Deck 5: Nomenclature
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/219
Play
Full screen (f)
Deck 5: Nomenclature
1
The correct name for LiCl is
A) lithium monochloride
B) lithium(I) chloride
C) monolithium chloride
D) lithium chloride
E) monolithium monochloride
A) lithium monochloride
B) lithium(I) chloride
C) monolithium chloride
D) lithium chloride
E) monolithium monochloride
lithium chloride
2
The name for  2+ is
2+ is
A) mercury(I) ion
B) mercury ion
C) mercury(II) ion
D) hydrogen ion
E) hydrogen(II) ion
 2+ is
2+ isA) mercury(I) ion
B) mercury ion
C) mercury(II) ion
D) hydrogen ion
E) hydrogen(II) ion
mercury(I) ion
3
The correct name for the Al3+ species is
A) aluminum ion
B) aluminum(III) ion
C) aluminum
D) trialuminum ion
E) aluminum(3A) ion
A) aluminum ion
B) aluminum(III) ion
C) aluminum
D) trialuminum ion
E) aluminum(3A) ion
aluminum ion
4
The formula for calcium hydrogen sulfate is
A) Ca(SO4)2
B) CaS2
C) Ca(HSO4)2
D) Ca2HSO4
E) Ca2S
A) Ca(SO4)2
B) CaS2
C) Ca(HSO4)2
D) Ca2HSO4
E) Ca2S

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
5
The name for SO2 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
6
A compound contains an unknown ion X and has the formula XCl2. Ion X contains 20 electrons. What is the identity of X?
A) Ti2+
B) Sc+
C) Ca2+
D) Cr2+
E) Mn3+
A) Ti2+
B) Sc+
C) Ca2+
D) Cr2+
E) Mn3+

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
7
The charge on a strontium ion in its ionic compound is
A) +2
B) +1
C) +3
D) -2
E) Various charges are possible.
A) +2
B) +1
C) +3
D) -2
E) Various charges are possible.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
8
In naming ionic compounds, the cation is always named first and the anion second.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
9
Titanium(IV) oxide has the formula
A) Ti4O
B) TiO4
C) Ti(IV)O
D) TiO2
E) Ti4O2
A) Ti4O
B) TiO4
C) Ti(IV)O
D) TiO2
E) Ti4O2

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
10
The correct name for FeO is
A) iron oxide
B) iron(II) oxide
C) iron(III) oxide
D) iron monoxide
E) iron(I) oxide
A) iron oxide
B) iron(II) oxide
C) iron(III) oxide
D) iron monoxide
E) iron(I) oxide

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is not the correct chemical formula for the compound named?
A) hydrocyanic acid HCN
B) calcium sulfate CaSO4
C) beryllium oxide BeO
D) nickel(II) peroxide Ni2O
E) ammonium chromate (NH4)2CrO4
A) hydrocyanic acid HCN
B) calcium sulfate CaSO4
C) beryllium oxide BeO
D) nickel(II) peroxide Ni2O
E) ammonium chromate (NH4)2CrO4

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
12
The compound PI3 is named
A) potassium iodide
B) monophosphorus iodide
C) phosphorus iodide
D) phosphorus triiodide
E) none of these
A) potassium iodide
B) monophosphorus iodide
C) phosphorus iodide
D) phosphorus triiodide
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
13
The name for Li2O is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
14
The symbol for the rubidium ion is
A) Rb2+
B) R+
C) R2+
D) Ru
E) Rb+
A) Rb2+
B) R+
C) R2+
D) Ru
E) Rb+

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
15
Na+ represents a
A) sodium ion
B) sodium atom
C) potassium ion
D) potassium atom
E) naval ion
A) sodium ion
B) sodium atom
C) potassium ion
D) potassium atom
E) naval ion

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
16
What is the formula for selenium trioxide?
A) SeO3
B) Se3O
C) Se2O3
D) Se3O2
E) SeO
A) SeO3
B) Se3O
C) Se2O3
D) Se3O2
E) SeO

