Deck 4: Models of the Atom
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/173
Play
Full screen (f)
Deck 4: Models of the Atom
1
What is the term for a unit of mass exactly equal to 1/12 the mass of a carbon-12 atom?
A)atomic mass unit
B)hydrogen atom
C)neutron
D)proton
E)none of the above
A)atomic mass unit
B)hydrogen atom
C)neutron
D)proton
E)none of the above
atomic mass unit
2
What is the term for a symbolic method of expressing the composition of an atomic nucleus?
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above
atomic notation
3
What is the negatively charged subatomic particle having a negligible mass?
A)electron
B)neutron
C)proton
D)quark
E)none of the above
A)electron
B)neutron
C)proton
D)quark
E)none of the above
electron
4
What is the term for the region of very high density in the center of the atom?
A)atomic center
B)atomic core
C)atomic kernel
D)atomic nucleus
E)none of the above
A)atomic center
B)atomic core
C)atomic kernel
D)atomic nucleus
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
5
What is the term for an electron energy level that results from splitting a main energy level?
A)energy sublevel
B)orbital
C)shell
D)subshell
E)none of the above
A)energy sublevel
B)orbital
C)shell
D)subshell
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
6
What is the neutral subatomic particle having an approximate mass of 1 amu?
A)electron
B)neutron
C)proton
D)quark
E)none of the above
A)electron
B)neutron
C)proton
D)quark
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
7
What is the positively charged subatomic particle having an approximate mass of 1 amu?
A)electron
B)neutron
C)proton
D)quark
E)none of the above
A)electron
B)neutron
C)proton
D)quark
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
8
What is the term for a particle of radiant energy?
A)electron
B)neutron
C)photon
D)proton
E)none of the above
A)electron
B)neutron
C)photon
D)proton
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
9
What is the term for the shorthand description of the arrangement of electrons by sublevels according to increasing energy?
A)atomic notation
B)atomic number
C)continuous spectrum
D)electron configuration
E)none of the above
A)atomic notation
B)atomic number
C)continuous spectrum
D)electron configuration
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
10
What is the term for two atoms of the same element that differ by the number of neutrons in the nucleus?
A)atomic mass units
B)isotopes
C)nucleons
D)photons
E)none of the above
A)atomic mass units
B)isotopes
C)nucleons
D)photons
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
11
What is the term for the weighted average mass of all the naturally occurring isotopes of an element?
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
12
What is the general term that refers to either visible or invisible radiant energy?
A)continuous spectrum
B)emission spectrum
C)line spectrum
D)light
E)none of the above
A)continuous spectrum
B)emission spectrum
C)line spectrum
D)light
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
13
What is the term for the collection of narrow bands of light that results from excited atoms of a given element releasing energy?
A)continuous spectrum
B)electromagnetic spectrum
C)emission line spectrum
D)radiant energy spectrum
E)none of the above
A)continuous spectrum
B)electromagnetic spectrum
C)emission line spectrum
D)radiant energy spectrum
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
14
What is the term for the atomic model that describes electrons circling the nucleus in an orbit of specific energy?
A)Bohr atom
B)Rutherford atom
C)Thomson atom
D)quantum mechanical atom
E)none of the above
A)Bohr atom
B)Rutherford atom
C)Thomson atom
D)quantum mechanical atom
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
15
What is the term for an orbit that electrons occupy at a fixed distance from the nucleus;designated 1,2,3,4 ...?
A)energy level
B)orbital
C)shell
D)subshell
E)none of the above
A)energy level
B)orbital
C)shell
D)subshell
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
16
What is the term for the value which indicates the number of protons for an atom of a given element?
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
17
What is the term for the spatial region about the nucleus of an atom where there is a high probability of finding an electron with a given energy?
A)electron shell
B)electron subshell
C)orbital
D)quantum level
E)none of the above
A)electron shell
B)electron subshell
C)orbital
D)quantum level
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
18
What is the term for a broad uninterrupted band of radiant energy?
