Deck 15: Advanced Problem Solving
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/111
Play
Full screen (f)
Deck 15: Advanced Problem Solving
1
What is the term for the value corresponding to the number of carbon atoms in 12.01g of carbon?
A)Avogadro's number
B)atomic number
C)mass number
D)mole number
E)none of the above
A)Avogadro's number
B)atomic number
C)mass number
D)mole number
E)none of the above
Avogadro's number
2
Which of the following is described by the equation: PV = nRT?
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)ideal gas law
E)none of the above
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)ideal gas law
E)none of the above
ideal gas law
3
What is the term for the constant R in the equation PV = nRT?
A)combined gas constant
B)ideal gas constant
C)real gas constant
D)universal gas constant
E)none of the above
A)combined gas constant
B)ideal gas constant
C)real gas constant
D)universal gas constant
E)none of the above
ideal gas constant
4
Which of the following laws states that a compound always contains the same elements in the same proportion by mass?
A)law of conservation of energy
B)law of conservation of mass
C)law of conservation of mass and energy
D)law of definite composition
E)none of the above
A)law of conservation of energy
B)law of conservation of mass
C)law of conservation of mass and energy
D)law of definite composition
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
5
What is the general term for a substance dissolved in water?
A)aqueous salt
B)aqueous substance
C)aqueous solution
D)water solution
E)none of the above
A)aqueous salt
B)aqueous substance
C)aqueous solution
D)water solution
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
6
What is the term for the amount of substance that contains 6.02 x 1023 particles?
A)Avogadro's number
B)formula mass
C)molar mass
D)mole
E)none of the above
A)Avogadro's number
B)formula mass
C)molar mass
D)mole
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following states that the pressure exerted by a gas is inversely proportional to its volume and directly proportional to its Kelvin temperature?
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)combined gas law
E)none of the above
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)combined gas law
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
8
What general term refers to the mass of 1 mol of any substance?
A)gram-atomic mass
B)gram-molecular mass
C)gram-formula mass
D)molar mass
E)none of the above
A)gram-atomic mass
B)gram-molecular mass
C)gram-formula mass
D)molar mass
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
9
What principle states that mass is neither gained or lost during a chemical reaction?
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
10
What is the term for the concentration expression that relates the moles of solute dissolved in each liter of solution?
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following terms defines the amount of mass in a unit volume?
A)density
B)specific mass
C)specific gravity
D)specific volume
E)none of the above
A)density
B)specific mass
C)specific gravity
D)specific volume
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
12
What is the term for a chemical formula that expresses the simplest whole number ratio of atoms of each element in a molecule,or of ions in a formula unit?
A)atomic formula
B)elemental formula
C)empirical formula
D)molecular formula
E)none of the above
A)atomic formula
B)elemental formula
C)empirical formula
D)molecular formula
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
13
What principle states that equal volumes of gases,at the same temperature and pressure,contain equal numbers of molecules?
A)Avogadro's theory
B)Boyle's theory
C)Charles' theory
D)Dalton's theory
E)none of the above
A)Avogadro's theory
B)Boyle's theory
C)Charles' theory
D)Dalton's theory
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
14
What is the term for the volume occupied by 1 mol of any gas at STP?
A)Avogadro's volume
B)molar volume
C)standard volume
D)STP volume
E)none of the above
A)Avogadro's volume
B)molar volume
C)standard volume
D)STP volume
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
15
What is the term for the concentration expression that relates the mass of solute dissolved in each 100 grams of solution?
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
16
What term expresses the ratio of moles of reactants and products according to the coefficients in the balanced chemical equation?
A)mole ratio
B)reactant ratio
C)product ratio
D)yield ratio
E)none of the above
A)mole ratio
B)reactant ratio
C)product ratio
D)yield ratio
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
17
What is the term that describes the relationship between two variables such that when one variable doubles,the other variable doubles?
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
18
What is the term for the chemical formula of a compound that expresses the actual number of atoms of each element in a molecule?
A)atomic formula
B)elemental formula
C)empirical formula
D)molecular formula
E)none of the above
A)atomic formula
B)elemental formula
C)empirical formula
D)molecular formula
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
19
What is the term that describes the relationship between two variables such that when one variable doubles,the other variable halves?
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
20
What is the term for the substance in a chemical reaction that controls the maximum amount of product?
A)limiting reactant
B)limiting product
C)maximum reactant
D)maximum product
E)none of the above
A)limiting reactant
B)limiting product
C)maximum reactant
D)maximum product
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
21
After organizing a solution to a chemistry problem,what should be done before using a calculator?
A)Decide on the relevant given value.
B)Estimate an approximate answer.
C)Refer to a reference book.
D)Write a balanced chemical equation.
E)none of the above
A)Decide on the relevant given value.
B)Estimate an approximate answer.
C)Refer to a reference book.
D)Write a balanced chemical equation.
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following units is an expression of mass?
A)mg
B)mL
C)mm
D)all of the above
E)none of the above
A)mg
B)mL
C)mm
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
23
If the unknown quantity is cm/s,what is true of the relevant given value?
A)The given value is a ratio of two units.
