Deck 9: Chemical Equation Calculations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/152
Play
Full screen (f)
Deck 9: Chemical Equation Calculations
1
What term refers to the amount of product experimentally measured in a laboratory procedure?
A)actual yield
B)percent yield
C)stoichiometric yield
D)theoretical yield
E)none of the above
A)actual yield
B)percent yield
C)stoichiometric yield
D)theoretical yield
E)none of the above
actual yield
2
Assuming similar conditions,how many liters of carbon monoxide gas react to produce 2 L of carbon dioxide gas? 2 CO(g)+ O₂(g) 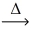 2 CO₂(g)
2 CO₂(g)
A)1 L
B)2 L
C)3 L
D)4 L
E)none of the above
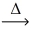 2 CO₂(g)
2 CO₂(g)A)1 L
B)2 L
C)3 L
D)4 L
E)none of the above
2 L
3
How many moles of carbon monoxide react with 1 mol of oxygen gas according to the balanced chemical equation? 2 CO(g)+ O₂(g) 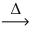 2 CO₂(g)
2 CO₂(g)
A)1 mol
B)2 mol
C)3 mol
D)4 mol
E)none of the above
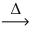 2 CO₂(g)
2 CO₂(g)A)1 mol
B)2 mol
C)3 mol
D)4 mol
E)none of the above
2 mol
4
What term refers to a type of stoichiometry calculation that relates the mass of a substance to the volume of a gas according to a balanced chemical equation?
A)mass-mass problem
B)mass-volume problem
C)mole-mole problem
D)volume-volume problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)mole-mole problem
D)volume-volume problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
5
What is the term for the actual yield compared to the theoretical yield expressed as a percent?
A)experimental yield
B)percent yield
C)ratio yield
D)stoichiometric yield
E)none of the above
A)experimental yield
B)percent yield
C)ratio yield
D)stoichiometric yield
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
6
What principle states that equal volumes of gases,at the same temperature and pressure,contain equal numbers of molecules?
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
7
How many moles of chlorine gas react with 1 mol of hydrogen gas according to the balanced chemical equation? H₂(g)+ Cl₂(g)→ 2 HCl(g)
A)1 mol
B)2 mol
C)3 mol
D)4 mol
E)none of the above
A)1 mol
B)2 mol
C)3 mol
D)4 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
8
What term refers to a type of stoichiometry calculation that relates the volumes of two gases according to a balanced chemical equation?
A)mass-mass problem
B)mass-volume problem
C)mole-mole problem
D)volume-volume problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)mole-mole problem
D)volume-volume problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
9
What term refers to a type of stoichiometry calculation that relates the masses of two substances according to a balanced chemical equation?
A)mass-mass problem
B)mass-volume problem
C)mole-mole problem
D)volume-volume problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)mole-mole problem
D)volume-volume problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
10
What is the term for the amount of product calculated to be obtained from a given amount of reactant?
A)actual yield
B)percent yield
C)stoichiometric yield
D)theoretical yield
E)none of the above
A)actual yield
B)percent yield
C)stoichiometric yield
D)theoretical yield
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
11
What principle states the volumes of gases that combine in a chemical reaction are in the ratio of small whole numbers?
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of volume
D)law of constant conditions
E)none of the above
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of volume
D)law of constant conditions
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
12
What is the term for a temperature of 0 °C and a pressure of 1 atm?
A)atmospheric temperature and pressure
B)experimental temperature and pressure
C)ideal gas temperature and pressure
D)standard temperature and pressure
E)none of the above
A)atmospheric temperature and pressure
B)experimental temperature and pressure
C)ideal gas temperature and pressure
D)standard temperature and pressure
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
13
Assuming similar conditions,how many liters of chlorine gas react to produce 2 L of hydrogen chloride gas? H₂(g)+ Cl₂(g)→ 2 HCl(g)
A)1 L
B)2 L
C)3 L
D)4 L
E)none of the above
A)1 L
B)2 L
C)3 L
D)4 L
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
14
What principle states that mass is neither gained or lost during a chemical reaction?