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
17
The binary compound PCl3 is called
A) phosphorus chloride
B) triphosphorus chloride
C) monophosphorus trichloride
D) phosphorus trichloride
E) none of these
A) phosphorus chloride
B) triphosphorus chloride
C) monophosphorus trichloride
D) phosphorus trichloride
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following formulas is incorrect?
A) AlO3
B) KBr
C) KNO3
D) Li2O
E) CaSO4
A) AlO3
B) KBr
C) KNO3
D) Li2O
E) CaSO4

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
19
The correct name for P2O5 is
A) phosphorus(II) oxide
B) phosphorus(V) oxide
C) diphosphorus oxide
D) diphosphorus pentoxide
E) phosphorus pentoxide
A) phosphorus(II) oxide
B) phosphorus(V) oxide
C) diphosphorus oxide
D) diphosphorus pentoxide
E) phosphorus pentoxide

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a binary compound?
A) O2
B) HCN
C) H2SO4
D) H2S
E) NaOH
A) O2
B) HCN
C) H2SO4
D) H2S
E) NaOH

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
21
The name of the PO43- ion is
A) phosphate
B) phosphite
C) phosphide
D) phosphoric
E) phosphorous
A) phosphate
B) phosphite
C) phosphide
D) phosphoric
E) phosphorous

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
22
The correct name for an aqueous solution of HC2H3O2 is
A) hydrocarbonate acid
B) hydrocarbonic acid
C) hydroacetic acid
D) acetic acid
E) none of these
A) hydrocarbonate acid
B) hydrocarbonic acid
C) hydroacetic acid
D) acetic acid
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
23
What is the name for 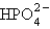 ?
?
A) phosphate ion
B) phosphite ion
C) hydrogen phosphate ion
D) hydrogen phosphite ion
E) hydrogen phosphorus oxide ion
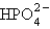 ?
?A) phosphate ion
B) phosphite ion
C) hydrogen phosphate ion
D) hydrogen phosphite ion
E) hydrogen phosphorus oxide ion

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
24
The name for ClO4- is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
25
The correct name for an aqueous solution of HCN is
A) hydrocyanic acid
B) cyanic acid
C) cyanate acid
D) hydrocyanous acid
E) cyanous acid
A) hydrocyanic acid
B) cyanic acid
C) cyanate acid
D) hydrocyanous acid
E) cyanous acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
26
The name for CO32- is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
27
The correct name for an aqueous solution of H2CO3 is
A) carbonate acid
B) hydrocarbonic acid
C) carbonous acid
D) carbonic acid
E) hydrocarbonous acid
A) carbonate acid
B) hydrocarbonic acid
C) carbonous acid
D) carbonic acid
E) hydrocarbonous acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
28
The correct name for an aqueous solution of HCl is
A) chloric acid
B) hydrochloric acid
C) hypochloric acid
D) hypochlorous acid
E) perchloric acid
A) chloric acid
B) hydrochloric acid
C) hypochloric acid
D) hypochlorous acid
E) perchloric acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
29
The name for NO3- is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
30
The correct formula for the ammonium ion is
A) NH42+
B) N4H+
C) NH4+
D) NH4
E) Am+
A) NH42+
B) N4H+
C) NH4+
D) NH4
E) Am+

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
31
The correct name for an aqueous solution of H3PO4 is
A) hydrophosphoric acid
B) phosphorous acid
C) phosphate acid
D) hydrophosphorus acid
E) phosphoric acid
A) hydrophosphoric acid
B) phosphorous acid
C) phosphate acid
D) hydrophosphorus acid
E) phosphoric acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
32
What is the correct name of the compound that results from the most stable ion for sulfur and the metal ion that contains 24 electrons?
A) iron(II) sulfate
B) iron(III) sulfide
C) chromium(II) sulfide
D) nickel(III) sulfate
E) iron(II) sulfide
A) iron(II) sulfate
B) iron(III) sulfide
C) chromium(II) sulfide
D) nickel(III) sulfate
E) iron(II) sulfide