A)continuous spectrum
B)radiant energy spectrum
C)ultraviolet spectrum
D)visible spectrum
E)none of the above
A)continuous spectrum
B)radiant energy spectrum
C)ultraviolet spectrum
D)visible spectrum
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
19
What is the term for the value which indicates the total number of protons and neutrons in an atom of a given element?
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above
A)atomic notation
B)atomic number
C)atomic mass
D)mass number
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
20
What is the term for the number of times a light wave travels a complete cycle in one second?
A)energy
B)frequency
C)velocity
D)wavelength
E)none of the above
A)energy
B)frequency
C)velocity
D)wavelength
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following subatomic particles are found outside the nucleus?
A)electron and neutron
B)neutron and proton
C)proton and electron
D)all of the above
E)none of the above
A)electron and neutron
B)neutron and proton
C)proton and electron
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
22
According to the Thomson model,what is the simplest positive particle in an atom?
A)alpha
B)electron
C)neutron
D)proton
E)none of the above
A)alpha
B)electron
C)neutron
D)proton
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
23
What is the term for the atomic model that describes the energy of an electron in terms of its probable location about the nucleus?
A)Bohr atom
B)nuclear atom
C)planetary atom
D)quantum mechanical atom
E)none of the above
A)Bohr atom
B)nuclear atom
C)planetary atom
D)quantum mechanical atom
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
24
What is the term for light energy that appears violet to red?
A)infrared spectrum
B)radiant energy spectrum
C)ultraviolet spectrum
D)visible spectrum
E)none of the above
A)infrared spectrum
B)radiant energy spectrum
C)ultraviolet spectrum
D)visible spectrum
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following of Dalton's proposals is still valid?
A)Atoms of different elements combine to form compounds.
B)Atoms can combine in small whole number ratios.
C)Atoms can combine in more than one whole number ratio.
D)all of the above
E)none of the above
A)Atoms of different elements combine to form compounds.
B)Atoms can combine in small whole number ratios.
C)Atoms can combine in more than one whole number ratio.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
26
What is the term for the statement that it is impossible to precisely measure both the location and energy of a particle at the same time?
A)exclusion principle
B)quantum principle
C)spectrum rule
D)uncertainty principle
E)none of the above
A)exclusion principle
B)quantum principle
C)spectrum rule
D)uncertainty principle
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following of Dalton's proposals is still valid?
A)An element is composed of tiny particles called atoms.
B)Atoms of different elements combine to form compounds.
C)Compounds contain atoms in small whole number ratios.
D)all of the above
E)none of the above
A)An element is composed of tiny particles called atoms.
B)Atoms of different elements combine to form compounds.
C)Compounds contain atoms in small whole number ratios.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
28
What does the pudding represent in the plum pudding model of the atom?
A)homogeneous negative charge
B)homogeneous positive charge
C)heterogeneous negative charge
D)heterogeneous positive charge
E)none of the above
A)homogeneous negative charge
B)homogeneous positive charge
C)heterogeneous negative charge
D)heterogeneous positive charge
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
29
According to the Thomson model of the atom,what is the simplest negative particle in an atom?
A)alpha
B)electron
C)neutron
D)proton
E)none of the above
A)alpha
B)electron
C)neutron
D)proton
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
30
Which subatomic particle has a relative charge of +1 and a mass of ~1 amu?
A)alpha
B)electron
C)neutron
D)proton
E)none of the above
A)alpha
B)electron
C)neutron
D)proton
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
31
According to the Thomson model,what is the relative charge on a proton?
A)-1
B)+1
C)-2
D)+2
E)none of the above
A)-1
B)+1
C)-2
D)+2
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
32
Which subatomic particle has a relative charge of 0 and a mass of ~1 amu?
A)alpha
B)electron
C)neutron
D)proton
E)none of the above
A)alpha
B)electron
C)neutron
D)proton
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
33
What is the term for the range of light energy extending from short wavelength gamma rays to long wavelength microwaves?
