of a compound that expresses the smallest whole-number ratio of the atoms present is called the empirical formula.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
13
Which represents the greatest mass?
A) 1.0 mol Rb
B) 1.0 mol Ti
C) 1.0 mol Fe
D) 1.0 mol Al
E) all the same
A) 1.0 mol Rb
B) 1.0 mol Ti
C) 1.0 mol Fe
D) 1.0 mol Al
E) all the same

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
14
The molar mass of ammonium phosphate is
A) 149.09 g/mol
B) 113.01 g/mol
C) 302.95 g/mol
D) 165.09 g/mol
E) 133.09 g/mol
A) 149.09 g/mol
B) 113.01 g/mol
C) 302.95 g/mol
D) 165.09 g/mol
E) 133.09 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
15
You have two beakers on your lab table. Beaker #1 contains 32.07 g of sulfur and Beaker #2 contains 74.92 g of arsenic (As). Which beaker contains the greatest number of atoms? Choose the best answer.
A) Beaker #1 because one sulfur atom weighs less than one arsenic atom so you need more of the sulfur atoms to fill the beaker.
B) Beaker #1 because it contains more moles of sulfur atoms than the number of moles of arsenic atoms in Beaker #2.
C) Beaker #2 because arsenic has a greater mass than sulfur.
D) Beaker #2 because it contains more moles of arsenic atoms than the number of moles of sulfur atoms in Beaker #1.
E) Beakers #1 and #2 contain the same number of atoms because there is one mole in each.
A) Beaker #1 because one sulfur atom weighs less than one arsenic atom so you need more of the sulfur atoms to fill the beaker.
B) Beaker #1 because it contains more moles of sulfur atoms than the number of moles of arsenic atoms in Beaker #2.
C) Beaker #2 because arsenic has a greater mass than sulfur.
D) Beaker #2 because it contains more moles of arsenic atoms than the number of moles of sulfur atoms in Beaker #1.
E) Beakers #1 and #2 contain the same number of atoms because there is one mole in each.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
16
What is the mass of 8 atoms of copper in grams?
A) 8.44 * 10-22 g
B) 1.33 * 10-23 g
C) 508 g
D) 7.58 *1022 g
E) 63.5 g
A) 8.44 * 10-22 g
B) 1.33 * 10-23 g
C) 508 g
D) 7.58 *1022 g
E) 63.5 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
17
A 36.6-mol sample of Co represents how many atoms?
A) 2.20 * 1025 atoms
B) 6.08 *10-23 atoms
C) 1.65 * 1022 atoms
D) 1.30 * 1027 atoms
E) 6.72 * 102 atoms
A) 2.20 * 1025 atoms
B) 6.08 *10-23 atoms
C) 1.65 * 1022 atoms
D) 1.30 * 1027 atoms
E) 6.72 * 102 atoms

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
18
What is the mass of 5.826 moles of Ca(OH)2?
A) 431.7 g
B) 7.863 * 10-2 g
C) 12.718 g
D) 863.4 g
E) 74.10 g
A) 431.7 g
B) 7.863 * 10-2 g
C) 12.718 g
D) 863.4 g
E) 74.10 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
19
How many atoms are there in 71.9 g of nickel?
A) 7.38 * 1023
B) 4.22* 103
C) 1.23
D) 4.33 * 1025
E) none of these
A) 7.38 * 1023
B) 4.22* 103
C) 1.23
D) 4.33 * 1025
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate the mass of 3.53 *1026 atoms of silver.
A) 6.32* 104 g
B) 3.81 * 1028 g
C) 1.97 * 1048 g
D) 5.86 *102 g
E) none of these
A) 6.32* 104 g
B) 3.81 * 1028 g
C) 1.97 * 1048 g
D) 5.86 *102 g
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
21
The mass in grams of 3.52 mol of nitrogen gas ( N2) is
A) 98.6 g/mol
B) 49.3 g/mol
C) 2.12 *1024 g/mol
D) 6.02 * 1023 g/mol
E) none of these
A) 98.6 g/mol
B) 49.3 g/mol
C) 2.12 *1024 g/mol
D) 6.02 * 1023 g/mol
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
22
Consider equal mole samples of dinitrogen monoxide, aluminum nitrate, and potassium cyanide. Rank these from least to greatest number of nitrogen atoms in each sample.
A) potassium cyanide, aluminum nitrate, dinitrogen monoxide
B) aluminum nitrate, dinitrogen monoxide, potassium cyanide
C) potassium cyanide, dinitrogen monoxide, aluminum nitrate
D) aluminum nitrate, potassium cyanide, dinitrogen monoxide
E) dinitrogen monoxide, aluminum nitrate, potassium cyanide
A) potassium cyanide, aluminum nitrate, dinitrogen monoxide
B) aluminum nitrate, dinitrogen monoxide, potassium cyanide
C) potassium cyanide, dinitrogen monoxide, aluminum nitrate
D) aluminum nitrate, potassium cyanide, dinitrogen monoxide
E) dinitrogen monoxide, aluminum nitrate, potassium cyanide

