Deck 13: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/120
Play
Full screen (f)
Deck 13: Solutions
1
What is the term for the concentration expression that relates the moles of solute dissolved in each liter of solution?
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above
molarity (M)
2
What is the term for the scattering of a beam of light by colloidal-size particles?
A)colloidal scattering
B)dispersion effect
C)Henry's law
D)Tyndall effect
E)none of the above
A)colloidal scattering
B)dispersion effect
C)Henry's law
D)Tyndall effect
E)none of the above
Tyndall effect
3
What is the term that refers to liquids that do not dissolve in one another and separate into two layers?
A)immiscible
B)insoluble
C)miscible
D)soluble
E)none of the above
A)immiscible
B)insoluble
C)miscible
D)soluble
E)none of the above
immiscible
4
What is the term for a cluster of solvent molecules surrounding a solute particle in solution?
A)colloid
B)dipole
C)solvent cage
D)Tyndall effect
E)none of the above
A)colloid
B)dipole
C)solvent cage
D)Tyndall effect
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
5
What is the term for a solution that contains the maximum solute that can dissolve at a given temperature?
A)concentrated
B)saturated
C)supersaturated
D)unsaturated
E)none of the above
A)concentrated
B)saturated
C)supersaturated
D)unsaturated
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
6
What is the term that refers to liquids that dissolve in one another?
A)immiscible
B)insoluble
C)miscible
D)soluble
E)none of the above
A)immiscible
B)insoluble
C)miscible
D)soluble
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
7
What is the term for the component of a solution that is the greater quantity?
A)primary component
B)secondary component
C)solute
D)solvent
E)none of the above
A)primary component
B)secondary component
C)solute
D)solvent
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
8
What is the term for a solution that contains more than the maximum solute that can dissolve at a given temperature?
A)concentrated
B)saturated
C)supersaturated
D)unsaturated
E)none of the above
A)concentrated
B)saturated
C)supersaturated
D)unsaturated
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
9
What principle states that the solubility of a gas in a liquid is proportional to the partial pressure of the gas above the liquid?
A)colloid principle
B)Henry's law
C)solubility principle
D)Tyndall effect
E)none of the above
A)colloid principle
B)Henry's law
C)solubility principle
D)Tyndall effect
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
10
What is the term for a solution that contains less than the maximum solute that can dissolve at a given temperature?
A)dilute
B)saturated
C)supersaturated
D)unsaturated
E)none of the above
A)dilute
B)saturated
C)supersaturated
D)unsaturated
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
11
What is the term for a region in a molecule having partial negative and partial positive charge resulting from a polar bond?
A)delta plus
B)delta minus
C)dipole
D)net dipole
E)none of the above
A)delta plus
B)delta minus
C)dipole
D)net dipole
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
12
What is the term for the component of a solution that is the lesser quantity?
A)primary component
B)secondary component
C)solute
D)solvent
E)none of the above
A)primary component
B)secondary component
C)solute
D)solvent
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
13
What is the term for the general principle that solubility is greatest when the polarity of the solute and solvent are similar?
A)like dissolves like rule
B)polarity rule
C)solute rule
D)solvent rule
E)none of the above
A)like dissolves like rule
B)polarity rule
C)solute rule
D)solvent rule
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
14
What is the term for a liquid composed of nonpolar molecules?
A)inorganic solvent
B)organic solvent
C)nonpolar solvent
D)polar solvent
E)none of the above
A)inorganic solvent
B)organic solvent
C)nonpolar solvent
D)polar solvent
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
15
What is the term for the overall direction of partial negative charge in a molecule having one or more dipoles?
A)charge dipole
B)directional dipole
C)electron dipole
D)net dipole
E)none of the above
A)charge dipole
B)directional dipole
C)electron dipole
D)net dipole
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
16
What is the term for the concentration expression that relates the mass of solute in grams dissolved in each 100 grams of solution?
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above
A)mass/mass percent (m/m %)
B)molarity (M)
C)molality (m)
D)parts per million (ppm)
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
17
What is the term for a homogeneous mixture in which the dispersed particles range from 1 to 100 nm in diameter?
A)colloid
B)saturated solution
C)supersaturated solution
D)true solution
E)none of the above
A)colloid
B)saturated solution
C)supersaturated solution
D)true solution
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
18
What is the term for a liquid composed of polar molecules?
A)inorganic solvent
B)organic solvent
C)nonpolar solvent
D)polar solvent
E)none of the above
A)inorganic solvent
B)organic solvent
C)nonpolar solvent
D)polar solvent
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
19
What is the term that refers to the maximum amount of a given solute that dissolves in a solvent at a specified temperature?
A)miscibility
B)polarizability
C)probability
D)solubility
E)none of the above
A)miscibility
B)polarizability
C)probability
D)solubility
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
20
What is the general term for a solute dissolved in a solvent?
A)colloid
B)mixture
C)solution
D)suspension
E)none of the above
A)colloid
B)mixture
C)solution
D)suspension
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
21
Apply the like dissolves like rule to predict which of the following vitamins is soluble in water.
A)thiamine,C12H17N4OS
B)riboflavin,C17H₂0N4O₆
C)niacinamide,C₆H₆N₂O
D)cyanocobalamin,C₆3H₈8CoN14O14P
E)all of the above
A)thiamine,C12H17N4OS
B)riboflavin,C17H₂0N4O₆
C)niacinamide,C₆H₆N₂O
D)cyanocobalamin,C₆3H₈8CoN14O14P
E)all of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following illustrates the like dissolves like rule for a solid solute in a liquid solvent?
A)An ionic compound is soluble in a nonpolar solvent.
B)A polar compound is soluble in a nonpolar solvent.
C)A nonpolar compound is soluble in a polar solvent.
D)all of the above
E)none of the above
A)An ionic compound is soluble in a nonpolar solvent.
B)A polar compound is soluble in a nonpolar solvent.
C)A nonpolar compound is soluble in a polar solvent.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
23
Apply the like dissolves like rule to predict which of the following liquids is miscible with liquid bromine,Br₂.
A)benzene,C₆H₆
B)carbon tetrachloride,CCl4
C)hexane,C₆H14
D)all of the above
E)none of the above
A)benzene,C₆H₆
B)carbon tetrachloride,CCl4
C)hexane,C₆H14
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
24
Yeast and sugar are added to champagne to give the sparkle of carbonation.Under what conditions is carbon dioxide gas most soluble?
A)low temperature,high pressure
B)low temperature,low pressure
C)high temperature,low pressure
D)high temperature,high pressure
E)none of the above
A)low temperature,high pressure
B)low temperature,low pressure
C)high temperature,low pressure
D)high temperature,high pressure
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following illustrates the like dissolves like rule for a solid solute in a liquid solvent?
A)An ionic compound is soluble in a nonpolar solvent.
B)A polar compound is soluble in a nonpolar solvent.
C)A nonpolar compound is soluble in a polar solvent.
D)A nonpolar compound is soluble in a nonpolar solvent.
E)none of the above
A)An ionic compound is soluble in a nonpolar solvent.
B)A polar compound is soluble in a nonpolar solvent.
C)A nonpolar compound is soluble in a polar solvent.
D)A nonpolar compound is soluble in a nonpolar solvent.
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following illustrates the like dissolves like rule for two liquids?
A)A polar solute is miscible with a nonpolar solvent.
B)A polar solute is immiscible with a polar solvent.
C)A nonpolar solute is miscible with a nonpolar solvent.
D)A nonpolar solvent is miscible with a polar solvent.
E)none of the above
A)A polar solute is miscible with a nonpolar solvent.
B)A polar solute is immiscible with a polar solvent.
C)A nonpolar solute is miscible with a nonpolar solvent.
D)A nonpolar solvent is miscible with a polar solvent.
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
27
Apply the like dissolves like rule to predict which of the following liquids is immiscible with water.
A)acetic acid,CH₃COOH
B)acetone,C₃H₆O
C)methanol,CH₃OH
D)all of the above
E)none of the above
A)acetic acid,CH₃COOH
B)acetone,C₃H₆O
C)methanol,CH₃OH
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
28
Apply the like dissolves like rule to predict which of the following solids is soluble in hexane,C₆H14.
A)potassium iodide,KI
B)potassium iodite,KIO₂
C)potassium iodate,KIO₃
D)potassium periodate,KIO₄
E)iodine,I₂
A)potassium iodide,KI
B)potassium iodite,KIO₂
C)potassium iodate,KIO₃
D)potassium periodate,KIO₄
E)iodine,I₂

