Deck 9: Chemical Quantities
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/84
Play
Full screen (f)
Deck 9: Chemical Quantities
1
Consider the following reaction, where X represents an unknown element: 6X(s) + 2B2O3(s)  B4X3(s) + 3XO2(g)
B4X3(s) + 3XO2(g)
If 165 grams of X reacts completely with diboron trioxide to produce 2.29 mol of B4X3, what is the identity of X?
A) C
B) Mg
C) Ge
D) Si
E) N
 B4X3(s) + 3XO2(g)
B4X3(s) + 3XO2(g)If 165 grams of X reacts completely with diboron trioxide to produce 2.29 mol of B4X3, what is the identity of X?
A) C
B) Mg
C) Ge
D) Si
E) N
C
2
Refer to the following equation: 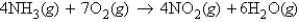 How many molecules of NO2 are produced when 5.38 mol of ammonia is completely reacted?
How many molecules of NO2 are produced when 5.38 mol of ammonia is completely reacted?
A) 21.52
B)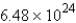
C)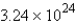
D) 247
E) none of these
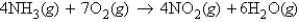 How many molecules of NO2 are produced when 5.38 mol of ammonia is completely reacted?
How many molecules of NO2 are produced when 5.38 mol of ammonia is completely reacted?A) 21.52
B)
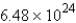
C)
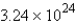
D) 247
E) none of these
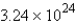
3
What mass of carbon dioxide will be produced when 29.9 g of butane reacts with an excess of oxygen in the following reaction? 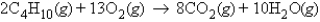
A) 181.1 g CO2
B) 90.6 g CO2
C) 11.32 g CO2
D) 119.6 g CO2
E) none of these
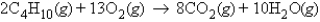
A) 181.1 g CO2
B) 90.6 g CO2
C) 11.32 g CO2
D) 119.6 g CO2
E) none of these
90.6 g CO2
4
Refer to the following unbalanced equation: 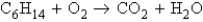 What mass of oxygen (O2) is required to react completely with 11.7 g of C6H14?
What mass of oxygen (O2) is required to react completely with 11.7 g of C6H14?
A)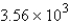 g
g
B) 20.6 g
C) 4.34 g
D) 41.3 g
E) 0.136 g
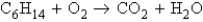 What mass of oxygen (O2) is required to react completely with 11.7 g of C6H14?
What mass of oxygen (O2) is required to react completely with 11.7 g of C6H14?A)
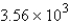 g
gB) 20.6 g
C) 4.34 g
D) 41.3 g
E) 0.136 g

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the mass of water produced when 9.47 g of methane, CH4, reacts with an excess of oxygen in the following unbalanced reaction. 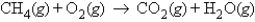
A) 10.64 g H2O
B)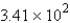 g H2O
g H2O
C) 21.3 g H2O
D) 0.526 g H2O
E) 1.18 g H2O
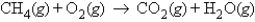
A) 10.64 g H2O
B)
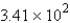 g H2O
g H2OC) 21.3 g H2O
D) 0.526 g H2O
E) 1.18 g H2O

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
6
The balanced equation 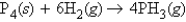 tells us that 5.0 mol H2
tells us that 5.0 mol H2
A) reacts with 2.5 mol P4
B) produces 10.0 mol PH3
C) cannot react with phosphorus
D) produces 3.3 mol PH3
E) reacts with 5.0 mol P4
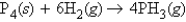 tells us that 5.0 mol H2
tells us that 5.0 mol H2A) reacts with 2.5 mol P4
B) produces 10.0 mol PH3
C) cannot react with phosphorus
D) produces 3.3 mol PH3
E) reacts with 5.0 mol P4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
7
For the reaction 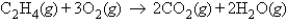 if 5.5 mol of CO2 are produced, how many moles of O2 were reacted?
if 5.5 mol of CO2 are produced, how many moles of O2 were reacted?
A) 3.6 mol
B) 6.8 mol
C) 8.2 mol
D) 13.7 mol
E) none of these
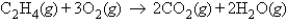 if 5.5 mol of CO2 are produced, how many moles of O2 were reacted?
if 5.5 mol of CO2 are produced, how many moles of O2 were reacted?A) 3.6 mol
B) 6.8 mol
C) 8.2 mol
D) 13.7 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
8
The rusting of iron is represented by the equation 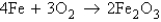 . If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?
. If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?
A) 0.483 mol
B) 0.725 mol
C) 0.97 mol
D) 1.45 mol
E) 2.18 mol
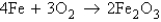 . If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?
. If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?A) 0.483 mol
B) 0.725 mol
C) 0.97 mol
D) 1.45 mol
E) 2.18 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
9
The equation 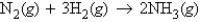 can be interpreted by saying that 1 mol of N2 reacts with 3 mol of H2 to form 2 mol of NH3.
can be interpreted by saying that 1 mol of N2 reacts with 3 mol of H2 to form 2 mol of NH3.
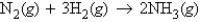 can be interpreted by saying that 1 mol of N2 reacts with 3 mol of H2 to form 2 mol of NH3.
can be interpreted by saying that 1 mol of N2 reacts with 3 mol of H2 to form 2 mol of NH3.
Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
10
Refer to the following unbalanced equation: 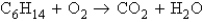 What mass of carbon dioxide (CO2) can be produced from 25.0 g of C6H14 and excess oxygen?
What mass of carbon dioxide (CO2) can be produced from 25.0 g of C6H14 and excess oxygen?
A)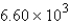 g
g
B) 12.8 g
C) 76.6 g
D) 38.3 g
E) 0.290 g
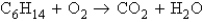 What mass of carbon dioxide (CO2) can be produced from 25.0 g of C6H14 and excess oxygen?
What mass of carbon dioxide (CO2) can be produced from 25.0 g of C6H14 and excess oxygen?A)
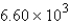 g
gB) 12.8 g
C) 76.6 g
D) 38.3 g
E) 0.290 g

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
11
Refer to the following equation: 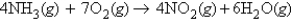 How many moles of ammonia will be required to produce 11.9 mol of water?
How many moles of ammonia will be required to produce 11.9 mol of water?