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
23
How many molecules of O2 are there in 2.2 mol of O2?
A) 1.3 *1024
B) 3.7 * 10-24
C) 70
D) 14.55
E) 2.7 *1023
A) 1.3 *1024
B) 3.7 * 10-24
C) 70
D) 14.55
E) 2.7 *1023

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
24
What is the molar mass of nitroglycerin, C3H5(NO3)3?
A) 227 g/mol
B) 179 g/mol
C) 199 g/mol
D) 185 g/mol
E) none of these
A) 227 g/mol
B) 179 g/mol
C) 199 g/mol
D) 185 g/mol
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
25
What is the molar mass of Ba(OH)2?
A) 171.35 g/mol
B) 291.67 g/mol
C) 308.68 g/mol
D) 154.34 g/mol
E) 188.36 g/mol
A) 171.35 g/mol
B) 291.67 g/mol
C) 308.68 g/mol
D) 154.34 g/mol
E) 188.36 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
26
The molar mass of strontium hydroxide, Sr(OH)2 is
A) 121.6 g/mol
B) 192.3 g/mol
C) 209.3 g/mol
D) 105.6 g/mol
E) 104.6 g/mol
A) 121.6 g/mol
B) 192.3 g/mol
C) 209.3 g/mol
D) 105.6 g/mol
E) 104.6 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
27
The molar mass of barium nitrate, Ba(NO3)2 is
A) 261.35 g/mol
B) 336.67 g/mol
C) 293.35 g/mol
D) 229.35 g/mol
E) 199.34 g/mol
A) 261.35 g/mol
B) 336.67 g/mol
C) 293.35 g/mol
D) 229.35 g/mol
E) 199.34 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
28
The mass of 0.74 mol of H2 is
A) 1.5 g
B) 0.74 g
C) 2.7 g
D) 0.37 g
E) none of these
A) 1.5 g
B) 0.74 g
C) 2.7 g
D) 0.37 g
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
29
The molar mass of AsBr5 is
A) 474.4 g/mol
B) 154.8 g/mol
C) 774.1 g/mol
D) 454.5 g/mol
E) 399.5 g/mol
A) 474.4 g/mol
B) 154.8 g/mol
C) 774.1 g/mol
D) 454.5 g/mol
E) 399.5 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
30
The term formula weight is also used instead of molar mass for ionic compounds.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
31
What is the molar mass of BaBr2?
A) 297.13 g/mol
B) 354.56 g/mol
C) 217.23 g/mol
D) 434.46 g/mol
E) 594.26 g/mol
A) 297.13 g/mol
B) 354.56 g/mol
C) 217.23 g/mol
D) 434.46 g/mol
E) 594.26 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
32
What is the mass of 1.819 mol of potassium sulfide?
A) 200.6 g
B) 129.4 g
C) 187.8 g
D) 60.62 g
E) none of these
A) 200.6 g
B) 129.4 g
C) 187.8 g
D) 60.62 g
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
33
What is the molar mass of Li2SO4?
A) 109.88 g/mol
B) 77.88 g/mol
C) 102.94 g/mol
D) 93.88 g/mol
E) 198.94 g/mol
A) 109.88 g/mol
B) 77.88 g/mol
C) 102.94 g/mol
D) 93.88 g/mol
E) 198.94 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
34
The molar mass of blood sugar, C6H12O6, also known as glucose and dextrose, is
A) 180.16 g/mol
B) 6.02 * 1023 g/mol
C) 108.10 g/mol
D) 168.06 g/mol
E) none of these
A) 180.16 g/mol
B) 6.02 * 1023 g/mol
C) 108.10 g/mol
D) 168.06 g/mol
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
35