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
29
Apply the like dissolves like rule to predict which of the following liquids is immiscible with liquid bromine,Br₂.
A)chloroform,CHCl3
B)toluene,C₆H5CH₃
C)water,H₂O
D)all of the above
E)none of the above
A)chloroform,CHCl3
B)toluene,C₆H5CH₃
C)water,H₂O
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
30
Pepsi-Cola is carbonated by injection with carbon dioxide gas.Under what conditions is carbon dioxide gas least soluble?
A)low temperature,low pressure
B)low temperature,high pressure
C)high temperature,high pressure
D)high temperature,low pressure
E)none of the above
A)low temperature,low pressure
B)low temperature,high pressure
C)high temperature,high pressure
D)high temperature,low pressure
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following illustrates the like dissolves like rule for two liquids?
A)A polar solute is miscible with a nonpolar solvent.
B)A polar solute is immiscible with a polar solvent.
C)A nonpolar solute is miscible with a polar solvent.
D)A nonpolar solute is immiscible with a nonpolar solvent.
E)none of the above
A)A polar solute is miscible with a nonpolar solvent.
B)A polar solute is immiscible with a polar solvent.
C)A nonpolar solute is miscible with a polar solvent.
D)A nonpolar solute is immiscible with a nonpolar solvent.
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
32
Coca-Cola is carbonated by injection with carbon dioxide gas.Under what conditions is carbon dioxide gas most soluble?
A)low temperature,low pressure
B)low temperature,high pressure
C)high temperature,high pressure
D)high temperature,low pressure
E)none of the above
A)low temperature,low pressure
B)low temperature,high pressure
C)high temperature,high pressure
D)high temperature,low pressure
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
33
Apply the like dissolves like rule to predict which of the following solids is soluble in water.
A)alanine (an amino acid),CH₃CH(NH₂)COOH
B)benzopyrene (in charcoal),C₂0H₁₂
C)DDT (an insecticide),C14H9Cl5
D)naphthalene (moth repellent),C10H₈
E)paradichlorobenzene (fumigant),C₆H₄Cl₂
A)alanine (an amino acid),CH₃CH(NH₂)COOH
B)benzopyrene (in charcoal),C₂0H₁₂
C)DDT (an insecticide),C14H9Cl5
D)naphthalene (moth repellent),C10H₈
E)paradichlorobenzene (fumigant),C₆H₄Cl₂