B)The given value is expressed in metric units.
C)The given value contains an exponent.
D)all of the above
E)none of the above
A)The given value is a ratio of two units.
B)The given value is expressed in metric units.
C)The given value contains an exponent.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
24
After carefully reading a chemistry problem,what is the first question to ask?
A)What is the unknown quantity?
B)What is the relevant given value?
C)What steps are necessary to proceed from the given to the unknown?
D)What units can be canceled?
E)none of the above
A)What is the unknown quantity?
B)What is the relevant given value?
C)What steps are necessary to proceed from the given to the unknown?
D)What units can be canceled?
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
25
What problem-solving technique requires rearranging variables in an equation to calculate an unknown quantity?
A)algebraic analysis
B)concept analysis
C)strategy analysis
D)unit analysis
E)none of the above
A)algebraic analysis
B)concept analysis
C)strategy analysis
D)unit analysis
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
26
What is the term for a gas at 273 K and 760 mm Hg pressure?
A)atmospheric temperature and pressure
B)experimental temperature and pressure
C)ideal gas temperature and pressure
D)standard temperature and pressure
E)none of the above
A)atmospheric temperature and pressure
B)experimental temperature and pressure
C)ideal gas temperature and pressure
D)standard temperature and pressure
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
27
If the unknown quantity is g/cm3,what is true of the relevant given value?
A)The given value is a ratio of two units.
B)The given value is expressed in metric units.
C)The given value contains an exponent.
D)all of the above
E)none of the above
A)The given value is a ratio of two units.
B)The given value is expressed in metric units.
C)The given value contains an exponent.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
28
What is the term for the relationship of quantities (i.e. ,mass of substance or volume of gas)in a chemical reaction according to the balanced chemical equation?
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)stoichiometry
E)none of the above
A)mass-mass problem
B)mass-volume problem
C)volume-volume problem
D)stoichiometry
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following units is an expression of volume?
A)mg
B)mL
C)mm
D)all of the above
E)none of the above
A)mg
B)mL
C)mm
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
30
What problem-solving technique converts a given value to an unknown value by applying one or more unit factors?
A)algebraic analysis
B)concept analysis
C)strategy analysis
D)unit analysis
E)none of the above
A)algebraic analysis
B)concept analysis
C)strategy analysis
D)unit analysis
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following units is an expression of length?
A)mg
B)mL
C)mm
D)all of the above
E)none of the above
A)mg
B)mL
C)mm
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
32
After carefully reading a chemistry problem,what is the second question to ask?
A)What is the unknown quantity?
B)What is the relevant given value?
C)What steps are necessary to proceed from the given to the unknown?
D)What units can be canceled?
E)none of the above
A)What is the unknown quantity?
B)What is the relevant given value?
C)What steps are necessary to proceed from the given to the unknown?
D)What units can be canceled?
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
33
After carefully reading a stoichiometry problem,what must be done before planning a solution?
A)Decide on the relevant given value.
B)Estimate an approximate answer.
C)Refer to a reference book.
D)Write a balanced chemical equation.
E)none of the above
A)Decide on the relevant given value.
B)Estimate an approximate answer.
C)Refer to a reference book.
D)Write a balanced chemical equation.
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
34
What problem-solving technique helps to understand concepts that cannot be observed directly,such as molecules in a gas?
A)formation
B)imagination
C)revelation
D)visualization
E)none of the above
A)formation
B)imagination
C)revelation
D)visualization
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following units is an expression of density?
A)g/cm3
B)g/mL
C)mg/dL
D)all of the above
E)none of the above
A)g/cm3
B)g/mL
C)mg/dL
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
36
After carefully reading a chemistry problem,what is the third question to ask?
A)What is the unknown quantity?
B)What is the relevant given value?
C)What steps are necessary to proceed from the given to the unknown?
D)What units can be canceled?
E)none of the above
A)What is the unknown quantity?
B)What is the relevant given value?
C)What steps are necessary to proceed from the given to the unknown?
D)What units can be canceled?
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
37
What problem-solving technique shows the relationship of chemical concepts,often shown by interrelated boxes?
A)concept map
B)hypothesis map
C)strategy map
D)theory map
E)none of the above
A)concept map
B)hypothesis map
C)strategy map
D)theory map
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
38
If the unknown quantity is g/mL,what is true of the relevant given value?
A)The given value is a ratio of two units.
B)The given value is expressed in metric units.
C)The given value contains an exponent.
D)all of the above
E)none of the above
A)The given value is a ratio of two units.
B)The given value is expressed in metric units.
C)The given value contains an exponent.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following units is an expression of gas pressure?
A)atm
B)mm Hg
C)psi
D)all of the above
E)none of the above
A)atm
B)mm Hg
C)psi
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following units is an expression of temperature?
A)°C
B)°F
C)K
D)all of the above
E)none of the above
A)°C
B)°F
C)K
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
41
What are the units of the unknown quantity for the following problem? Calculate the molar mass of oxygen gas given that 1.00 g of gas occupies 0.700 L.
A)g/L
B)L/g
C)g/mol
D)mol/g
E)none of the above
A)g/L
B)L/g
C)g/mol
D)mol/g
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
42
If 5.00 g of ammonia,NH₃,is dissolved in 50.0 mL of aqueous solution,what is the molarity of the ammonia solution?
A)0.170 M
B)0.235 M
C)4.25 M
D)5.88 M
E)6.80 M
A)0.170 M
B)0.235 M
C)4.25 M
D)5.88 M
E)6.80 M