A)gamma ray spectrum
B)microwave spectrum
C)radiant energy spectrum
D)visible spectrum
E)none of the above
A)gamma ray spectrum
B)microwave spectrum
C)radiant energy spectrum
D)visible spectrum
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following subatomic particles is found outside the nucleus?
A)electron
B)neutron
C)proton
D)all of the above
E)none of the above
A)electron
B)neutron
C)proton
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following subatomic particles are found inside the nucleus?
A)electron and neutron
B)neutron and proton
C)proton and electron
D)all of the above
E)none of the above
A)electron and neutron
B)neutron and proton
C)proton and electron
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
36
What do the raisins represent in the raisin pudding model of the atom?
A)electrons
B)neutrons
C)protons
D)nuclei
E)none of the above
A)electrons
B)neutrons
C)protons
D)nuclei
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following of Dalton's proposals is still valid?
A)Atoms are indivisible.
B)Atoms are indestructible.
C)All atoms of an element are identical.
D)all of the above
E)none of the above
A)Atoms are indivisible.
B)Atoms are indestructible.
C)All atoms of an element are identical.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
38
What is the term for the distance a light wave travels to complete one cycle?
A)energy
B)frequency
C)velocity
D)wavelength
E)none of the above
A)energy
B)frequency
C)velocity
D)wavelength
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
39
Which subatomic particle has a relative charge of -1 and a mass of ~0 amu?
A)alpha
B)electron
C)neutron
D)proton
E)none of the above
A)alpha
B)electron
C)neutron
D)proton
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
40
According to the Thomson model,what is the relative charge on an electron?
A)-1
B)+1
C)-2
D)+2
E)none of the above
A)-1
B)+1
C)-2
D)+2
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
41
Why are atomic masses expressed on a relative atomic mass scale?
A)Atoms are electrically positive.
B)Atoms are electrically neutral.
C)Atoms are too reactive to weigh directly.
D)Atoms are too small to weigh directly.
E)none of the above
A)Atoms are electrically positive.
B)Atoms are electrically neutral.
C)Atoms are too reactive to weigh directly.
D)Atoms are too small to weigh directly.
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
42
Given that the only naturally occurring isotope of aluminum is 27Al,what is its isotopic mass? (Hint: Refer to the Periodic Table. )
A)13.00 amu
B)13.98 amu
C)14.00 amu
D)26.98 amu
E)39.98 amu
A)13.00 amu
B)13.98 amu
C)14.00 amu
D)26.98 amu
E)39.98 amu

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
43
Element Y has two natural isotopes: Y-63 (62.940 amu)and Y-65 (64.928 amu).Calculate the atomic mass of element Y given the abundance of Y-63 is 69.17%.
A)63.55 amu
B)64.00 amu
C)64.32 amu
D)107.85 amu
E)108.46 amu
A)63.55 amu
B)64.00 amu
C)64.32 amu
D)107.85 amu
E)108.46 amu

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
44
Element X has two natural isotopes: X-6 (6.015 amu)and X-7 (7.016 amu).Calculate the atomic mass of element X given the abundance of X-7 is 92.5%.
A)6.09 amu
B)6.50 amu
C)6.52 amu
D)6.94 amu
E)12.5 amu
A)6.09 amu
B)6.50 amu
C)6.52 amu
D)6.94 amu
E)12.5 amu

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
45
How many neutrons are in the nucleus of an atom of 195Pt?
A)78
B)117
C)195
D)273
E)none of the above
A)78
B)117
C)195
D)273
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
46
Element Z has two natural isotopes: Z-79 (78.918 amu)and Z-81 (80.916 amu).Calculate the atomic mass of element Z given the abundance of Z-81 is 49.31%.
A)79.00 amu
B)79.90 amu
C)80.00 amu
D)119.93 amu
E)120.92 amu
A)79.00 amu
B)79.90 amu
C)80.00 amu
D)119.93 amu
E)120.92 amu

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
47
Using atomic notation,indicate the isotope having 11 p+,12 n0,and 11 e-.
A)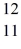 Na
Na
B)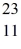 Na
Na
C)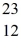 Na
Na
D)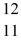 Mg
Mg
E)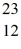 Mg
Mg
A)
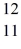 Na
NaB)
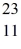 Na
NaC)
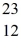 Na
NaD)
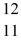 Mg
MgE)
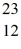 Mg
Mg
Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
48
How many neutrons are in the nucleus of an atom of 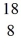 O?
O?
A)8
B)10
C)18
D)26
E)none of the above
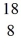 O?
O?A)8
B)10
C)18
D)26
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
49
How many neutrons are in the nucleus of an atom of silver-107?
A)47
B)60
C)107
D)154
E)none of the above
A)47
B)60
C)107
D)154
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
50
Using atomic notation,indicate the isotope having 26 p+,32 n0,and 26 e-.
A)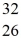 Fe
Fe
B)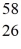 Fe
Fe
C)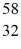 Fe
Fe
D)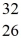 S
S
E)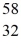 S
S
A)
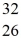 Fe
FeB)
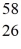 Fe
FeC)
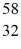 Fe
FeD)
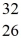 S
SE)
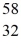 S
S
Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
51
How many neutrons are in the nucleus of an atom of iron-56?
A)26
B)30
C)56
D)82
E)none of the above
A)26
B)30
C)56
D)82
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
52
How many neutrons are in the nucleus of an atom of 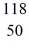 Sn?
Sn?
A)50
B)68
C)118
D)168
E)none of the above
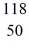 Sn?
Sn?A)50
B)68
C)118
D)168
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
53
What is the assigned mass of the reference isotope for the atomic mass scale?
A)1.01 amu
B)4.00 amu
C)12.01 amu
D)16.00 amu
E)12 amu exactly
A)1.01 amu
B)4.00 amu
C)12.01 amu
D)16.00 amu
E)12 amu exactly

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
54
Given that the only naturally occurring isotope of gold is 197Au,what is its isotopic mass? (Hint: Refer to the Periodic Table. )
A)79.00 amu
B)117.97 amu
C)118.00 amu
D)196.97 amu
E)275.97 amu
A)79.00 amu
B)117.97 amu
C)118.00 amu
D)196.97 amu
E)275.97 amu

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
55
How many neutrons are in the nucleus of an atom of 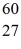 Co?
Co?
A)27
B)33
C)60
D)87
E)none of the above
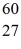 Co?
Co?A)27
B)33
C)60
D)87
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
56
How many neutrons are in the nucleus of an atom of copper-65?
A)29
B)36
C)65
D)94
E)none of the above
A)29
B)36
C)65
D)94
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
57
Given that the only naturally occurring isotope of sodium is 23Na,what is its isotopic mass? (Hint: Refer to the Periodic Table. )
A)11.00 amu
B)11.99 amu
C)12.00 amu
D)22.99 amu
E)34.99 amu
A)11.00 amu
B)11.99 amu
C)12.00 amu
D)22.99 amu
E)34.99 amu

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
58
Using atomic notation,indicate the isotope having 30 p+,35 n0,and 30 e-.
A)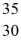 Zn
Zn
B)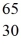 Zn
Zn
C)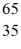 Zn
Zn
D)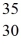 Br
Br
E)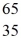 Br
Br
A)
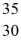 Zn
ZnB)
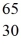 Zn
ZnC)
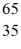 Zn
ZnD)
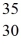 Br
BrE)
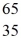 Br
Br
Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
59
How many neutrons are in the nucleus of an atom of 200Hg?
A)80
B)120
C)200
D)280
E)none of the above
A)80
B)120
C)200
D)280
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
60
What is the current reference isotope for the atomic mass scale?
A)hydrogen-1
B)helium-4
C)carbon-12
D)oxygen-16
E)none of the above
A)hydrogen-1
B)helium-4
C)carbon-12
D)oxygen-16
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following wavelengths has the lowest velocity?
A)440 nm
B)470 nm
C)540 nm
D)650 nm
E)All wavelengths have the same velocity.
A)440 nm
B)470 nm
C)540 nm
D)650 nm
E)All wavelengths have the same velocity.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following colors of light has the highest energy?
A)violet
B)blue
C)green
D)red
E)All colors have the same energy.
A)violet
B)blue
C)green
D)red
E)All colors have the same energy.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
63
Copper occurs naturally as 63Cu and 65Cu.Which isotope is more abundant? (Hint: Refer to the Periodic Table. )
A)copper-34
B)copper-36
C)copper-63
D)copper-65
E)none of the above
A)copper-34
B)copper-36
C)copper-63
D)copper-65
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following wavelengths of light is in the infrared region of the radiant energy spectrum?
A)180 nm
B)380 nm
C)580 nm
D)780 nm
E)all of the above
A)180 nm
B)380 nm
C)580 nm
D)780 nm
E)all of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following colors of light has the longest wavelength?
A)violet
B)blue
C)orange
D)red
E)All colors have the same wavelength.
A)violet
B)blue
C)orange
D)red
E)All colors have the same wavelength.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following types of radiation has the highest frequency?
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same frequency.
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same frequency.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following wavelengths of light has the lowest frequency?
A)440 nm
B)470 nm
C)540 nm
D)650 nm
E)All wavelengths have the same frequency.
A)440 nm
B)470 nm
C)540 nm
D)650 nm
E)All wavelengths have the same frequency.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following instruments produces quantized musical notes?
A)cello
B)guitar
C)piano
D)violin
E)all of the above
A)cello
B)guitar
C)piano
D)violin
E)all of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following colors of light has the shortest wavelength?
A)violet
B)green
C)yellow
D)red
E)All colors have the same wavelength.
A)violet
B)green
C)yellow
D)red
E)All colors have the same wavelength.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following types of radiation has the highest energy?
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same energy.
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same energy.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following types of radiation has the longest wavelength?
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same wavelength.
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same wavelength.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
72
Boron occurs naturally as 10B and 11B.Which isotope is more abundant? (Hint: Refer to the Periodic Table. )
A)boron-5
B)boron-6
C)boron-10
D)boron-11
E)none of the above
A)boron-5
B)boron-6
C)boron-10
D)boron-11
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
73
Lithium occurs naturally as 6Li and 7Li .Which isotope is more abundant? (Hint: Refer to the Periodic Table. )
A)lithium-3
B)lithium-4
C)lithium-6
D)lithium-7
E)none of the above
A)lithium-3
B)lithium-4
C)lithium-6
D)lithium-7
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following colors of light has the highest velocity?
A)red
B)orange
C)yellow
D)green
E)All colors have the same velocity.
A)red
B)orange
C)yellow
D)green
E)All colors have the same velocity.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following types of radiation has the highest velocity?
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same velocity.
A)X rays
B)ultraviolet
C)visible
D)infrared
E)All radiation has the same velocity.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following wavelengths of light has the lowest energy?
A)440 nm
B)470 nm
C)540 nm
D)650 nm
E)All wavelengths have the same energy.
A)440 nm
B)470 nm
C)540 nm
D)650 nm
E)All wavelengths have the same energy.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following colors of light has the highest frequency?
A)violet
B)green
C)yellow
D)red
E)All colors have the same frequency.
A)violet
B)green
C)yellow
D)red
E)All colors have the same frequency.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is an example of a quantized change in energy?
A)a ball rolling up a ramp
B)a ball rolling down a ramp
C)a ball rolling down a flight of stairs
D)a ball rolling across a playground
E)none of the above
A)a ball rolling up a ramp
B)a ball rolling down a ramp
C)a ball rolling down a flight of stairs
D)a ball rolling across a playground
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following wavelengths of light is in the visible region of the radiant energy spectrum?
A)250 nm
B)550 nm
C)850 nm
D)all of the above
E)none of the above
A)250 nm
B)550 nm
C)850 nm
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following wavelengths of light is in the ultraviolet region of the radiant energy spectrum?
A)380 nm
B)580 nm
C)780 nm
D)980 nm
E)all of the above
A)380 nm
B)580 nm
C)780 nm
D)980 nm
E)all of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck