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
33
The correct formula for the carbonate ion is
A) CO3-
B) CO32-
C) CO4-
D) CO42-
E) CO33-
A) CO3-
B) CO32-
C) CO4-
D) CO42-
E) CO33-

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
34
The correct name for an aqueous solution of H2SO4 is
A) sulfurous acid
B) hydrosulfurous acid
C) sulfuric acid
D) hydrosulfuric acid
E) none of these
A) sulfurous acid
B) hydrosulfurous acid
C) sulfuric acid
D) hydrosulfuric acid
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
35
The correct name for the Sn4+ species is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is an incorrect name for an acid?
A) hydrocarbonate acid
B) hydrocyanic acid
C) acetic acid
D) phosphoric acid
E) sulfurous acid
A) hydrocarbonate acid
B) hydrocyanic acid
C) acetic acid
D) phosphoric acid
E) sulfurous acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
37
The name of the compound NH4BrO2 is
A) ammonium bromite
B) ammonium hypobromite
C) ammonium perbromate
D) ammonium bromate
E) ammonium bromide
A) ammonium bromite
B) ammonium hypobromite
C) ammonium perbromate
D) ammonium bromate
E) ammonium bromide

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
38
The carbonate ion has the formula CO32-. What is the correct formula for sodium carbonate?
A) Na(CO3)2
B) Na2(CO3)2
C) Na2CO3
D) Na3(CO)2
E) NaCO3
A) Na(CO3)2
B) Na2(CO3)2
C) Na2CO3
D) Na3(CO)2
E) NaCO3

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
39
The name for the Fe2+ species is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
40
The correct formula for ammonium sulfate is
A) NH4SO3
B) NH4SO4
C) (NH4)2SO3
D) (NH4)2SO4
E) (NH3)2SO3
A) NH4SO3
B) NH4SO4
C) (NH4)2SO3
D) (NH4)2SO4
E) (NH3)2SO3

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
41
The name for the acid H2SO3 is
A) sulfuric acid
B) sulfurous acid
C) hydrosulfuric acid
D) hydrosulfurous acid
E) sulfurite acid
A) sulfuric acid
B) sulfurous acid
C) hydrosulfuric acid
D) hydrosulfurous acid
E) sulfurite acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
42
Sodium chlorite has the formula
A) NaCl
B) NaClO
C) NaClO2
D) NaClO3
E) NaClO4
A) NaCl
B) NaClO
C) NaClO2
D) NaClO3
E) NaClO4

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following represents the hypochlorite ion?
A) ClO-
B) ClO2-
C) ClO3-
D) ClO4-
E) Cl-
A) ClO-
B) ClO2-
C) ClO3-
D) ClO4-
E) Cl-

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
44
The name for the acid HNO3 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
45
The name for the compound NH4F is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
46
The name for the acid H2SO4 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
47
The name for the compound Sn(NO3)2 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
48
The name for the acid HC2H3O2 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
49
The name for the acid HNO2 is
A) nitrous acid
B) nitric acid
C) hydronitrous acid
D) hydronitric acid
E) hydrogen nitrite acid
A) nitrous acid
B) nitric acid
C) hydronitrous acid
D) hydronitric acid
E) hydrogen nitrite acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
50
The formula for the compound formed from ammonium and sulfate ions is
A) NH4SO4
B) (NH4)2SO4
C) NH4(SO4)2
D) (NH4)3SO4
E) none of these
A) NH4SO4
B) (NH4)2SO4
C) NH4(SO4)2
D) (NH4)3SO4
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
51
Potassium chlorate has the formula
A) KCl
B) KClO
C) KClO2
D) KClO3
E) KClO4
A) KCl
B) KClO
C) KClO2
D) KClO3
E) KClO4