A sample of an element with a mass equal to that element's average atomic mass expressed in grams contains one mole of atoms.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
36
The molar mass of C10H22 is
A) 142.32 g/mol
B) 130.31 g/mol
C) 120.10 g/mol
D) 286.44 g/mol
E) 130.20 g/mol
A) 142.32 g/mol
B) 130.31 g/mol
C) 120.10 g/mol
D) 286.44 g/mol
E) 130.20 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
37
The molar mass of calcium phosphate, Ca3(PO4)2 is
A) 310.18 g/mol
B) 365.07 g/mol
C) 230.02 g/mol
D) 215.21 g/mol
E) 278.18 g/mol
A) 310.18 g/mol
B) 365.07 g/mol
C) 230.02 g/mol
D) 215.21 g/mol
E) 278.18 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
38
What is the molar mass of C3H6O3?
A) 90.09 g/mol
B) 94.08 g/mol
C) 54.06 g/mol
D) 84.03 g/mol
E) none of these
A) 90.09 g/mol
B) 94.08 g/mol
C) 54.06 g/mol
D) 84.03 g/mol
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
39
What is the molar mass of Rb3PO4?
A) 351.38 g/mol
B) 320.41 g/mol
C) 6.02 *1023 g/mol
D) 265.91 g/mol
E) 319.38 g/mol
A) 351.38 g/mol
B) 320.41 g/mol
C) 6.02 *1023 g/mol
D) 265.91 g/mol
E) 319.38 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
40
What is the molar mass of N2O?
A) 44.02 g/mol
B) 30.01 g/mol
C) 28.02 g/mol
D) 60.02 g/mol
E) 46.01 g/mol
A) 44.02 g/mol
B) 30.01 g/mol
C) 28.02 g/mol
D) 60.02 g/mol
E) 46.01 g/mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
41
Convert: 7.38 mol Cu(NO3)2 = ____________ g Cu(NO3)2
A) 1.38 * 103
B) 25.4
C) 3.93 * 10-2
D) 1.27 * 103
E) none of these
A) 1.38 * 103
B) 25.4
C) 3.93 * 10-2
D) 1.27 * 103
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
42
Convert 47.5 g O2 to mol O2.
A) 1.48 mol
B) 0.674 mol
C) 2.97 mol
D) 1.52 *103 mol
E) 2.86* 1025 mol
A) 1.48 mol
B) 0.674 mol
C) 2.97 mol
D) 1.52 *103 mol
E) 2.86* 1025 mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
43
Convert: 19.9 g NaCl = ____ mol NaCl
A) 0.341
B)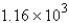
C) 2.94
D)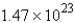
E) none of these
A) 0.341
B)
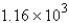
C) 2.94
D)
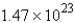
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
44
Convert: 11.59 mol CaF2 = ____ g CaF2
A) 684.7
B) 904.9
C) 0.1484
D) 6.737
E) none of these
A) 684.7
B) 904.9
C) 0.1484
D) 6.737
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
45
Convert: 0.205 mol Ca(OH)2 = ___________ g Ca(OH)2
A) 15.2
B) 2.77 *10-3
C) 11.7
D) 361
E) none of these
A) 15.2
B) 2.77 *10-3
C) 11.7
D) 361
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
46
You have 1.0 mole of each compound below. Which has the greatest mass?
A) sodium hydroxide
B) iron(III) sulfate
C) ammonium nitrate
D) barium carbonate
E) lead(IV) oxide
A) sodium hydroxide
B) iron(III) sulfate
C) ammonium nitrate
D) barium carbonate
E) lead(IV) oxide