A) 4.76 mol
B) 11.9 mol
C) 7.93 mol
D) 5.95 mol
E) none of these
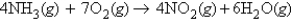 How many moles of ammonia will be required to produce 11.9 mol of water?
How many moles of ammonia will be required to produce 11.9 mol of water?A) 4.76 mol
B) 11.9 mol
C) 7.93 mol
D) 5.95 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
12
A 3.1-mol sample of KClO3 was decomposed according to the equation 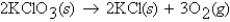 How many moles of O2 are formed assuming 100% yield?
How many moles of O2 are formed assuming 100% yield?
A) 2.1 mol
B) 2.6 mol
C) 3.1 mol
D) 1.6 mol
E) 4.7 mol
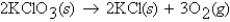 How many moles of O2 are formed assuming 100% yield?
How many moles of O2 are formed assuming 100% yield?A) 2.1 mol
B) 2.6 mol
C) 3.1 mol
D) 1.6 mol
E) 4.7 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
13
The balanced equation 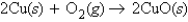 tells us that 4.0 mol of Cu
tells us that 4.0 mol of Cu
A) reacts with 4.0 mol of O2
B) produces 4.0 mol of CuO
C) must react with 128 g of O2
D) cannot react with oxygen
E) produces 8.0 mol of CuO
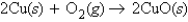 tells us that 4.0 mol of Cu
tells us that 4.0 mol of CuA) reacts with 4.0 mol of O2
B) produces 4.0 mol of CuO
C) must react with 128 g of O2
D) cannot react with oxygen
E) produces 8.0 mol of CuO

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
14
An excess of Al and 7.9 mol of Br2 are reacted according to the equation 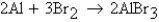 How many moles of AlBr3 will be formed assuming 100% yield?
How many moles of AlBr3 will be formed assuming 100% yield?
A) 2.6 mol
B) 4.0 mol
C) 5.3 mol
D) 7.9 mol
E) 11.9 mol
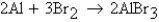 How many moles of AlBr3 will be formed assuming 100% yield?
How many moles of AlBr3 will be formed assuming 100% yield?A) 2.6 mol
B) 4.0 mol
C) 5.3 mol
D) 7.9 mol
E) 11.9 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
15
A mole ratio is used to convert the moles of a starting substance to the moles of a desired substance.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
16
How many molecules of carbon dioxide will be formed if 2.21 g of propane is burned in the following reaction? 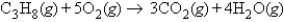
A)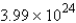 molecules
molecules
B)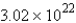 molecules
molecules
C)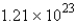 molecules
molecules
D)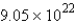 molecules
molecules
E)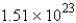 molecules
molecules
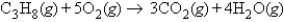
A)
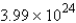 molecules
moleculesB)
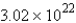 molecules
moleculesC)
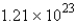 molecules
moleculesD)
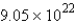 molecules
moleculesE)
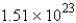 molecules
molecules
Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
17
A balanced chemical equation is one that has the same number of moles of molecules on each side of the equation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following reaction mixtures would produce the greatest amount of product, assuming all went to completion? Each involves the reaction symbolized by the equation 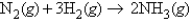
A) 3 moles of N2 and 3 moles of H2
B) 1 mole of N2 and 6 moles of H2
C) 5 moles of N2 and 3 moles of H2
D) 1 mole of N2 and 3 moles of H2
E) All would produce the same amount of product.
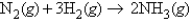
A) 3 moles of N2 and 3 moles of H2
B) 1 mole of N2 and 6 moles of H2
C) 5 moles of N2 and 3 moles of H2
D) 1 mole of N2 and 3 moles of H2
E) All would produce the same amount of product.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
19
Refer to the following equation: 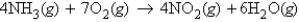 How many molecules of water are produced if 9.12 mol of NO2 is given off?
How many molecules of water are produced if 9.12 mol of NO2 is given off?
A)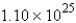
B)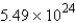
C) 164.2
D)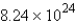
E) none of these
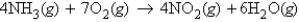 How many molecules of water are produced if 9.12 mol of NO2 is given off?
How many molecules of water are produced if 9.12 mol of NO2 is given off?A)
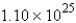
B)
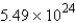
C) 164.2
D)
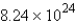
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
20
In the reaction 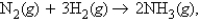 how many moles of ammonia would be produced from 1.21 mol of hydrogen and excess nitrogen?
how many moles of ammonia would be produced from 1.21 mol of hydrogen and excess nitrogen?
A) 1.57 mol
B) 3.63 mol
C) 0.807 mol
D) 2.42 mol
E) 0.403 mol
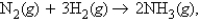 how many moles of ammonia would be produced from 1.21 mol of hydrogen and excess nitrogen?
how many moles of ammonia would be produced from 1.21 mol of hydrogen and excess nitrogen?A) 1.57 mol
B) 3.63 mol
C) 0.807 mol
D) 2.42 mol
E) 0.403 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the molecules of oxygen required to react with 25.0 g of sulfur in the following reaction. 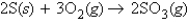
A)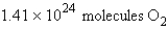
B)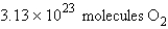
C)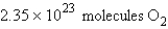
D)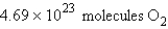
E)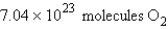
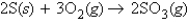
A)
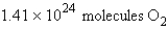
B)
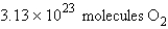
C)
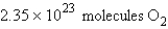
D)
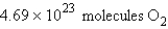
E)
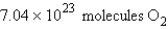

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
22
In the reaction 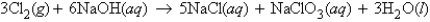 how many grams of sodium chloride can be produced from 37.2 g of NaOH?
how many grams of sodium chloride can be produced from 37.2 g of NaOH?
A) 45.3 g NaCl
B) 65.2 g NaCl
C) 54.4 g NaCl
D) 21.2 g NaCl
E) 14.0 g NaCl
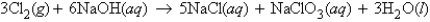 how many grams of sodium chloride can be produced from 37.2 g of NaOH?
how many grams of sodium chloride can be produced from 37.2 g of NaOH?A) 45.3 g NaCl
B) 65.2 g NaCl
C) 54.4 g NaCl
D) 21.2 g NaCl
E) 14.0 g NaCl

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
23
When 3.4 mol of Fe reacts with Cl2 according to the equation 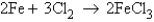 how many moles of Cl2 are required to react with all of the iron?
how many moles of Cl2 are required to react with all of the iron?