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
52
The formula for the compound formed from the polyatomic ions NH4+ and 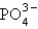 is
is
A) NH4(PO4)3
B) NH4PO4
C) (NH4)3PO4
D) (NH4)2(PO4)2
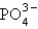 is
isA) NH4(PO4)3
B) NH4PO4
C) (NH4)3PO4
D) (NH4)2(PO4)2

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
53
The name of the BrO3- ion is
A) bromate ion
B) bromite ion
C) hypobromite ion
D) perbromate ion
E) bromoxide ion
A) bromate ion
B) bromite ion
C) hypobromite ion
D) perbromate ion
E) bromoxide ion

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
54
The correct name for HClO4(aq) is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
55
The name of the ClO2- ion is
A) chlorite
B) chloride
C) hypochlorate
D) perchlorate
E) none of these
A) chlorite
B) chloride
C) hypochlorate
D) perchlorate
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
56
The substance ClO3- is best described as
A) a molecule
B) a polyatomic ion
C) a polyatomic molecule
D) a mixture
A) a molecule
B) a polyatomic ion
C) a polyatomic molecule
D) a mixture

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is named incorrectly? What should its name be?
a. FeSO4; iron(II) sulfate
b. Sn3(PO4)4; tin(IV) phosphate
c. K3P; potassium phosphide
d. Fe(OH)2; iron(III) hydroxide
e. All are correct.
a. FeSO4; iron(II) sulfate
b. Sn3(PO4)4; tin(IV) phosphate
c. K3P; potassium phosphide
d. Fe(OH)2; iron(III) hydroxide
e. All are correct.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
58
The name for the compound Fe2O3 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
59
The correct name for NiO is
A) nickel(II) oxide
B) nickel(I) oxide
C) nickel oxide
D) nickel monoxide
E) nickel(I) monoxide
A) nickel(II) oxide
B) nickel(I) oxide
C) nickel oxide
D) nickel monoxide
E) nickel(I) monoxide

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
60
The correct name for an aqueous solution of HBr is
A) bromic acid
B) hydrobromic acid
C) hypobromic acid
D) hydrobromous acid
E) hypobromous acid
A) bromic acid
B) hydrobromic acid
C) hypobromic acid
D) hydrobromous acid
E) hypobromous acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
61
The name for MnBr2 is
A) manganese(II) bromide
B) manganese(I) bromide
C) magnesium bromide
D) manganese bromide
E) manganese(III) bromide
A) manganese(II) bromide
B) manganese(I) bromide
C) magnesium bromide
D) manganese bromide
E) manganese(III) bromide

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
62
The name for NH4Br is
A) nitrogen hydrogen bromide
B) ammonium bromide
C) ammonium(I) bromide
D) nitrogen tetrahydrogen bromide
E) none of these
A) nitrogen hydrogen bromide
B) ammonium bromide
C) ammonium(I) bromide
D) nitrogen tetrahydrogen bromide
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
63
The name for NaHCO3 is
A) sodium hydrogen carbonate (sodium bicarbonate)
B) sodium carbonate
C) sodium(I) hydrogen carbonate
D) sodium(I) bicarbonate
E) none of these
A) sodium hydrogen carbonate (sodium bicarbonate)
B) sodium carbonate
C) sodium(I) hydrogen carbonate
D) sodium(I) bicarbonate
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
64
The name for SrCl2 is
A) strontium dichloride
B) strontium(I) chloride
C) strontium(II) chloride
D) strontium chloride
E) strontium(I) chloride(II)
A) strontium dichloride
B) strontium(I) chloride
C) strontium(II) chloride
D) strontium chloride
E) strontium(I) chloride(II)