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following illustrates mass-mass stoichiometry?
A)g reactant → g product → mol product → mol reactant
B)g reactant → mol product → mol reactant → g product
C)g reactant → mol product → g product → mol reactant
D)g reactant → mol reactant → g product → mol product
E)g reactant → mol reactant → mol product → g product
A)g reactant → g product → mol product → mol reactant
B)g reactant → mol product → mol reactant → g product
C)g reactant → mol product → g product → mol reactant
D)g reactant → mol reactant → g product → mol product
E)g reactant → mol reactant → mol product → g product

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
44
Given 25.0 mL of 0.200 M H₂SO₄,what is the mass of SO₃ dissolved in the sulfuric acid solution?
A)0.00500 g
B)0.0100 g
C)0.401 g
D)0.491 g
E)0.801 g
A)0.00500 g
B)0.0100 g
C)0.401 g
D)0.491 g
E)0.801 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
45
What are the units of the unknown quantity for the following problem? Calculate the molarity of a solution that contains 0.100 g of KI in 10.0 mL.
A)g KI/mL
B)g KI/L
C)mol KI/mL
D)mol KI/L
E)none of the above
A)g KI/mL
B)g KI/L
C)mol KI/mL
D)mol KI/L
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
46
If 1.00 x 1022 molecules of CO₂ gas are dissolved in 150.0 mL of aqueous solution,what is the STP volume of carbon dioxide dissolved in solution?
A)0.0166 L
B)0.372 L
C)0.731 L
D)2.69 L
E)37.2 L
A)0.0166 L
B)0.372 L
C)0.731 L
D)2.69 L
E)37.2 L