A) 2.3 mol
B) 3.4 mol
C) 5.1 mol
D) 0.9 mol
E) none of these
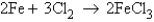 how many moles of Cl2 are required to react with all of the iron?
how many moles of Cl2 are required to react with all of the iron?A) 2.3 mol
B) 3.4 mol
C) 5.1 mol
D) 0.9 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
24
How many atoms of aluminum can be produced by the decomposition of 31.5 g of Al2O3? (Hint: Write and balance the equation first.)
A)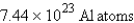
B)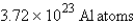
C)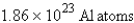
D)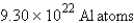
E) none of these
A)
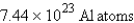
B)
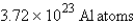
C)
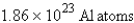
D)
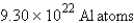
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
25
For the reaction 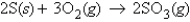 how many moles of SO3 will be produced from 2.2 mol O2 and excess S?
how many moles of SO3 will be produced from 2.2 mol O2 and excess S?
A) 3.3 mol SO3
B) 1.5 mol SO3
C) 2.2 mol SO3
D) 0.7 mol SO3
E) none of these
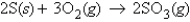 how many moles of SO3 will be produced from 2.2 mol O2 and excess S?
how many moles of SO3 will be produced from 2.2 mol O2 and excess S?A) 3.3 mol SO3
B) 1.5 mol SO3
C) 2.2 mol SO3
D) 0.7 mol SO3
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
26
If 19.2 g of CO2 is produced in the reaction of C2H2 with O2 to form CO2 and H2O, how many grams of H2O are produced in this reaction?
A) 7.86 g
B) 3.93 g
C) 15.7 g
D) 2.62 g
E) none of these
A) 7.86 g
B) 3.93 g
C) 15.7 g
D) 2.62 g
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
27
In the reaction 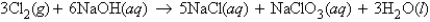 how many molecules of water can be produced starting with 19.8 kilograms of sodium hydroxide and excess Cl2?
how many molecules of water can be produced starting with 19.8 kilograms of sodium hydroxide and excess Cl2?
A)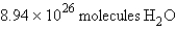
B)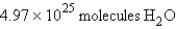
C)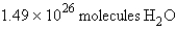
D)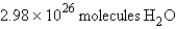
E) none of these
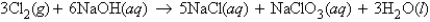 how many molecules of water can be produced starting with 19.8 kilograms of sodium hydroxide and excess Cl2?
how many molecules of water can be produced starting with 19.8 kilograms of sodium hydroxide and excess Cl2?A)
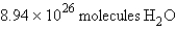
B)
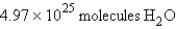
C)
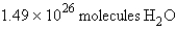
D)
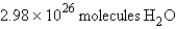
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
28
Nitrogen and hydrogen gases are combined at high temperatures and pressures to produce ammonia, NH3. If 104.7 g of N2 are reacted with excess H2, how many moles of NH3 will be formed?
A) 3.737 mol
B) 2.491 mol
C) 7.473 mol
D) 5.605 mol
E) none of these
A) 3.737 mol
B) 2.491 mol
C) 7.473 mol
D) 5.605 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
29
For the reaction 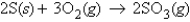 how many moles of SO3 can be produced from 7.2 g O2 and excess S?
how many moles of SO3 can be produced from 7.2 g O2 and excess S?
A) 0.30 mol SO3
B) 4.8 mol SO3
C) 0.15 mol SO3
D) 0.34 mol SO3
E) none of these
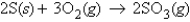 how many moles of SO3 can be produced from 7.2 g O2 and excess S?
how many moles of SO3 can be produced from 7.2 g O2 and excess S?A) 0.30 mol SO3
B) 4.8 mol SO3
C) 0.15 mol SO3
D) 0.34 mol SO3
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the reaction 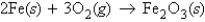 If 11.9 g of iron(III) oxide (rust) is produced from a certain amount of iron, how many grams of oxygen are needed for this reaction?
If 11.9 g of iron(III) oxide (rust) is produced from a certain amount of iron, how many grams of oxygen are needed for this reaction?
A) 3.58 g
B) 7.15 g
C) 1.59 g
D) 2.38 g
E) none of these
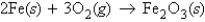 If 11.9 g of iron(III) oxide (rust) is produced from a certain amount of iron, how many grams of oxygen are needed for this reaction?
If 11.9 g of iron(III) oxide (rust) is produced from a certain amount of iron, how many grams of oxygen are needed for this reaction?A) 3.58 g
B) 7.15 g
C) 1.59 g
D) 2.38 g
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the mass of carbon dioxide produced from 22.3 g of octane, C8H18, in the following reaction. 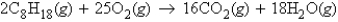
A) 68.7 g CO2
B) 137 g CO2
C) 77.3 g CO2
D) 1.074 g CO2
E) 1.562 g CO2
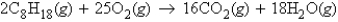
A) 68.7 g CO2
B) 137 g CO2
C) 77.3 g CO2
D) 1.074 g CO2
E) 1.562 g CO2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
32
In the reaction 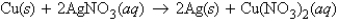 what number of grams of silver can be produced from the reaction of 55.0 g of copper?
what number of grams of silver can be produced from the reaction of 55.0 g of copper?
A) 187 g Ag
B) 93.4 g Ag
C) 46.7 g Ag
D) 64.8 g Ag
E) 0.87 g Ag
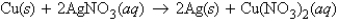 what number of grams of silver can be produced from the reaction of 55.0 g of copper?
what number of grams of silver can be produced from the reaction of 55.0 g of copper?A) 187 g Ag
B) 93.4 g Ag
C) 46.7 g Ag
D) 64.8 g Ag
E) 0.87 g Ag

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
33
For the reaction 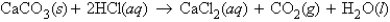 how many grams of CaCl2 can be obtained if 14.6 g HCl is allowed to react with excess CaCO3?
how many grams of CaCl2 can be obtained if 14.6 g HCl is allowed to react with excess CaCO3?