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following illustrates the like dissolves like rule for two liquids?
A)A polar solute is miscible with a polar solvent
B)A nonpolar solute is miscible with a nonpolar solvent
C)A polar solute is immiscible with a nonpolar solvent
D)all of the above
E)none of the above
A)A polar solute is miscible with a polar solvent
B)A nonpolar solute is miscible with a nonpolar solvent
C)A polar solute is immiscible with a nonpolar solvent
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
35
Apply the like dissolves like rule to predict which of the following vitamins is soluble in water.
A)retinol,C₂0H₃0O
B)ascorbic acid,C₆H₈O₆
C)calciferol,C₂7H₄4O
D)α-tocopherol,C₂9H50O₂
E)none of the above
A)retinol,C₂0H₃0O
B)ascorbic acid,C₆H₈O₆
C)calciferol,C₂7H₄4O
D)α-tocopherol,C₂9H50O₂
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
36
Apply the like dissolves like rule to predict which of the following solids is insoluble in water.
A)cholesterol,C₂7H₄6O
B)citric acid,C₆H₈O₇
C)fructose,C₆H₁₂O₆
D)glycine,CH₂(NH₂)COOH
E)lactic acid,CH₃CH(OH)COOH
A)cholesterol,C₂7H₄6O
B)citric acid,C₆H₈O₇
C)fructose,C₆H₁₂O₆
D)glycine,CH₂(NH₂)COOH
E)lactic acid,CH₃CH(OH)COOH