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
47
Convert: 92.1 g CO = ____ molecules CO
A)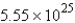
B)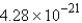
C)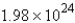
D)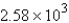
E)
A)
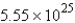
B)
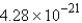
C)
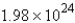
D)
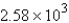
E)


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
48
Convert: 460.2 g MgCl2 = ____ units of MgCl2
A)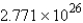
B) 4.834
C)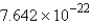
D)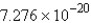
E)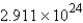
A)
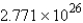
B) 4.834
C)
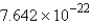
D)
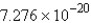
E)
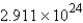

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the mass of 3.64*1019 molecules of HCl.
A) 2.20 *10-3 g
B) 6.04* 10-5 g
C) 2.19 * 1043 g
D) 1.66 * 10-6 g
E) none of these
A) 2.20 *10-3 g
B) 6.04* 10-5 g
C) 2.19 * 1043 g
D) 1.66 * 10-6 g
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
50
Convert: 3.248 mol Mg(NO3)2 = ____ g Mg(NO3)2
A) 429.8
B) 45.67
C) 481.8
D)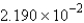
E) none of these
A) 429.8
B) 45.67
C) 481.8
D)
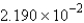
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
51
Convert: 6.911 mol K2O = _____________ g K2O
A) 651.0
B) 13.63
C) 7.337 * 10-2
D) 6.911
E) 4.162 * 1024
A) 651.0
B) 13.63
C) 7.337 * 10-2
D) 6.911
E) 4.162 * 1024

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
52
The number of moles in 20.03 g of C2H6O (molar mass = 46.1 g/mol) is
A) 0.835
B) 9.23 * 102
C) 4.81 * 102
D) 0.434
E) none of these
A) 0.835
B) 9.23 * 102
C) 4.81 * 102
D) 0.434
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
53
Convert: 44.4 g NO2 = ___________ mol NO2
A) 0.965
B) 2.04 * 103
C) 2.67 * 1025
D) 44.4
E) 1.04
A) 0.965
B) 2.04 * 103
C) 2.67 * 1025
D) 44.4
E) 1.04

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the mass, in grams, of 1.83 *1026 formula units of sulfur dioxide.
A) 1.95* 104 g
B) 1.17 * 1028 g
C) 3.04 *102 g
D) 4.74 * 100 g
E) none of these
A) 1.95* 104 g
B) 1.17 * 1028 g
C) 3.04 *102 g
D) 4.74 * 100 g
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
55
Convert: 6.09 g O3 = ___________ molecules O3
A) 7.64 *1022
B) 3.67 * 1024
C) 1.76 *1026
D) 2.11 * 10-25
E) 1.15 * 1023
A) 7.64 *1022
B) 3.67 * 1024
C) 1.76 *1026
D) 2.11 * 10-25
E) 1.15 * 1023