A) 44 g CaCl2
B) 89 g CaCl2
C) 0.200 g CaCl2
D) 22.2 g CaCl2
E) none of these
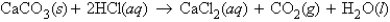 how many grams of CaCl2 can be obtained if 14.6 g HCl is allowed to react with excess CaCO3?
how many grams of CaCl2 can be obtained if 14.6 g HCl is allowed to react with excess CaCO3?A) 44 g CaCl2
B) 89 g CaCl2
C) 0.200 g CaCl2
D) 22.2 g CaCl2
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
34
Methane, CH4, the major component of natural gas, burns in air to form CO2 and H2O. What mass of water is formed in the complete combustion of 7.07 *103 g of CH4?
A)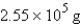
B)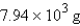
C)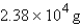
D)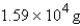
E) none of these
A)
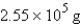
B)
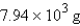
C)
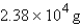
D)
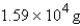
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
35
How many moles of O2 are required for the complete reaction of 43.5 g of C2H4 to form CO2 and H2O?
A) 0.775 mol
B) 3.10 mol
C) 6.20 mol
D) 4.65 mol
E) none of these
A) 0.775 mol
B) 3.10 mol
C) 6.20 mol
D) 4.65 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
36
How many moles of oxygen are produced by decomposing 28.5 g of H2O2 (molar mass = 34.0 g/mol) according to the equation: 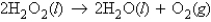
A) 0.838 mol O2
B) 485 mol O2
C) 0.419 mol O2
D) 1.68 mol O2
E) none of these
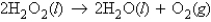
A) 0.838 mol O2
B) 485 mol O2
C) 0.419 mol O2
D) 1.68 mol O2
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
37
In the reaction 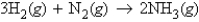 how many molecules of hydrogen are required to react with 3.66 g of nitrogen?
how many molecules of hydrogen are required to react with 3.66 g of nitrogen?
A)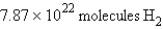
B)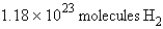
C)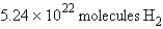
D)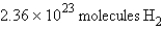
E) none of these
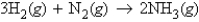 how many molecules of hydrogen are required to react with 3.66 g of nitrogen?
how many molecules of hydrogen are required to react with 3.66 g of nitrogen?A)
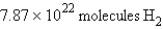
B)
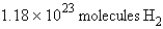
C)
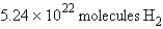
D)
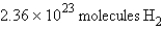
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
38
For the reaction 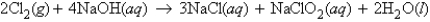 how many moles of Cl2 are needed to react with 11.6 g NaOH?
how many moles of Cl2 are needed to react with 11.6 g NaOH?
A) 0.58 mol Cl2
B) 0.290 mol Cl2
C) 0.193 mol Cl2
D) 0.073 mol Cl2
E) 0.145 mol Cl2
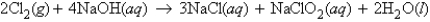 how many moles of Cl2 are needed to react with 11.6 g NaOH?
how many moles of Cl2 are needed to react with 11.6 g NaOH?A) 0.58 mol Cl2
B) 0.290 mol Cl2
C) 0.193 mol Cl2
D) 0.073 mol Cl2
E) 0.145 mol Cl2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
39
What number of moles of ammonia can be produced from 7.46 g of hydrogen gas and excess nitrogen gas?
A) 1.233 mol NH3
B) 7.40 mol NH3
C) 4.93 mol NH3
D) 5.55 mol NH3
E) 2.47 mol NH3
A) 1.233 mol NH3
B) 7.40 mol NH3
C) 4.93 mol NH3
D) 5.55 mol NH3
E) 2.47 mol NH3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
40
In the reaction 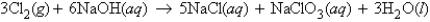 how many moles of chlorine molecules are needed to react with 13.3 g of NaOH?
how many moles of chlorine molecules are needed to react with 13.3 g of NaOH?
A) 1.00 mol Cl2
B) 0.333 mol Cl2
C) 0.67 mol Cl2
D) 0.166 mol Cl2
E) none of these
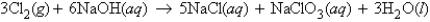 how many moles of chlorine molecules are needed to react with 13.3 g of NaOH?
how many moles of chlorine molecules are needed to react with 13.3 g of NaOH?A) 1.00 mol Cl2
B) 0.333 mol Cl2
C) 0.67 mol Cl2
D) 0.166 mol Cl2
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
41
For the reaction 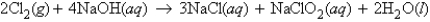 how many molecules of H2O can be produced from 32.7 g of NaOH and excess Cl2?
how many molecules of H2O can be produced from 32.7 g of NaOH and excess Cl2?
A)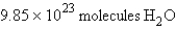
B)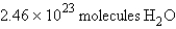
C)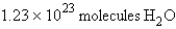
D)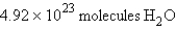
E)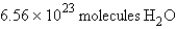
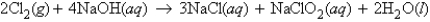 how many molecules of H2O can be produced from 32.7 g of NaOH and excess Cl2?
how many molecules of H2O can be produced from 32.7 g of NaOH and excess Cl2?A)
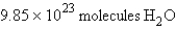
B)
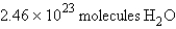
C)
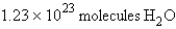
D)
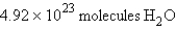
E)
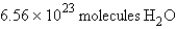

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
42
For the reaction of C2H4(g) with O2(g) to form CO2(g) and H2O(g), what number of moles of CO2 can be produced by the reaction of 5.00 mol C2H4 and 12.1 mol O2?
A) 18.2 mol
B) 12.1 mol
C) 8.07 mol
D) 10.0 mol
E) none of these
A) 18.2 mol
B) 12.1 mol
C) 8.07 mol
D) 10.0 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
43
For the reaction 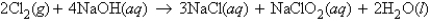 how many grams of NaCl can be produced from 19.3 g of Cl2 and excess NaOH?
how many grams of NaCl can be produced from 19.3 g of Cl2 and excess NaOH?
A) 23.9 g NaCl
B) 10.6 g NaCl
C) 15.9 g NaCl
D) 7.95 g NaCl
E) none of these
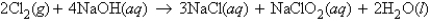 how many grams of NaCl can be produced from 19.3 g of Cl2 and excess NaOH?
how many grams of NaCl can be produced from 19.3 g of Cl2 and excess NaOH?A) 23.9 g NaCl
B) 10.6 g NaCl
C) 15.9 g NaCl
D) 7.95 g NaCl
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the reaction 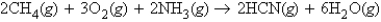 If 416.3 g NH3 is reacted with excess CH4 and O2, what mass of HCN can be produced?