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following illustrates volume-volume stoichiometry?
A)L reactant → L reactant
B)mol reactant → mol product
C)L reactant → mol product
D)mol reactant → L product
E)g reactant → g product
A)L reactant → L reactant
B)mol reactant → mol product
C)L reactant → mol product
D)mol reactant → L product
E)g reactant → g product

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
48
If 1.12 L of HCl gas at STP is dissolved in 75.0 mL of aqueous solution,what is the mass of hydrogen chloride dissolved in solution?
A)0.0131 g
B)0.687 g
C)0.548 g
D)1.83 g
E)916 g
A)0.0131 g
B)0.687 g
C)0.548 g
D)1.83 g
E)916 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
49
If 1.00 x 1022 molecules of CO₂ gas is dissolved in 150.0 mL of aqueous solution,what is the molarity of the carbonic acid solution?
A)0.111 M
B)0.166 M
C)2.49 M
D)4.01 M
E)9.03 M
A)0.111 M
B)0.166 M
C)2.49 M
D)4.01 M
E)9.03 M

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following illustrates mass-volume stoichiometry?
A)g reactant → L product → mol product → mol reactant
B)g reactant → mol reactant → L product → mol product
C)g reactant → mol reactant → mol product → L product
D)g reactant → mol product → mol reactant → L product
E)g reactant → mol product → L product → mol reactant
A)g reactant → L product → mol product → mol reactant
B)g reactant → mol reactant → L product → mol product
C)g reactant → mol reactant → mol product → L product
D)g reactant → mol product → mol reactant → L product
E)g reactant → mol product → L product → mol reactant

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
51
Given 25.0 mL of 0.200 M H₂SO₄,what is the STP volume of SO₃ dissolved in the sulfuric acid solution?
A)0.112 L
B)0.179 L
C)0.223 L
D)0.491 L
E)5.00 L
A)0.112 L
B)0.179 L
C)0.223 L
D)0.491 L
E)5.00 L

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
52
If 1.00 x 1022 molecules of CO₂ gas are dissolved in 150.0 mL of aqueous solution,what is the mass of carbon dioxide dissolved in solution?
A)0.0166 g
B)0.372 g
C)0.731 g
D)1.37 g
E)26.5 g
A)0.0166 g
B)0.372 g
C)0.731 g
D)1.37 g
E)26.5 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following illustrates the conversion of molecules of oxygen gas to mass of O₂?
A)g O₂ → mol O₂ → molecules O₂
B)molecules O₂ → mol O₂ → g O₂
C)molecules O₂ → g O₂ → mol O₂
D)mol O₂ → molecules O₂ → g O₂
E)mol O₂ → g O₂ → molecules O₂
A)g O₂ → mol O₂ → molecules O₂
B)molecules O₂ → mol O₂ → g O₂
C)molecules O₂ → g O₂ → mol O₂
D)mol O₂ → molecules O₂ → g O₂
E)mol O₂ → g O₂ → molecules O₂