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
37
Apply the like dissolves like rule to predict which of the following liquids is miscible with water.
A)carbon tetrachloride,CCl4
B)ethanol,C₂H5OH
C)toluene,C7H₈
D)all of the above
E)none of the above
A)carbon tetrachloride,CCl4
B)ethanol,C₂H5OH
C)toluene,C7H₈
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
38
Apply the like dissolves like rule to predict which of the following liquids is miscible with water.
A)formic acid,HCHO₂
B)glycerin,C₃H5(OH)3
C)methyl ethyl ketone,C4H₈O
D)all of the above
E)none of the above
A)formic acid,HCHO₂
B)glycerin,C₃H5(OH)3
C)methyl ethyl ketone,C4H₈O
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
39
Apply the like dissolves like rule to predict which of the following liquids is miscible with liquid bromine,Br₂.
A)heptane,C7H16
B)methylene chloride,CH₂Cl₂
C)xylene,C8H10
D)all of the above
E)none of the above
A)heptane,C7H16
B)methylene chloride,CH₂Cl₂
C)xylene,C8H10
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following illustrates the like dissolves like rule for a solid solute in a liquid solvent?
A)An ionic compound is soluble in a polar solvent.
B)A polar compound is soluble in a polar solvent.
C)A nonpolar compound is soluble in a nonpolar solvent.
D)all of the above
E)none of the above
A)An ionic compound is soluble in a polar solvent.
B)A polar compound is soluble in a polar solvent.
C)A nonpolar compound is soluble in a nonpolar solvent.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
41
What is the mass of a 10.0% blood plasma sample that contains 2.50 g of dissolved solute?
A)0.250 g
B)0.278 g
C)22.5 g
D)25.0 g
E)250 g
A)0.250 g
B)0.278 g
C)22.5 g
D)25.0 g
E)250 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
42
When sodium bromide,NaBr,dissolves in water,which of the following is formed in aqueous solution?
A)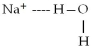
B)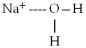
C)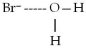
D)all of the above
E)none of the above
A)
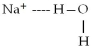
B)
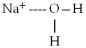
C)
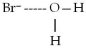
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is not a unit factor related to a 10.0% aqueous solution of sodium hydroxide,NaOH?
A)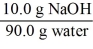
B)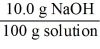
C)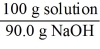
D)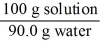
E)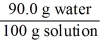
A)
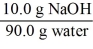
B)
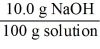
C)
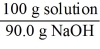
D)
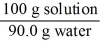
E)
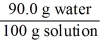

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
44
If 25.0 mL of urine has a mass of 25.725 g and contains 1.929 g of solute,what is the mass/mass percent concentration of solute in the urine sample?
A)3.887%
B)7.499%
C)7.72%
D)13.34%
E)97.2%
A)3.887%
B)7.499%
C)7.72%
D)13.34%
E)97.2%

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
45
If 25.0 mL of seawater has a mass of 25.895 g and contains 1.295 g of solute,what is the mass/mass percent concentration of solute in the seawater sample?
A)3.862%
B)5.001%
C)5.18%
D)20.00%
E)96.5%
A)3.862%
B)5.001%
C)5.18%
D)20.00%
E)96.5%

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
46
What is the mass of a 12.5% blood plasma sample that contains 10.0 g of dissolved solute?
A)1.25 g
B)1.43 g
C)70.0 g
D)80.0 g
E)125 g
A)1.25 g
B)1.43 g
C)70.0 g
D)80.0 g
E)125 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
47
Apply the like dissolves like rule to predict which of the following vitamins is insoluble in water.
A)retinol,C₂0H₃0O
B)thiamine,C12H17N4OS
C)niacinamide,C₆H₆N₂O
D)pyridoxine,C8H11NO₃
E)cyanocobalamin,C₆3H₈8CoN14O14P
A)retinol,C₂0H₃0O
B)thiamine,C12H17N4OS
C)niacinamide,C₆H₆N₂O
D)pyridoxine,C8H11NO₃
E)cyanocobalamin,C₆3H₈8CoN14O14P