If 416.3 g NH3 is reacted with excess CH4 and O2, what mass of HCN can be produced?
A) 660.5 g HCN
B) 1321.1 g HCN
C) 330.3 g HCN
D) 440.4 g HCN
E) none of these
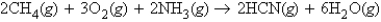 If 416.3 g NH3 is reacted with excess CH4 and O2, what mass of HCN can be produced?
If 416.3 g NH3 is reacted with excess CH4 and O2, what mass of HCN can be produced?A) 660.5 g HCN
B) 1321.1 g HCN
C) 330.3 g HCN
D) 440.4 g HCN
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
45
Sodium and water react according to the equation 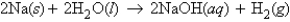 What number of moles of H2 will be produced when 4.0 mol Na is added to 1.2 mol H2O?
What number of moles of H2 will be produced when 4.0 mol Na is added to 1.2 mol H2O?
A) 0.6 mol
B) 2.4 mol
C) 2.0 mol
D) 1.2 mol
E) 8.0 mol
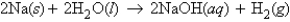 What number of moles of H2 will be produced when 4.0 mol Na is added to 1.2 mol H2O?
What number of moles of H2 will be produced when 4.0 mol Na is added to 1.2 mol H2O?A) 0.6 mol
B) 2.4 mol
C) 2.0 mol
D) 1.2 mol
E) 8.0 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
46
For the reaction of C2H4(g) with O2(g) to form CO2(g) and H2O(g), what number of moles of CO2 can be produced by the reaction of 0.480 mol C2H4 and 1.01 mol O2?
A) 1.52 mol
B) 0.960 mol
C) 0.673 mol
D) 1.01 mol
E) none of these
A) 1.52 mol
B) 0.960 mol
C) 0.673 mol
D) 1.01 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
47
For the reaction 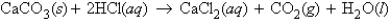 45.9 g solid CaCO3 is mixed with 51.6 g HCl. What number of grams of CO2 will be produced?
45.9 g solid CaCO3 is mixed with 51.6 g HCl. What number of grams of CO2 will be produced?
A) 16.3 g CO2
B) 40.4 g CO2
C) 10.1 g CO2
D) 31.1 g CO2
E) 20.2 g CO2
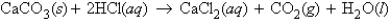 45.9 g solid CaCO3 is mixed with 51.6 g HCl. What number of grams of CO2 will be produced?
45.9 g solid CaCO3 is mixed with 51.6 g HCl. What number of grams of CO2 will be produced?A) 16.3 g CO2
B) 40.4 g CO2
C) 10.1 g CO2
D) 31.1 g CO2
E) 20.2 g CO2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
48
For the reaction 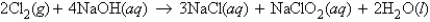 11.9 g Cl2 is reacted with 12.0 g NaOH. How many moles of NaCl are produced?
11.9 g Cl2 is reacted with 12.0 g NaOH. How many moles of NaCl are produced?
A) 0.300 mol NaCl
B) 0.400 mol NaCl
C) 0.150 mol NaCl
D) 0.252 mol NaCl
E) 0.225 mol NaCl
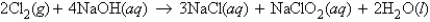 11.9 g Cl2 is reacted with 12.0 g NaOH. How many moles of NaCl are produced?
11.9 g Cl2 is reacted with 12.0 g NaOH. How many moles of NaCl are produced?A) 0.300 mol NaCl
B) 0.400 mol NaCl
C) 0.150 mol NaCl
D) 0.252 mol NaCl
E) 0.225 mol NaCl

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
49
Fe2O3 (molar mass = 159.7 g/mol) reacts with CO (molar mass = 28.0 g/mol) according to the equation 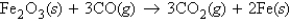 When 470.0 g Fe2O3 reacts with excess CO, what number of moles of Fe (iron) is produced?
When 470.0 g Fe2O3 reacts with excess CO, what number of moles of Fe (iron) is produced?
A) 8.829 mol Fe
B) 5.886 mol Fe
C) 1.472 mol Fe
D) 11.772 mol Fe
E) none of these
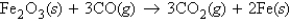 When 470.0 g Fe2O3 reacts with excess CO, what number of moles of Fe (iron) is produced?
When 470.0 g Fe2O3 reacts with excess CO, what number of moles of Fe (iron) is produced?A) 8.829 mol Fe
B) 5.886 mol Fe
C) 1.472 mol Fe
D) 11.772 mol Fe
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction 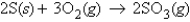 if 5.42 g of S is reacted with 10.0 g of O2, how many grams of SO3 will be produced?
if 5.42 g of S is reacted with 10.0 g of O2, how many grams of SO3 will be produced?
A) 27.1 g
B) 6.77 g
C) 16.7 g
D) 13.5 g
E) none of these
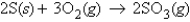 if 5.42 g of S is reacted with 10.0 g of O2, how many grams of SO3 will be produced?
if 5.42 g of S is reacted with 10.0 g of O2, how many grams of SO3 will be produced?A) 27.1 g
B) 6.77 g
C) 16.7 g
D) 13.5 g
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
51
Look at the reaction below: 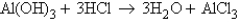 Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.
Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.
A) Aluminum hydroxide is the limiting reactant because 1.2 g of aluminum hydroxide produces 0.55 g of water.
B) Aluminum hydroxide is the limiting reactant because it has a smaller coefficient than the hydrochloric acid.
C) Aluminum hydroxide is not the limiting reactant because more than 0.55 g of water could have been produced from 1.2 g of aluminum hydroxide.
D) Aluminum hydroxide is not the limiting reactant because we are given an amount of leftover aluminum hydroxide.
E) There is not enough information given to answer this question.
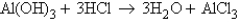 Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.
Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.A) Aluminum hydroxide is the limiting reactant because 1.2 g of aluminum hydroxide produces 0.55 g of water.
B) Aluminum hydroxide is the limiting reactant because it has a smaller coefficient than the hydrochloric acid.
C) Aluminum hydroxide is not the limiting reactant because more than 0.55 g of water could have been produced from 1.2 g of aluminum hydroxide.
D) Aluminum hydroxide is not the limiting reactant because we are given an amount of leftover aluminum hydroxide.
E) There is not enough information given to answer this question.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
52
For the reaction of C2H4(g) with O2(g) to form CO2(g) and H2O(g), what number of grams of CO2 could be produced from 2.0 g of C2H4 and 5.2 g of O2?
A) 10.7 g
B) 4.8 g
C) 7.2 g
D) 6.3 g
E) none of these
A) 10.7 g
B) 4.8 g
C) 7.2 g
D) 6.3 g
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the reaction 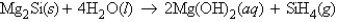 Calculate the number of grams of silane gas, SiH4, formed when 35.9 g of Mg2Si reacts with excess H2O.
Calculate the number of grams of silane gas, SiH4, formed when 35.9 g of Mg2Si reacts with excess H2O.
A) 30.1 g SiH4
B) 10.1 g SiH4
C) 15.0 g SiH4
D) 19.5 g SiH4
E) none of these
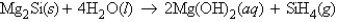 Calculate the number of grams of silane gas, SiH4, formed when 35.9 g of Mg2Si reacts with excess H2O.
Calculate the number of grams of silane gas, SiH4, formed when 35.9 g of Mg2Si reacts with excess H2O.A) 30.1 g SiH4
B) 10.1 g SiH4
C) 15.0 g SiH4
D) 19.5 g SiH4
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
54
Determine the mass of CO2 produced when 75.5 g of CaO is reacted with 50.0 g of C according to the unbalanced equation 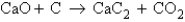
A) 29.6 g CO2
B) 119 g CO2
C) 59.3 g CO2
D) 36.6 g CO2
E) none of these
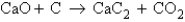
A) 29.6 g CO2
B) 119 g CO2
C) 59.3 g CO2
D) 36.6 g CO2
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the reaction 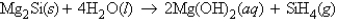 How many moles of excess reactant are left over if we start with 39.1 g of each reactant?
How many moles of excess reactant are left over if we start with 39.1 g of each reactant?
A) 0.13 mol
B) 2.04 mol
C) 1.66 mol
D) 2.17 mol
E) none of these
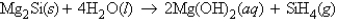 How many moles of excess reactant are left over if we start with 39.1 g of each reactant?
How many moles of excess reactant are left over if we start with 39.1 g of each reactant?A) 0.13 mol
B) 2.04 mol
C) 1.66 mol
D) 2.17 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
56
How many moles of SbCl3 is formed when 4.00 mol Sb are reacted with 4.43 mol Cl2 according to the unbalanced equation 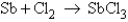
A) 6.65 mol SbCl3
B) 4.43 mol SbCl3
C) 2.95 mol SbCl3
D) 4.00 mol SbCl3
E) Cannot be determined based on the information given.
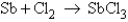
A) 6.65 mol SbCl3
B) 4.43 mol SbCl3
C) 2.95 mol SbCl3
D) 4.00 mol SbCl3
E) Cannot be determined based on the information given.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
57
Fe3O4 reacts with CO according to the equation 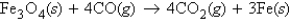 If 335.2 g Fe3O4 is reacted with excess CO, what mass of CO2 will be produced?
If 335.2 g Fe3O4 is reacted with excess CO, what mass of CO2 will be produced?
A) 63.71 g CO2
B) 191.1 g CO2
C) 127.42 g CO2
D) 254.8 g CO2
E) none of these
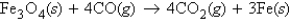 If 335.2 g Fe3O4 is reacted with excess CO, what mass of CO2 will be produced?
If 335.2 g Fe3O4 is reacted with excess CO, what mass of CO2 will be produced?A) 63.71 g CO2
B) 191.1 g CO2
C) 127.42 g CO2
D) 254.8 g CO2
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
58
Fe3O4 reacts with CO according to the equation 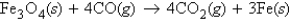 If 482.3 g CO is reacted with excess Fe3O4, what mass of CO2 will be produced?
If 482.3 g CO is reacted with excess Fe3O4, what mass of CO2 will be produced?
A) 1515.6 g CO2
B) 378.9 g CO2
C) 1010.4 g CO2
D) 568.4 g CO2
E) 757.8 g CO2
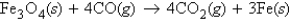 If 482.3 g CO is reacted with excess Fe3O4, what mass of CO2 will be produced?
If 482.3 g CO is reacted with excess Fe3O4, what mass of CO2 will be produced?A) 1515.6 g CO2
B) 378.9 g CO2
C) 1010.4 g CO2
D) 568.4 g CO2
E) 757.8 g CO2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the equation: 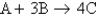 . If 3.0 moles of A is reacted with 6.0 moles of B, which of the following is true after the reaction is complete?
. If 3.0 moles of A is reacted with 6.0 moles of B, which of the following is true after the reaction is complete?
A) A is the leftover reactant because you need only 2 moles of A and have 3.
B) A is the leftover reactant because for every 1 mole of A, 4 moles of C are produced.
C) B is the leftover reactant because you have more moles of B than A.
D) B is the leftover reactant because 3 moles of B react with every 1 mole of A.
E) Neither reactant is leftover.
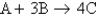 . If 3.0 moles of A is reacted with 6.0 moles of B, which of the following is true after the reaction is complete?
. If 3.0 moles of A is reacted with 6.0 moles of B, which of the following is true after the reaction is complete?A) A is the leftover reactant because you need only 2 moles of A and have 3.
B) A is the leftover reactant because for every 1 mole of A, 4 moles of C are produced.
C) B is the leftover reactant because you have more moles of B than A.
D) B is the leftover reactant because 3 moles of B react with every 1 mole of A.
E) Neither reactant is leftover.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
60
Fe2O3 (molar mass = 159.7 g/mol) reacts with CO (molar mass = 28.0 g/mol) according to the equation 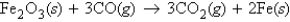 When 185.5 g of CO reacts with excess Fe2O3, how many moles of Fe (iron) will be produced?
When 185.5 g of CO reacts with excess Fe2O3, how many moles of Fe (iron) will be produced?
A) 13.25 mol Fe
B) 2.208 mol Fe
C) 4.415 mol Fe
D) 9.93 mol Fe
E) 3.311 mol Fe
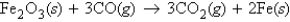 When 185.5 g of CO reacts with excess Fe2O3, how many moles of Fe (iron) will be produced?