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following illustrates the conversion of a volume of ammonia gas at STP to molecules of NH₃?
A)mol NH₃ → molecules NH₃ → L NH₃
B)mol NH₃ → L NH₃ → molecules NH₃
C)molecules NH₃ → mol NH₃ → L NH₃
D)L NH₃ → mol NH₃ → molecules NH₃
E)L NH₃ → molecules NH₃ → mol NH₃
A)mol NH₃ → molecules NH₃ → L NH₃
B)mol NH₃ → L NH₃ → molecules NH₃
C)molecules NH₃ → mol NH₃ → L NH₃
D)L NH₃ → mol NH₃ → molecules NH₃
E)L NH₃ → molecules NH₃ → mol NH₃

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following illustrates the conversion of a mass of argon gas to volume of Ar at STP?
A)g Ar → mol Ar → L Ar
B)g Ar → L Ar → mol Ar
C)L Ar → mol Ar → g Ar
D)mol Ar → L Ar → g Ar
E)mol Ar → g Ar → L Ar
A)g Ar → mol Ar → L Ar
B)g Ar → L Ar → mol Ar
C)L Ar → mol Ar → g Ar
D)mol Ar → L Ar → g Ar
E)mol Ar → g Ar → L Ar

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
56
What are the units of the unknown quantity for the following problem? Calculate the molar volume of oxygen gas given an STP density of 1.43 g/1.00 L.
A)g/L
B)L/g
C)L/mol
D)mol/L
E)none of the above
A)g/L
B)L/g
C)L/mol
D)mol/L
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
57
If 1.12 L of HCl gas at STP is dissolved in 75.0 mL of aqueous solution,what is the number of hydrogen chloride molecules dissolved in solution?
A)2.39 x 1022 molecules
B)3.01 x 1022 molecules
C)4.17 x 1023 molecules
D)1.20 x 1025 molecules
E)1.51 x 1025 molecules
A)2.39 x 1022 molecules
B)3.01 x 1022 molecules
C)4.17 x 1023 molecules
D)1.20 x 1025 molecules
E)1.51 x 1025 molecules

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
58
If 5.00 g of ammonia,NH₃,is dissolved in 50.0 mL of aqueous solution,what is the number of ammonia molecules dissolved in solution?
A)7.08 x 1021
B)1.77 x 1023
C)7.08 x 1023
D)2.05 x 1024
E)5.12 x 1025
A)7.08 x 1021
B)1.77 x 1023
C)7.08 x 1023
D)2.05 x 1024
E)5.12 x 1025

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
59
If 1.12 L of HCl gas at STP is dissolved in 75.0 mL of aqueous solution,what is the molarity of the hydrochloric acid solution?
A)0.335 M
B)0.531 M
C)0.667 M
D)3.75 M
E)14.9 M
A)0.335 M
B)0.531 M
C)0.667 M
D)3.75 M
E)14.9 M

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
60
If 5.00 g of ammonia,NH₃,is dissolved in 50.0 mL of aqueous solution,what is the STP volume of ammonia gas before dissolving in solution?
A)0.0131 L
B)0.152 L
C)0.264 L
D)0.293 L
E)6.57 L
A)0.0131 L
B)0.152 L
C)0.264 L
D)0.293 L
E)6.57 L

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
61
If a sample of methane gas,CH₄,reacts completely with oxygen gas to give 1.75 g of water,what STP volume of methane reacted? CH₄(g)+ O₂(g) 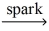 CO₂(g)+ H₂O(l)
CO₂(g)+ H₂O(l)
A)0.703 L
B)1.09 L
C)1.41 L
D)2.18 L
E)4.36 L
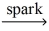 CO₂(g)+ H₂O(l)
CO₂(g)+ H₂O(l)A)0.703 L
B)1.09 L
C)1.41 L
D)2.18 L
E)4.36 L