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
15
What term expresses the ratio of moles of reactants and products according to the coefficients in the balanced chemical equation?
A)mole ratio
B)reactant ratio
C)product ratio
D)yield ratio
E)none of the above
A)mole ratio
B)reactant ratio
C)product ratio
D)yield ratio
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
16
What term refers to the mass of 1 mol of substance expressed in grams?
A)gram-atomic mass
B)gram-molecular mass
C)gram-formula mass
D)molar mass
E)none of the above
A)gram-atomic mass
B)gram-molecular mass
C)gram-formula mass
D)molar mass
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
17
How many moles of steam,H₂O,react with 1 mol of charcoal,C,according to the balanced chemical equation? C(s)+ H₂O(g) 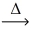 CO(g)+ H₂(g)
CO(g)+ H₂(g)
A)1 mol
B)2 mol
C)3 mol
D)4 mol
E)none of the above
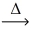 CO(g)+ H₂(g)
CO(g)+ H₂(g)A)1 mol
B)2 mol
C)3 mol
D)4 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
18
What is the term for the substance in a chemical reaction that controls the maximum amount of product?
A)limiting reactant
B)limiting product
C)maximum reactant
D)maximum product
E)none of the above
A)limiting reactant
B)limiting product
C)maximum reactant
D)maximum product
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
19
What term refers to the volume occupied by 1 mol of any gas at STP?
A)Avogadro's volume
B)molar volume
C)standard volume
D)STP volume
E)none of the above
A)Avogadro's volume
B)molar volume
C)standard volume
D)STP volume
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
20
What is the term for the relationship of quantities (i.e. ,mass of substance or volume of gas)in a chemical reaction according to the balanced chemical equation?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)stoichiometry
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)stoichiometry
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
21
In an experiment,0.243 g of magnesium reacts to give 0.403 g magnesium oxide.Using the conservation of mass law,predict the mass of reacting oxygen gas. 2 Mg(s)+ O₂(g) 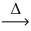 2 MgO(s)
2 MgO(s)
A)0.080 g
B)0.160 g
C)0.320 g
D)0.646 g
E)1.292 g
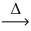 2 MgO(s)
2 MgO(s)A)0.080 g
B)0.160 g
C)0.320 g
D)0.646 g
E)1.292 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
22
How many moles of iodine vapor react with 1.00 mol of hydrogen gas? __H₂(g)+ __I₂(g) 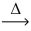 __HI(g)
__HI(g)
A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above
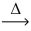 __HI(g)
__HI(g)A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
23
How many moles of hydrogen iodide are produced from 1.00 mol of iodine? __H₂(g)+ __I₂(g) 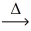 __HI(g)
__HI(g)
A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above
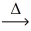 __HI(g)
__HI(g)A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
24
In an experiment,0.327 g of zinc metal reacts to produce 0.407 g of zinc oxide.Using the conservation of mass law,predict the mass of reacting oxygen gas. 2 Zn(s)+ O₂(g) 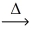 2 ZnO(s)
2 ZnO(s)
A)0.040 g
B)0.080 g
C)0.160 g
D)0.734 g
E)1.468 g
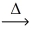 2 ZnO(s)
2 ZnO(s)A)0.040 g
B)0.080 g
C)0.160 g
D)0.734 g
E)1.468 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
25
How many moles of water react with 5.00 mol of lithium metal? __Li(s)+ __H₂O(l)→ __LiOH(aq)+ __H₂(g)
A)2.50 mol
B)5.00 mol
C)10.0 mol
D)20.0 mol
E)none of the above
A)2.50 mol
B)5.00 mol
C)10.0 mol
D)20.0 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
26
In an experiment,5.585 g of iron metal reacts with 3.207 g of yellow sulfur.Using the conservation of mass law,predict the mass of product. Fe(s)+ S(s) 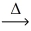 FeS(s)
FeS(s)
A)2.198 g
B)2.378 g
C)4.396 g
D)8.792 g
E)17.584 g
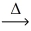 FeS(s)
FeS(s)A)2.198 g
B)2.378 g
C)4.396 g
D)8.792 g
E)17.584 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of water are produced from 1.00 mol of hydrogen peroxide? __H₂O₂(l) 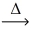 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)
A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above