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
56
Convert: 4.13 mol PCl5 = ___________ molecules PCl5
A) 2.49 *1024
B) 6.86 * 10-24
C) 1.46 *1023
D) 1.19 * 1022
E) none of these
A) 2.49 *1024
B) 6.86 * 10-24
C) 1.46 *1023
D) 1.19 * 1022
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate the number of moles of water molecules in 72.0 g of water.
A) 4.00 mol
B) 1.30 *103 mol
C) 0.250 mol
D) 35.7 mol
E) 90.0 mol
A) 4.00 mol
B) 1.30 *103 mol
C) 0.250 mol
D) 35.7 mol
E) 90.0 mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
58
Convert: 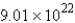 molecules NH3 = ____ mol NH3
molecules NH3 = ____ mol NH3
A)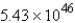
B) 6.68
C) 2.55
D) 0.150
E) none of these
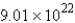 molecules NH3 = ____ mol NH3
molecules NH3 = ____ mol NH3A)
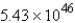
B) 6.68
C) 2.55
D) 0.150
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the mass of 5.986 mol of sulfur dioxide.
A) 383.5 g
B) 9.343 * 10-2 g
C) 10.70 g
D) 3.605 * 1024 g
E) 1.006 *1023 g
A) 383.5 g
B) 9.343 * 10-2 g
C) 10.70 g
D) 3.605 * 1024 g
E) 1.006 *1023 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
60
A 84.9-g sample of SO2 contains how many moles of SO2?
A) 1.33 mol
B)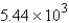 mol
mol
C)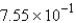 mol
mol
D)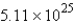 mol
mol
E) none of these
A) 1.33 mol
B)
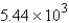 mol
molC)
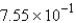 mol
molD)
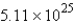 mol
molE) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
61
Consider separate 100.0-g samples of each of the following: NH3, N2O, HCN, N2H4, and HNO3. Which of the samples has the least mass of nitrogen?
A) NH3
B) N2O
C) HCN
D) N2H4
E) HNO3
A) NH3
B) N2O
C) HCN
D) N2H4
E) HNO3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
62
Calculate the mass of 3.663 moles of silver nitrate.
A)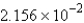 g
g
B) 622.4 g
C) 46.4 g
D) 563.8 g
E) none of these
A)
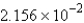 g
gB) 622.4 g
C) 46.4 g
D) 563.8 g
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
63
Calculate the percentage composition (by mass) of Mg in Mg3(AsO4)2.
A) 20.8 %
B) 34.4 %
C) 36.3 %
D) 6.9 %
E) 22.9 %
A) 20.8 %
B) 34.4 %
C) 36.3 %
D) 6.9 %
E) 22.9 %

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the number of moles of Cl2 molecules in a sample that contains 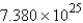 molecules of Cl2.
molecules of Cl2.
A)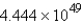 mol
mol
B)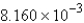 mol
mol
C)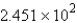 mol
mol
D) 122.6 mol
E) none of these
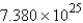 molecules of Cl2.
molecules of Cl2.A)
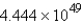 mol
molB)
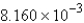 mol
molC)
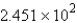 mol
molD) 122.6 mol
E) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
65
A hydrocarbon has the formula C5H12. What is the percent by mass of carbon in the compound?
A) 83.2 %
B) 41.6 %
C) 16.8 %
D) 29.4 %
E) 15.7 %
A) 83.2 %
B) 41.6 %
C) 16.8 %
D) 29.4 %
E) 15.7 %

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
66
A sample with 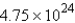 atoms of copper represents how many moles of copper?
atoms of copper represents how many moles of copper?
A)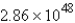 mol
mol
B)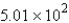 mol
mol
C)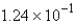 mol
mol
D)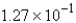 mol
mol
E) 7.89 mol
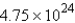 atoms of copper represents how many moles of copper?
atoms of copper represents how many moles of copper?A)
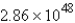 mol
molB)
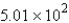 mol
molC)
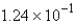 mol
molD)
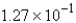 mol
molE) 7.89 mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
67
Calculate the number of molecules in 0.000400 grams of gaseous oxygen.
A)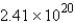 molecules
molecules
B)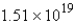 molecules
molecules
C)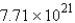 molecules
molecules
D)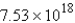 molecules
molecules
E)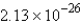 molecules
molecules
A)
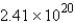 molecules
moleculesB)
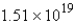 molecules
moleculesC)
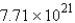 molecules
moleculesD)
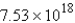 molecules
moleculesE)
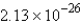 molecules
molecules
Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the number of molecules of CH4 in 41 g CH4.
A)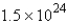 molecules
molecules
B)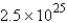 molecules
molecules
C)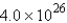 molecules
molecules
D)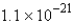 molecules
molecules
E) none of these
A)
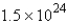 molecules
moleculesB)
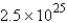 molecules
moleculesC)
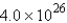 molecules
moleculesD)
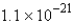 molecules
moleculesE) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
69
How many molecules are present in 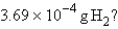
A)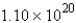 molecules
molecules
B)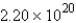 molecules
molecules
C)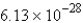 molecules
molecules
D)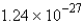 molecules
molecules
E) none of these
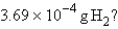
A)
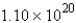 molecules
moleculesB)
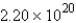 molecules
moleculesC)
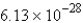 molecules
moleculesD)
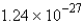 molecules
moleculesE) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
70
A 50.3-g sample of H2O contains how many molecules of water?
A)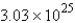 molecules
molecules
B)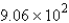 molecules
molecules
C)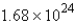 molecules
molecules
D)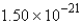 molecules
molecules
E) none of these
A)
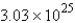 molecules
moleculesB)
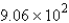 molecules
moleculesC)
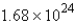 molecules
moleculesD)
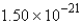 molecules
moleculesE) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
71
Compound X2Y is known to be 60% X by mass. Calculate the percent Y by mass of the compound X2Y2.
A) 20%
B) 30%
C) 40%
D) 60%
E) 80%
A) 20%
B) 30%
C) 40%
D) 60%
E) 80%