When 185.5 g of CO reacts with excess Fe2O3, how many moles of Fe (iron) will be produced?A) 13.25 mol Fe
B) 2.208 mol Fe
C) 4.415 mol Fe
D) 9.93 mol Fe
E) 3.311 mol Fe

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
61
Consider a reaction in which two reactants make one product (for example, consider the unbalanced reaction 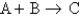 ). You know the following: 2.0 mol A (with an excess of B) can produce a maximum of 2.0 mol C
). You know the following: 2.0 mol A (with an excess of B) can produce a maximum of 2.0 mol C
3)0 mol B (with an excess of A) can produce a maximum of 4.0 mol C
If you react 2.0 mol A with 3.0 mol B, what is the maximum amount of C that can be produced?
A) 2.0 mol
B) 4.0 mol
C) 5.0 mol
D) 6.0 mol
E) More information is needed to answer this question.
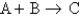 ). You know the following: 2.0 mol A (with an excess of B) can produce a maximum of 2.0 mol C
). You know the following: 2.0 mol A (with an excess of B) can produce a maximum of 2.0 mol C3)0 mol B (with an excess of A) can produce a maximum of 4.0 mol C
If you react 2.0 mol A with 3.0 mol B, what is the maximum amount of C that can be produced?
A) 2.0 mol
B) 4.0 mol
C) 5.0 mol
D) 6.0 mol
E) More information is needed to answer this question.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
62
Consider the equation: A + 4B  3C + 3D When equal masses of A and B are reacted, which is limiting?
3C + 3D When equal masses of A and B are reacted, which is limiting?
A) If the molar mass of A is less than the molar mass of B, then B must be limiting.
B) If the molar mass of A is greater than the molar mass of B, then A must be limiting.
C) If the molar mass of A is greater than the molar mass of B, then B must be limiting.
D) If the molar mass of A is less than the molar mass of B, then A must be limiting.
E) Neither reactant is limiting.
 3C + 3D When equal masses of A and B are reacted, which is limiting?
3C + 3D When equal masses of A and B are reacted, which is limiting?A) If the molar mass of A is less than the molar mass of B, then B must be limiting.
B) If the molar mass of A is greater than the molar mass of B, then A must be limiting.
C) If the molar mass of A is greater than the molar mass of B, then B must be limiting.
D) If the molar mass of A is less than the molar mass of B, then A must be limiting.
E) Neither reactant is limiting.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following unbalanced equation: 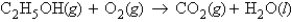 If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?
If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?
A) 0.0404 mol
B) 0.121 mol
C) 0.0135 mol
D) 0.447 mol
E) 0.894 mol
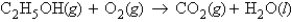 If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?
If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?A) 0.0404 mol
B) 0.121 mol
C) 0.0135 mol
D) 0.447 mol
E) 0.894 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following reaction at 1.10 atm and 19°C: 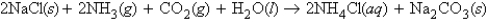 0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
A) 0.139 mol
B) 0.278 mol
C) 0.228 mol
D) 6.06 mol
E) 0.0918 mol
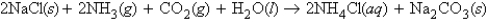 0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?A) 0.139 mol
B) 0.278 mol
C) 0.228 mol
D) 6.06 mol
E) 0.0918 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the equation: 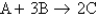 . The molar mass of B is 50.0 g/mol. Which of the following statements is true when equal masses of A and B are reacted?
. The molar mass of B is 50.0 g/mol. Which of the following statements is true when equal masses of A and B are reacted?
A) If the molar mass of A is greater than the molar mass of B, then A must determine how much C is produced.
B) If the molar mass of A is less than the molar mass of B, then A must determine how much C is produced.
C) If the molar mass of A is the same as the molar mass of B, then A and B react in a perfect stoichiometric ratio and both determine how much C is produced.
D) If the molar mass of A is greater than the molar mass of B, then B must determine how much C is produced.
E) If the molar mass of A is less than the molar mass of B, then B must determine how much C is produced.
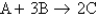 . The molar mass of B is 50.0 g/mol. Which of the following statements is true when equal masses of A and B are reacted?
. The molar mass of B is 50.0 g/mol. Which of the following statements is true when equal masses of A and B are reacted?A) If the molar mass of A is greater than the molar mass of B, then A must determine how much C is produced.
B) If the molar mass of A is less than the molar mass of B, then A must determine how much C is produced.
C) If the molar mass of A is the same as the molar mass of B, then A and B react in a perfect stoichiometric ratio and both determine how much C is produced.
D) If the molar mass of A is greater than the molar mass of B, then B must determine how much C is produced.
E) If the molar mass of A is less than the molar mass of B, then B must determine how much C is produced.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the following unbalanced equation: 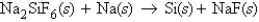 How many grams of sodium are required to completely react with 0.300 mol Na2SiF6?
How many grams of sodium are required to completely react with 0.300 mol Na2SiF6?
A) 0.300 g
B) 1.20 g
C) 1.72 g
D) 6.90 g
E) 27.6 g
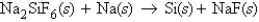 How many grams of sodium are required to completely react with 0.300 mol Na2SiF6?
How many grams of sodium are required to completely react with 0.300 mol Na2SiF6?A) 0.300 g
B) 1.20 g
C) 1.72 g
D) 6.90 g
E) 27.6 g

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is always true concerning a reaction represented by the following balanced chemical equation? 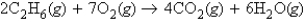
A) If we have equal masses of C2H6 and O2, there is no limiting reactant.
B) If we have an equal number of moles of C2H6 and O2, there is no limiting reactant.
C) If we have more mass of C2H6, then O2 must be the limiting reactant.
D) If we have more mass of O2, then C2H6 must be the limiting reactant.
E) None of these statements (a-d) are true.
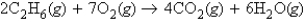
A) If we have equal masses of C2H6 and O2, there is no limiting reactant.
B) If we have an equal number of moles of C2H6 and O2, there is no limiting reactant.
C) If we have more mass of C2H6, then O2 must be the limiting reactant.
D) If we have more mass of O2, then C2H6 must be the limiting reactant.
E) None of these statements (a-d) are true.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
68
For the titration of sulfuric acid (H2SO4) with sodium hydroxide (NaOH), how many moles of sodium hydroxide would be required to react with 1.50 L of 0.500 M sulfuric acid to reach the endpoint?
A) 0.750 mol
B) 0.375 mol
C) 1.50 mol
D) 3.00 mol
E) none of these
A) 0.750 mol
B) 0.375 mol
C) 1.50 mol
D) 3.00 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
69
When 10.0 mol of calcium metal is reacted with 3.9 mol of oxygen gas, how much calcium oxide is produced?
A) 3.9 mol
B) 10.0 mol
C) 7.8 mol
D) 20.0 mol
E) 2.0 mol
A) 3.9 mol
B) 10.0 mol
C) 7.8 mol
D) 20.0 mol
E) 2.0 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
70
Consider that calcium metal reacts with oxygen gas in the air to form calcium oxide. Suppose we react 5.66 mol calcium with 4.00 mol oxygen gas. Determine the number of moles of oxygen left over after the reaction is complete.
A) 2.83 mol O2
B) 1.66 mol O2
C) 1.17 mol O2
D) 4.00 mol O2
E) none of these
A) 2.83 mol O2
B) 1.66 mol O2
C) 1.17 mol O2
D) 4.00 mol O2
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
71
Consider that calcium metal reacts with oxygen gas in the air to form calcium oxide. Suppose we react 6.17 mol calcium with 4.00 mol oxygen gas. Determine the number of moles of calcium left over after the reaction is complete.
A) 1.83 mol Ca
B) 0.00 mol Ca
C) 3.09 mol Ca
D) 2.06 mol Ca
E) none of these
A) 1.83 mol Ca
B) 0.00 mol Ca
C) 3.09 mol Ca
D) 2.06 mol Ca
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the following unbalanced equation: 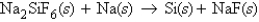 If 3.09 moles of Si were produced, how many moles of NaF were also created?
If 3.09 moles of Si were produced, how many moles of NaF were also created?
A) 0.515 mol
B) 3.09 mol
C) 2.06 mol
D) 18.5 mol
E) Impossible to determine without the moles of reactants.
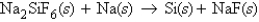 If 3.09 moles of Si were produced, how many moles of NaF were also created?
If 3.09 moles of Si were produced, how many moles of NaF were also created?A) 0.515 mol
B) 3.09 mol
C) 2.06 mol
D) 18.5 mol
E) Impossible to determine without the moles of reactants.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
73
In the reaction between CO and Fe3O4, the theoretical yield in an experiment is calculated to be 47.2 g Fe. When a careless chemistry student carries out the experiment, the actual yield is 37.0 g Fe. Calculate the percentage yield.
A) 78.4%
B) 21.6%
C) 52.3%
D) 39.2%
E) none of these
A) 78.4%
B) 21.6%
C) 52.3%
D) 39.2%
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
74
Equal masses of hydrogen gas and oxygen gas are reacted to form water. Which substance is limiting?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
75
Consider the following unbalanced equation: 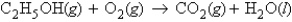 If 1.70 g of ethanol reacts with 11.2 g of oxygen, how many moles of carbon dioxide are produced?
If 1.70 g of ethanol reacts with 11.2 g of oxygen, how many moles of carbon dioxide are produced?
A) 0.0369 mol
B) 0.233 mol
C) 0.0738 mol
D) 0.350 mol
E) 0.0185 mol
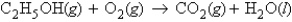 If 1.70 g of ethanol reacts with 11.2 g of oxygen, how many moles of carbon dioxide are produced?
If 1.70 g of ethanol reacts with 11.2 g of oxygen, how many moles of carbon dioxide are produced?A) 0.0369 mol
B) 0.233 mol
C) 0.0738 mol
D) 0.350 mol
E) 0.0185 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
76
Consider that calcium metal reacts with oxygen gas in the air to form calcium oxide. Suppose we react 6.02 mol calcium with 4.00 mol oxygen gas. Determine the number of moles of calcium oxide produced after the reaction is complete.
A) 6.02 mol CaO
B) 3.01 mol CaO
C) 4.01 mol CaO
D) 8.00 mol CaO
E) none of these
A) 6.02 mol CaO
B) 3.01 mol CaO
C) 4.01 mol CaO
D) 8.00 mol CaO
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
77
Ammonia reacts with oxygen to form nitrogen dioxide and water according to the following equation: 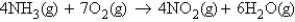 You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.
You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.
A) 35.0 g O2
B) 17.7 g O2
C) 40.9 g O2
D) 71.5 g O2
E) 47.7 g O2
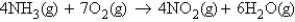 You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.
You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.A) 35.0 g O2
B) 17.7 g O2
C) 40.9 g O2
D) 71.5 g O2
E) 47.7 g O2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
78
You react 25.0 g hydrogen gas with 73.7 g oxygen gas. Determine the mass of water that can be produced from these reactants.
A) 41.49 g H2O
B) 20.7 g H2O
C) 9.3 g H2O
D) 83.0 g H2O
E) 223 g H2O
A) 41.49 g H2O
B) 20.7 g H2O
C) 9.3 g H2O
D) 83.0 g H2O
E) 223 g H2O

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
79
Consider the reaction of magnesium metal with hydrochloric acid to produce magnesium chloride and hydrogen gas. If 4.52 mol of magnesium and 4.52 mol of hydrochloric acid are reacted, how many moles of hydrogen gas are produced?
A) 9.04 mol
B) 2.26 mol
C) 4.52 mol
D) 6.52 mol
E) none of these
A) 9.04 mol
B) 2.26 mol
C) 4.52 mol
D) 6.52 mol
E) none of these

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
80
Reacting 3.00 mol nitrogen gas with 3.50 mol hydrogen gas will produce how many moles of ammonia according to the following balanced chemical equation? 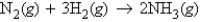
A) 3.50 mol NH3
B) 5.25 mol NH3
C) 2.33 mol NH3
D) 7.00 mol NH3
E) 6.00 mol NH3
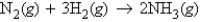
A) 3.50 mol NH3
B) 5.25 mol NH3
C) 2.33 mol NH3
D) 7.00 mol NH3
E) 6.00 mol NH3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck