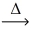 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of nitrogen dioxide gas are produced from 1.00mol NO? __NO(g)+ __O₂(g) 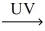 __NO₂(g)
__NO₂(g)
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above
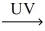 __NO₂(g)
__NO₂(g)A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
29
In an experiment,0.520 g of chromium metal reacts with 3.807 g of iodine.Using the conservation of mass law,predict the mass of product. 2 Cr(s)+ 3 I₂(s) 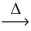 2 CrI₃(s)
2 CrI₃(s)
A)1.529 g
B)3.287 g
C)4.327 g
D)8.654 g
E)12.461 g
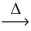 2 CrI₃(s)
2 CrI₃(s)A)1.529 g
B)3.287 g
C)4.327 g
D)8.654 g
E)12.461 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
30
How many moles of oxygen gas react with 1.00 mol NO? __NO(g)+ __O₂(g) 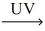 __NO₂(g)
__NO₂(g)
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above
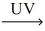 __NO₂(g)
__NO₂(g)A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
31
How many moles of hydrogen peroxide decompose to give 1.00 mol of oxygen? __H₂O₂(l) 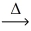 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)
A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above
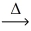 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
32
In an experiment,1.201 g of charcoal reacts with 6.414 g of powdered sulfur.Using the conservation of mass law,predict the mass of product. C(s)+ 2 S(s) 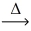 CS₂(g)
CS₂(g)
A)4.408 g
B)5.213 g
C)7.615 g
D)8.816 g
E)14.029 g
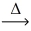 CS₂(g)
CS₂(g)A)4.408 g
B)5.213 g
C)7.615 g
D)8.816 g
E)14.029 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
33
Assuming similar conditions,how many liters of steam,H₂O,react to produce 1 L of hydrogen gas? C(s)+ H₂O(g) 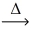 CO(g)+ H₂(g)
CO(g)+ H₂(g)
A)1 L
B)2 L
C)3 L
D)4 L
E)none of the above
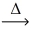 CO(g)+ H₂(g)
CO(g)+ H₂(g)A)1 L
B)2 L
C)3 L
D)4 L
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
34
How many moles of hydrogen gas are produced from 5.00 mol of water? __Li(s)+ __H₂O(l)→ __LiOH(aq)+ __H₂(g)
A)2.50 mol
B)5.00 mol
C)10.0 mol
D)20.0 mol
E)none of the above
A)2.50 mol
B)5.00 mol
C)10.0 mol
D)20.0 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
35
How many moles of lithium metal react to yield 5.00 mol of hydrogen gas? __Li(s)+ __H₂O(l)→ __LiOH(aq)+ __H₂(g)
A)2.50 mol
B)5.00 mol
C)10.0 mol
D)20.0 mol
E)none of the above
A)2.50 mol
B)5.00 mol
C)10.0 mol
D)20.0 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
36
How many moles of water react with 0.500 mol of calcium metal? __Ca(s)+ __H₂O(l)→ __Ca(OH)2(aq)+ __H₂(g)
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
37
How many moles of oxygen gas react to yield 1.00 mol NO₂? __NO(g)+ __O₂(g) 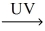 __NO₂(g)
__NO₂(g)
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above
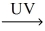 __NO₂(g)
__NO₂(g)A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
38
How many moles of oxygen are produced from 1.00 mol of hydrogen peroxide? __H₂O₂(l) 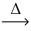 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)
A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above
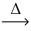 __H₂O(l)+ __O₂(g)
__H₂O(l)+ __O₂(g)A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
39
In an experiment,0.197 g of gold metal reacts to yield 0.303 g of gold(III)chloride.Using the conservation of mass law,predict the mass of reacting chlorine gas. 2 Au(s)+ 3 Cl₂(g) 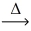 2 AuCl3(s)
2 AuCl3(s)
A)0.035 g
B)0.106 g
C)0.318 g
D)0.167 g
E)0.500 g
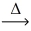 2 AuCl3(s)
2 AuCl3(s)A)0.035 g
B)0.106 g
C)0.318 g
D)0.167 g
E)0.500 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
40
How many moles of hydrogen gas react to yield 1.00 mol of hydrogen iodide? __H₂(g)+ __I₂(g) 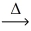 __HI(g)
__HI(g)
A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above
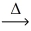 __HI(g)
__HI(g)A)0.500 mol
B)1.00 mol
C)2.00 mol
D)4.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
41
In general,how many unit factors are required to solve a mass-volume stoichiometry problem?
A)one
B)two
C)three
D)four
E)none of the above
A)one
B)two
C)three
D)four
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
42
In general,how many unit factors are required to solve a volume-mass stoichiometry problem?
A)one
B)two
C)three
D)four
E)none of the above
A)one
B)two
C)three
D)four
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following steps is necessary to solve a volume-volume stoichiometry problem?
A)Write a balanced chemical equation for the reaction.
B)Determine the ratio of gas volumes from the balanced equation.
C)Donvert the volume of known gas to volume of unknown gas.
D)all of the above
E)none of the above
A)Write a balanced chemical equation for the reaction.
B)Determine the ratio of gas volumes from the balanced equation.
C)Donvert the volume of known gas to volume of unknown gas.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
44
Classify the following type of stoichiometry problem: How many grams of aluminum must react with an excess volume of nitric acid to yield 0.500 g of aluminum nitrate?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
45
Classify the following type of stoichiometry problem: How many grams of zinc must react with an excess volume of hydrochloric acid to yield 0.500 g of zinc chloride?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following steps is necessary to solve a mass-mass stoichiometry problem?
A)Write a balanced equation for the reaction.
B)Calculate the moles of known substance.
C)Convert moles of known to moles of unknown.
D)Calculate the mass of unknown substance.
E)all of the above
A)Write a balanced equation for the reaction.
B)Calculate the moles of known substance.
C)Convert moles of known to moles of unknown.
D)Calculate the mass of unknown substance.
E)all of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
47
How many moles of calcium metal react to yield 0.500 mol of hydrogen gas? __Ca(s)+ __H₂O(l)→ __Ca(OH)2(aq)+ __H₂(g)
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
48
Classify the following type of stoichiometry problem: How many liters of carbon monoxide gas react with an excess volume of oxygen to give 125 mL of carbon dioxide gas?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
49
How many moles of hydrogen gas are produced from 0.500 mol of water? __Ca(s)+ __H₂O(l)→ __Ca(OH)2(aq)+ __H₂(g)
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above
A)0.250 mol
B)0.500 mol
C)1.00 mol
D)2.00 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
50
How many moles of water are produced from 0.100 mol pentane,C5H₁₂? __C5H₁₂ (g)+ __O₂(g) 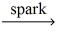 __CO₂(g)+ __H₂O(g)
__CO₂(g)+ __H₂O(g)
A)0.100 mol
B)0.500 mol
C)0.600 mol
D)0.800 mol
E)none of the above
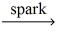 __CO₂(g)+ __H₂O(g)
__CO₂(g)+ __H₂O(g)A)0.100 mol
B)0.500 mol
C)0.600 mol
D)0.800 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
51
Classify the following type of stoichiometry problem: How many milliliters of nitrogen monoxide gas react with an excess mass of oxygen gas to give 50.0 cm3 of nitrogen dioxide gas?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
52
Classify the following type of stoichiometry problem: How many grams of tin must react with an excess volume of nitric acid to produce 50.0 mL of hydrogen gas?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
53
In general,how many unit factors are required to solve a mass-mass stoichiometry problem?