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
65
An aqueous solution of HF would be named
A) hydrogen fluoride
B) perfluoric acid
C) hypofluorous acid
D) hydrofluoric acid
E) none of these
A) hydrogen fluoride
B) perfluoric acid
C) hypofluorous acid
D) hydrofluoric acid
E) none of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
66
The correct formula for bromous acid is
A) HBrO2
B) HBrO
C) HBrO3
D) HBrO4
E) HBr
A) HBrO2
B) HBrO
C) HBrO3
D) HBrO4
E) HBr

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
67
The name for HClO3(aq) is
A) chloric acid
B) hydrogen chlorate
C) perchloric acid
D) Hydrogen chlorate
E) chlorous acid
A) chloric acid
B) hydrogen chlorate
C) perchloric acid
D) Hydrogen chlorate
E) chlorous acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
68
The name for Ba(NO3)2 is
A) barium dinitrate
B) barium(II) nitrate
C) barium nitrite
D) barium(I) nitrate
E) barium nitrate
A) barium dinitrate
B) barium(II) nitrate
C) barium nitrite
D) barium(I) nitrate
E) barium nitrate

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
69
Choose the correct name for HNO2.
A) hydronitric acid
B) ammonium acid
C) ammonia
D) nitric acid
E) nitrous acid
A) hydronitric acid
B) ammonium acid
C) ammonia
D) nitric acid
E) nitrous acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
70
The name for BaSO4 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
71
The name for the Sr2+ species is
A) strontium(II) ion
B) strontium ion
C) strontium(I) ion
D) strontium
E) distrontium ion
A) strontium(II) ion
B) strontium ion
C) strontium(I) ion
D) strontium
E) distrontium ion

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
72
The name for HBrO(aq) is
A) hydrogen bromous acid
B) bromous acid
C) bromic acid
D) hypobromous acid
E) perbromic acid
A) hydrogen bromous acid
B) bromous acid
C) bromic acid
D) hypobromous acid
E) perbromic acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
73
The name for FeCl3 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
74
The name for Pb4+ is
A) lead(IV) ion
B) palladium ion
C) palladium(IV) ion
D) phosphorus(IV) ion
E) rubidium ion
A) lead(IV) ion
B) palladium ion
C) palladium(IV) ion
D) phosphorus(IV) ion
E) rubidium ion

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is the correct formula for bromic acid?
A) HBr(aq)
B) HBrO(aq)
C) HBrO2(aq)
D) HBrO3(aq)
E) HBrO4(aq)
A) HBr(aq)
B) HBrO(aq)
C) HBrO2(aq)
D) HBrO3(aq)
E) HBrO4(aq)

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is named correctly?
A) HCl(aq); hypochlorous acid
B) (NH4)3PO3; ammonium phosphate
C) H2SO3(aq); sulfuric acid
D) NH3; ammonium ion
E) HNO3(aq); nitric acid
A) HCl(aq); hypochlorous acid
B) (NH4)3PO3; ammonium phosphate
C) H2SO3(aq); sulfuric acid
D) NH3; ammonium ion
E) HNO3(aq); nitric acid

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following compounds is not named as an acid?
A) HCl(aq)
B) HCN(aq)
C) H2SO4(aq)
D) HNO3(aq)
E) NH3(aq)
A) HCl(aq)
B) HCN(aq)
C) H2SO4(aq)
D) HNO3(aq)
E) NH3(aq)

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
78
The name for Al(OH)3 is
A) aluminum(III) hydroxide
B) aluminum trihydroxide
C) aluminum hydroxide
D) monaluminum trihydroxide
E) aluminum(I) hydroxide
A) aluminum(III) hydroxide
B) aluminum trihydroxide
C) aluminum hydroxide
D) monaluminum trihydroxide
E) aluminum(I) hydroxide

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
79
The name for Na3N is
A) sodium nitride
B) trisodium nitride
C) sodium(I) nitride
D) sodium(III) nitride
E) trisodium mononitride
A) sodium nitride
B) trisodium nitride
C) sodium(I) nitride
D) sodium(III) nitride
E) trisodium mononitride

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
80
The name for PCl5 is ______________.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck