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
62
Given the three-step Contact process for manufacturing sulfuric acid,H₂SO₄: S(s)+ O₂(g)→ SO₂(g)
2 SO₂(g)+ O₂(g)→ 2 SO₃(g)
SO₃(g)+ H₂O(l)→ H₂SO₄(aq)
What mass of sulfuric acid is produced from the reaction of 25.0 g of sulfur?
A)8.18 g
B)38.2 g
C)76.4 g
D)152 g
E)306 g
2 SO₂(g)+ O₂(g)→ 2 SO₃(g)
SO₃(g)+ H₂O(l)→ H₂SO₄(aq)
What mass of sulfuric acid is produced from the reaction of 25.0 g of sulfur?
A)8.18 g
B)38.2 g
C)76.4 g
D)152 g
E)306 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
63
Given the three-step "blast furnace" process that converts iron ore,Fe₂O₃,into molten "pig iron": 3 Fe₂O₃(l)+ CO(g)→ 2 Fe₃O₄(l)+ CO₂(g)
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
What mass of Fe₂O₃ is required to produce 1.00 metric ton (1000 kg)of iron?
A)175 kg
B)699 kg
C)1430 kg
D)2860 kg
E)5720 kg
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
What mass of Fe₂O₃ is required to produce 1.00 metric ton (1000 kg)of iron?
A)175 kg
B)699 kg
C)1430 kg
D)2860 kg
E)5720 kg

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
64
Given the three-step "blast furnace" process that converts iron ore,Fe₂O₃,into molten "pig iron": 3 Fe₂O₃(l)+ CO(g)→ 2 Fe₃O₄(l)+ CO₂(g)
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
How many moles of iron are produced from the reaction of 6.00 mol of Fe₂O₃?
A)3.00 mol
B)4.00 mol
C)6.00 mol
D)12.0 mol
E)none of the above
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
How many moles of iron are produced from the reaction of 6.00 mol of Fe₂O₃?
A)3.00 mol
B)4.00 mol
C)6.00 mol
D)12.0 mol
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
65
If a sample of ammonia gas,NH₃,reacts with oxygen and platinum catalyst to give 55.5 L of nitrogen monoxide gas at STP,what mass of ammonia reacted? NH₃(g)+ O₂(g) 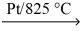 NO(g)+ H₂O(g)
NO(g)+ H₂O(g)
A)33.7 g
B)42.2 g
C)52.7 g
D)63.2 g
E)73.1 g
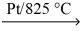 NO(g)+ H₂O(g)
NO(g)+ H₂O(g)A)33.7 g
B)42.2 g
C)52.7 g
D)63.2 g
E)73.1 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
66
Given that 50.0 mL of 0.125 M Hg(NO₃)2 reacts completely with 27.5 mL of aqueous NaI,what is the molarity of the sodium iodide solution? Hg(NO₃)2(aq)+ NaI(aq)→ HgI₂(s)+ NaNO₃(aq)
A)0.0688 M
B)0.114 M
C)0.138 M
D)0.227 M
E)0.455 M
A)0.0688 M
B)0.114 M
C)0.138 M
D)0.227 M
E)0.455 M

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
67
Given the following two-step process for converting coal to carbon dioxide: 2 C(s)+ O₂(g)→ 2 CO(g)
2 CO(g)+ O₂(g)→ 2 CO₂(g)
What volume of CO₂ at STP is produced from 1.00 kg of carbon?
A)933 L
B)1870 L
C)3670 L
D)3730 L
E)7330 L
2 CO(g)+ O₂(g)→ 2 CO₂(g)
What volume of CO₂ at STP is produced from 1.00 kg of carbon?
A)933 L
B)1870 L
C)3670 L
D)3730 L
E)7330 L

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
68
If a sample of ammonia gas,NH₃,reacts with oxygen and platinum catalyst to give 25.0 g of water vapor,what STP volume of ammonia reacted? NH₃(g)+ O₂(g) 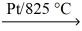 NO(g)+ H₂O(g)
NO(g)+ H₂O(g)
A)13.4 L
B)20.7 L
C)24.9 L
D)25.9 L
E)46.7 L
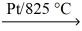 NO(g)+ H₂O(g)
NO(g)+ H₂O(g)A)13.4 L
B)20.7 L
C)24.9 L
D)25.9 L
E)46.7 L