A)one
B)two
C)three
D)four
E)none of the above
A)one
B)two
C)three
D)four
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
54
How many moles of oxygen gas react to yield 0.100 mol water? __C5H₁₂ (g)+ __O₂(g) 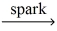 __CO₂(g)+ __H₂O(g)
__CO₂(g)+ __H₂O(g)
A)0.100 mol
B)0.500 mol
C)0.600 mol
D)0.800 mol
E)none of the above
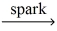 __CO₂(g)+ __H₂O(g)
__CO₂(g)+ __H₂O(g)A)0.100 mol
B)0.500 mol
C)0.600 mol
D)0.800 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
55
Classify the following type of stoichiometry problem: How many cubic centimeters of carbon dioxide gas are produced from heating 0.750 g of baking soda?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
56
Classify the following type of stoichiometry problem: How many grams of nickel must react with an excess volume of sulfuric acid to yield 0.500 g of nickel(II)sulfate?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
57
Classify the following type of stoichiometry problem: How many milliliters of hydrogen gas react with an excess mass of chlorine gas to give 250 mL of hydrogen chloride?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following steps is necessary to solve a mass-volume stoichiometry problem?
A)Write a balanced equation for the reaction.
B)Calculate the moles of known substance.
C)Convert moles of known to moles of unknown.
D)Calculate the volume of unknown gas.
E)all of the above
A)Write a balanced equation for the reaction.
B)Calculate the moles of known substance.
C)Convert moles of known to moles of unknown.
D)Calculate the volume of unknown gas.
E)all of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
59
How many moles of oxygen gas react with 0.100 mol of pentane,C5H₁₂? __C5H₁₂ (g)+ __O₂(g) 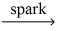 __CO₂(g)+ __H₂O(g)
__CO₂(g)+ __H₂O(g)
A)0.100 mol
B)0.500 mol
C)0.600 mol
D)0.800 mol
E)none of the above
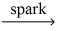 __CO₂(g)+ __H₂O(g)
__CO₂(g)+ __H₂O(g)A)0.100 mol
B)0.500 mol
C)0.600 mol
D)0.800 mol
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
60
Classify the following type of stoichiometry problem: How many grams of cobalt must react with an excess volume of hydrochloric acid to produce 25.0 mL of hydrogen gas?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)mole-mole problem
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
61
What is the mass of aluminum metal that reacts to give 1.00 g of hydrogen gas? __Al(s)+ __HCl(aq)→ __AlCl3(aq)+ __H₂(g)
A)4.46 g
B)8.90 g
C)13.4 g
D)20.0 g
E)26.7 g
A)4.46 g
B)8.90 g
C)13.4 g
D)20.0 g
E)26.7 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
62
What is the volume of oxygen gas at STP from the decomposition of 10.8 g of mercuric oxide (216.59 g/mol)? __HgO(s) 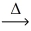 __Hg(l)+ __O₂(g)
__Hg(l)+ __O₂(g)
A)0.558 L
B)1.12 L
C)2.23 L
D)52.2 L
E)209 L
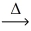 __Hg(l)+ __O₂(g)
__Hg(l)+ __O₂(g)A)0.558 L
B)1.12 L
C)2.23 L
D)52.2 L
E)209 L

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
63