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
72
The mole can be defined as the number equal to the number of oxygen atoms in 32.00 g of oxygen.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
73
How many moles of Al2O3 are present in 78.8 g of this compound?
A)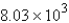 mol
mol
B) 0.773 mol
C) 1.29 mol
D)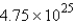 mol
mol
E)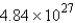 mol
mol
A)
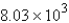 mol
molB) 0.773 mol
C) 1.29 mol
D)
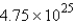 mol
molE)
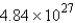 mol
mol
Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
74
A gaseous compound containing carbon and hydrogen was analyzed and found to consist of 83.65% carbon by mass. What is the empirical formula of the compound?
A) CH2
B) CH3
C) C7H16
D) CH
E) C3H7
A) CH2
B) CH3
C) C7H16
D) CH
E) C3H7

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
75
Calculate the mass of 0.314 mol of H2SO4.
A)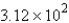 g
g
B) 30.8 g
C)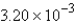 g
g
D)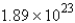 g
g
E)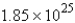 g
g
A)
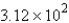 g
gB) 30.8 g
C)
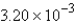 g
gD)
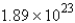 g
gE)
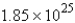 g
g
Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
76
Calculate the number of moles of CH4 in 67 g CH4.
A) 0.24 mol
B)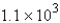 mol
mol
C)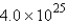 mol
mol
D)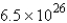 mol
mol
E) 4.2 mol
A) 0.24 mol
B)
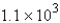 mol
molC)
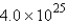 mol
molD)
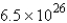 mol
molE) 4.2 mol

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
77
A 60.2-mL sample of Hg (density = 13.6 g/mL) contains how many atoms of Hg?
A)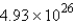 atoms
atoms
B)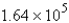 atoms
atoms
C)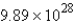 atoms
atoms
D)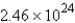 atoms
atoms
E) none of these
A)
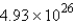 atoms
atomsB)
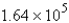 atoms
atomsC)
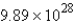 atoms
atomsD)
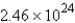 atoms
atomsE) none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
78
Calculate the mass of sulfur in 1.74 mol of H2SO4.
A)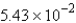 g
g
B) 18.4 g
C) 55.8 g
D)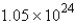 g
g
E)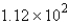 g
g
A)
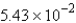 g
gB) 18.4 g
C) 55.8 g
D)
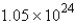 g
gE)
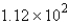 g
g
Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the number of moles in 2.28 g Rb2C2O4.
A)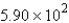 mol
mol
B) 113.6 mol
C)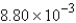 mol
mol
D)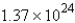 mol
mol
E)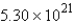 mol
mol
A)
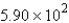 mol
molB) 113.6 mol
C)
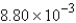 mol
molD)
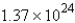 mol
molE)
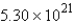 mol
mol
Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
80
A gaseous compound containing carbon and hydrogen was analyzed and found to consist of 83.65% carbon by mass. The molar mass of the compound is 86.2 g/mol. What is the molecular formula of the compound?
A) C3H7
B) CH2
C) C2H4
D) C6H14
E) C18H21
A) C3H7
B) CH2
C) C2H4
D) C6H14
E) C18H21

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck