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
69
Given that 25.0 mL of 0.100 M AgNO₃ reacts completely with 0.150 M k₂CrO₄,what volume of potassium chromate is required? AgNO₃(aq)+ k₂CrO₄(aq)→ Ag2CrO₄(s)+ KNO₃(aq)
A)8.33 mL
B)16.7 mL
C)33.3 mL
D)37.5 mL
E)75.0 mL
A)8.33 mL
B)16.7 mL
C)33.3 mL
D)37.5 mL
E)75.0 mL

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
70
Given that 50.0 mL of 0.125 M Hg(NO₃)2 reacts completely with 0.145 M NaI,what volume of sodium iodide is required? Hg(NO₃)2(aq)+ NaI(aq)→ HgI₂(s)+ NaNO₃(aq)
A)21.6 mL
B)29.0 mL
C)43.1 mL
D)58.0 mL
E)86.2 mL
A)21.6 mL
B)29.0 mL
C)43.1 mL
D)58.0 mL
E)86.2 mL

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
71
Given the three-step "blast furnace" process that converts iron ore,Fe₂O₃,into molten "pig iron": 3 Fe₂O₃(l)+ CO(g)→ 2 Fe₃O₄(l)+ CO₂(g)
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
What mass of iron is produced from 1.00 metric ton (1000 kg)of Fe₂O₃?
A)175 kg
B)699 kg
C)1430 kg
D)2860 kg
E)5720 kg
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
What mass of iron is produced from 1.00 metric ton (1000 kg)of Fe₂O₃?
A)175 kg
B)699 kg
C)1430 kg
D)2860 kg
E)5720 kg

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
72
Given the two-step process for converting aqueous hydrogen sulfide to SF6: 8 H₂S(aq)+ 8 Cl₂(g)→ 16 HCl(aq)+ S8(s)
S8(s)+ 24 F2(g)→ 8 SF6(g)
What mass of sulfur hexafluoride is produced from 10.0 L of 0.0100 M H₂S?
A)0.100 g
B)0.800 g
C)1.83 g
D)14.6 g
E)117 g
S8(s)+ 24 F2(g)→ 8 SF6(g)
What mass of sulfur hexafluoride is produced from 10.0 L of 0.0100 M H₂S?
A)0.100 g
B)0.800 g
C)1.83 g
D)14.6 g
E)117 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
73
Given the three-step Ostwald process for manufacturing nitric acid,HNO₃: 4 NH₃(g)+ 5 O₂(g)→ 4 NO(g)+ 6 H₂O(g)
2 NO(g)+ O₂(g)→ 2 NO₂(g)
3 NO₂(g)+ H₂O(l)→ 2 HNO₃(aq)+ NO(g)
What mass of ammonia is required to produce 75.0 g of HNO₃?
A)13.5 g
B)15.2 g
C)20.2 g
D)27.0 g
E)30.4 g
2 NO(g)+ O₂(g)→ 2 NO₂(g)
3 NO₂(g)+ H₂O(l)→ 2 HNO₃(aq)+ NO(g)
What mass of ammonia is required to produce 75.0 g of HNO₃?
A)13.5 g
B)15.2 g
C)20.2 g
D)27.0 g
E)30.4 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
74
If a sample of methane gas,CH₄,reacts completely with 1.55 L oxygen gas at STP,what mass of methane reacted? CH₄(g)+ O₂(g) 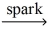 CO₂(g)+ H₂O(l)
CO₂(g)+ H₂O(l)
A)0.554 g
B)1.09 g
C)1.11 g
D)2.11 g
E)2.17 g
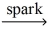 CO₂(g)+ H₂O(l)
CO₂(g)+ H₂O(l)A)0.554 g
B)1.09 g
C)1.11 g
D)2.11 g
E)2.17 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
75
Given 25.0 mL of 0.200 M H₂SO₄,how many SO₃ molecules are dissolved in the sulfuric acid solution?
A)3.01 x 1021 molecules
B)4.82 x 1021 molecules
C)8.30 x 1021 molecules
D)5.00 x 1022 molecules
E)3.01 x 1024 molecules
A)3.01 x 1021 molecules
B)4.82 x 1021 molecules
C)8.30 x 1021 molecules
D)5.00 x 1022 molecules
E)3.01 x 1024 molecules