What is the mass of insoluble silver bromide (187.77 g/mol)produced from 2.96 g of iron(III)bromide (295.55 g/mol)and aqueous silver nitrate? __FeBr3(s)+ __AgNO₃(aq)→ __AgBr(s)+ __Fe(NO₃)3(aq)
A)0.940 g
B)0.627 g
C)1.88 g
D)5.64 g
E)3.76 g
A)0.940 g
B)0.627 g
C)1.88 g
D)5.64 g
E)3.76 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
64
What is the mass of copper metal that yields 0.500 g of silver? __Cu(s)+ __AgNO₃(aq)→ __Cu(NO₃)2(aq)+ __Ag(s)
A)0.147 g
B)0.294 g
C)0.425 g
D)0.589 g
E)1.70 g
A)0.147 g
B)0.294 g
C)0.425 g
D)0.589 g
E)1.70 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
65
What is the volume of oxygen gas at STP from the decomposition of 1.70 g of sodium nitrate (85.00 g/mol)? __NaNO₃(s) 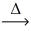 __NaNO₂(s)+ __O₂(g)
__NaNO₂(s)+ __O₂(g)
A)0.224 L
B)0.448 L
C)0.896 L
D)3.23 L
E)12.9 L
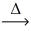 __NaNO₂(s)+ __O₂(g)
__NaNO₂(s)+ __O₂(g)A)0.224 L
B)0.448 L
C)0.896 L
D)3.23 L
E)12.9 L

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
66
What is the mass of aluminum metal that reacts to give 11.1 g of manganese metal? __MnO₂(l)+ __Al(l) 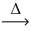 __Mn(l)+ __Al₂O₃(s)
__Mn(l)+ __Al₂O₃(s)
A)3.64 g
B)4.09 g
C)5.45 g
D)7.27 g
E)8.18 g
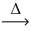 __Mn(l)+ __Al₂O₃(s)
__Mn(l)+ __Al₂O₃(s)A)3.64 g
B)4.09 g
C)5.45 g
D)7.27 g
E)8.18 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
67
What is the mass of iron(III)bromide (295.55 g/mol)that yields 0.188 g of silver bromide (187.77 g/mol)precipitate? __FeBr3(s)+ __AgNO₃(aq)→ __AgBr(s)+ __Fe(NO₃)3(aq)
A)0.0986 g
B)0.148 g
C)0.296 g
D)0.592 g
E)0.888 g
A)0.0986 g
B)0.148 g
C)0.296 g
D)0.592 g
E)0.888 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
68
What is the mass of aluminum metal that reacts to give 1.00 g of iron? __FeO(l)+ __Al(l) 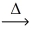 __Fe(l)+ __Al₂O₃(s)
__Fe(l)+ __Al₂O₃(s)
A)0.322 g
B)0.483 g
C)0.725 g
D)0.966 g
E)1.449 g
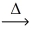 __Fe(l)+ __Al₂O₃(s)
__Fe(l)+ __Al₂O₃(s)A)0.322 g
B)0.483 g
C)0.725 g
D)0.966 g
E)1.449 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
69
What is the mass of aluminum oxide (101.96 g/mol)produced from 1.74 g of manganese(IV)oxide (86.94 g/mol)? __MnO₂(l)+ __Al(l) 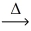 __Mn(l)+ __Al₂O₃(s)
__Mn(l)+ __Al₂O₃(s)
A)0.988 g
B)1.36 g
C)2.04 g
D)2.22 g
E)3.06 g
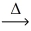 __Mn(l)+ __Al₂O₃(s)
__Mn(l)+ __Al₂O₃(s)A)0.988 g
B)1.36 g
C)2.04 g
D)2.22 g
E)3.06 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
70
What is the mass of mercuric oxide (216.59 g/mol)that decomposes to release 0.750L of oxygen gas at STP? __HgO(s) 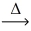 __Hg(l)+ __O₂(g)
__Hg(l)+ __O₂(g)
A)0.0388 g
B)0.155 g
C)3.63 g
D)7.25 g
E)14.5 g
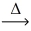 __Hg(l)+ __O₂(g)
__Hg(l)+ __O₂(g)A)0.0388 g
B)0.155 g
C)3.63 g
D)7.25 g
E)14.5 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
71
What is the mass of insoluble calcium phosphate (310.18 g/mol)produced from 0.555 g of calcium chloride (110.98 g/mol)? __CaCl₂(s)+ __Na3PO₄(aq)→ __Ca3(PO₄)2(s)+ __NaCl(aq)
A)0.0662 g
B)0.517 g
C)0.596 g
D)1.55 g
E)4.65 g
A)0.0662 g
B)0.517 g
C)0.596 g
D)1.55 g
E)4.65 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
72
What is the mass of potassium iodide (166.00 g/mol)that yields 0.500 g of lead(II)iodide (461.0 g/mol)precipitate? __Pb(NO₃)2(aq)+ __KI(s)→ __PbI₂(s)+ __KNO₃(aq)
A)0.0900 g
B)0.180 g
C)0.360 g
D)0.694 g
E)2.78 g
A)0.0900 g
B)0.180 g
C)0.360 g
D)0.694 g
E)2.78 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
73
What is the mass of silver metal produced from 6.35 g of copper? __Cu(s)+ __AgNO₃(aq)→ __Cu(NO₃)2(aq)+ __Ag(s)
A)0.187 g
B)0.540 g
C)0.747 g
D)1.08 g
E)21.6 g
A)0.187 g
B)0.540 g
C)0.747 g
D)1.08 g
E)21.6 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
74
What is the mass of sodium phosphate (163.94 g/mol)that yields 1.00 g of calcium phosphate (310.18 g/mol)precipitate? __CaCl₂(s)+ __Na3PO₄(aq)→ __Ca3(PO₄)2(s)+ __NaCl(aq)
A)0.264 g
B)0.358 g
C)0.931 g
D)1.06 g
E)8.38 g
A)0.264 g
B)0.358 g
C)0.931 g
D)1.06 g
E)8.38 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
75
In general,how many unit factors are required to solve a volume-volume stoichiometry problem?
A)one
B)two
C)three
D)four
E)none of the above
A)one
B)two
C)three
D)four
E)none of the above

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
76
What is the mass of insoluble lead(II)iodide (461.0 g/mol)produced from 0.830 g of potassium iodide (166.00 g/mol)and aqueous lead(II)nitrate? __Pb(NO₃)2(aq)+ __KI(s)→ __PbI₂(s)+ __KNO₃(aq)
A)0.149 g
B)0.598 g
C)1.15 g
D)2.31 g
E)4.61 g
A)0.149 g
B)0.598 g
C)1.15 g
D)2.31 g
E)4.61 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
77
What is the mass of sodium nitrate (85.00 g/mol)that decomposes to release 2.55 L of oxygen gas at STP? __NaNO₃(s) 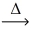 __NaNO₂(s)+ __O₂(g)
__NaNO₂(s)+ __O₂(g)
A)0.336 g
B)1.34 g
C)4.84 g
D)9.68 g
E)19.4 g
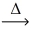 __NaNO₂(s)+ __O₂(g)
__NaNO₂(s)+ __O₂(g)A)0.336 g
B)1.34 g
C)4.84 g
D)9.68 g
E)19.4 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
78
What is the mass of aluminum oxide (101.96 g/mol)produced from 3.59 g of iron(II)oxide (71.85 g/mol)? __FeO(l)+ __Al(l) 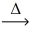 __Fe(l)+ __Al₂O₃(s)
__Fe(l)+ __Al₂O₃(s)
A)0.842 g
B)1.70 g
C)5.10 g
D)7.58 g
E)15.3 g
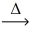 __Fe(l)+ __Al₂O₃(s)
__Fe(l)+ __Al₂O₃(s)A)0.842 g
B)1.70 g
C)5.10 g
D)7.58 g
E)15.3 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
79
What is the mass of hydrogen gas released from 2.70 g of aluminum metal and hydrochloric acid? __Al(s)+ __HCl(aq)→ __AlCl3(aq)+ __H₂(g)
A)0.101 g
B)0.135 g
C)0.202 g
D)0.303 g
E)0.606 g
A)0.101 g
B)0.135 g
C)0.202 g
D)0.303 g
E)0.606 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
80
What is the volume of oxygen gas at STP from the decomposition of 9.57 g of silver chlorate (191.32 g/mol)? __AgClO₃(s)→ __AgCl(s)+ __O₂(g)
A)0.560 L
B)0.747 L
C)1.12 L
D)1.68 L
E)3.36 L
A)0.560 L
B)0.747 L
C)1.12 L
D)1.68 L
E)3.36 L

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck