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
76
Given the three-step Solvay process for manufacturing soda ash,Na₂CO₃: CO₂(g)+ NH₃(g)+ 6 H₂O(l)→ NH₄HCO₃(aq)
NH₄HCO₃(aq)+ NaCl(aq)→ NaHCO₃(aq)+ NH₄Cl(aq)
2 NaHCO₃(aq)→ Na₂CO₃(s)+ CO₂(g)+ H₂O(l)
What mass of soda ash is produced from the reaction of 2.50 L of CO₂ at STP?
A)3.95 g
B)5.92 g
C)11.8 g
D)23.7 g
E)38.5 g
NH₄HCO₃(aq)+ NaCl(aq)→ NaHCO₃(aq)+ NH₄Cl(aq)
2 NaHCO₃(aq)→ Na₂CO₃(s)+ CO₂(g)+ H₂O(l)
What mass of soda ash is produced from the reaction of 2.50 L of CO₂ at STP?
A)3.95 g
B)5.92 g
C)11.8 g
D)23.7 g
E)38.5 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
77
Given that 25.0 mL of 0.500 M AgNO₃ reacts completely with 34.5 mL of aqueous k₂CrO₄,what is the molarity of the potassium chromate solution? AgNO₃(aq)+ k₂CrO₄(aq)→ Ag2CrO₄(s)+ KNO₃(aq)
A)0.181 M
B)0.345 M
C)0.362 M
D)0.690 M
E)0.725 M
A)0.181 M
B)0.345 M
C)0.362 M
D)0.690 M
E)0.725 M

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
78
Given that 0.500 g of NaHCO₃ reacts completely with 0.100 M H₂SO₄,what is the volume of sulfuric acid consumed? NaHCO₃(s)+ H₂SO₄(aq)→ Na₂SO₄(aq)+ H₂O(l)+ CO₂(g)
A)5.95 mL
B)29.8 mL
C)59.5 mL
D)119 mL
E)238 mL
A)5.95 mL
B)29.8 mL
C)59.5 mL
D)119 mL
E)238 mL

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
79
Given that 0.500 g of NaHCO₃ reacts completely with 0.100 M H₂SO₄,what is the STP volume of carbon dioxide gas produced? NaHCO₃(s)+ H₂SO₄(aq)→ Na₂SO₄(aq)+ H₂O(l)+ CO₂(g)
A)5.95 mL
B)13.3 mL
C)66.7 mL
D)133 mL
E)267 mL
A)5.95 mL
B)13.3 mL
C)66.7 mL
D)133 mL
E)267 mL

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
80
Given the three-step "blast furnace" process that converts iron ore,Fe₂O₃,into molten "pig iron": 3 Fe₂O₃(l)+ CO(g)→ 2 Fe₃O₄(l)+ CO₂(g)
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
How many moles of Fe₂O₃ are required to produce 6.00 mol of molten iron?
A)3.00 mol
B)4.00 mol
C)6.00 mol
D)12.0 mol
E)none of the above
Fe₃O₄(l)+ CO(g)→ 3 FeO(l)+ CO₂(g)
FeO(l)+ CO(g)→ Fe(l)+ CO₂(g)
How many moles of Fe₂O₃ are required to produce 6.00 mol of molten iron?
A)3.00 mol
B)4.00 mol
C)6.00 mol
D)12.0 mol
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck