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following decreases the rate of dissolving for a solid solute in a solvent?
A)grinding the solute
B)heating the solution
C)stirring the solution
D)all of the above
E)none of the above
A)grinding the solute
B)heating the solution
C)stirring the solution
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is not a unit factor related to a 15.0% aqueous solution of potassium iodide,KI?
A)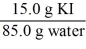
B)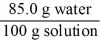
C)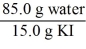
D)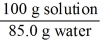
E)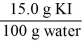
A)
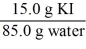
B)
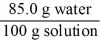
C)
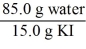
D)
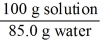
E)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
50
When fructose crystals,C₆H₁₂O₆,dissolve in water,which of the following is formed in solution?
A)hydrated clusters of C₆H₁₂O₆
B)hydrated clusters of CO₂
C)hydrated clusters of H₂O
D)hydrated clusters of H₂CO₃
E)none of the above
A)hydrated clusters of C₆H₁₂O₆
B)hydrated clusters of CO₂
C)hydrated clusters of H₂O
D)hydrated clusters of H₂CO₃
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is not a unit factor related to a 5.00% aqueous solution of lithium chloride,LiCl?
A)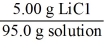
B)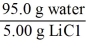
C)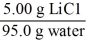
D)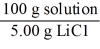
E)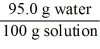
A)
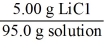
B)
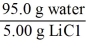
C)
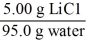
D)
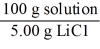
E)
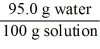

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
52
What is the mass of solute dissolved in 10.0 g of 5.00% sugar solution?
A)0.180 g
B)0.500 g
C)0.900 g
D)9.50 g
E)10.0 g
A)0.180 g
B)0.500 g
C)0.900 g
D)9.50 g
E)10.0 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following increases the rate of dissolving for a solid solute in a solvent?
A)grinding the solute
B)heating the solution
C)stirring the solution
D)all of the above
E)none of the above
A)grinding the solute
B)heating the solution
C)stirring the solution
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
54
What is the mass of a 7.50% urine sample that contains 122 g of dissolved solute?
A)9.15 g
B)9.89 g
C)915 g
D)1500 g
E)1630 g
A)9.15 g
B)9.89 g
C)915 g
D)1500 g
E)1630 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
55
When vitamin C crystals,C₆H₈O₆,dissolve in water,which of the following is formed in solution?
A)hydrated clusters of C₆H₈O₆
B)hydrated clusters of CO₂
C)hydrated clusters of H₂O
D)hydrated clusters of H₂CO₃
E)none of the above
A)hydrated clusters of C₆H₈O₆
B)hydrated clusters of CO₂
C)hydrated clusters of H₂O
D)hydrated clusters of H₂CO₃
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
56
Why does grinding solid crystals increase the rate of dissolving for a solid solute in water?
A)Smaller crystals have more surface area.
B)Smaller crystals have more energy.
C)Smaller crystals are more nonpolar.
D)Smaller crystals are more polar.
E)none of the above
A)Smaller crystals have more surface area.
B)Smaller crystals have more energy.
C)Smaller crystals are more nonpolar.
D)Smaller crystals are more polar.
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
57
When citric acid crystals,C₆H₈O₇,dissolve in water,which of the following is formed in solution?
A)hydrated clusters of C₆H₈O₇
B)hydrated clusters of CO₂
C)hydrated clusters of H₂O
D)hydrated clusters of H₂CO₃
E)none of the above
A)hydrated clusters of C₆H₈O₇
B)hydrated clusters of CO₂
C)hydrated clusters of H₂O
D)hydrated clusters of H₂CO₃
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
58
If 10.0 mL of blood plasma has a mass of 10.279 g and contains 0.870 g of protein,what is the mass/mass percent concentration of protein in the blood plasma?
A)0.870%
B)8.46%
C)8.70%
D)32.1%
E)97.3%
A)0.870%
B)8.46%
C)8.70%
D)32.1%
E)97.3%

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
59
When lithium chloride,LiCl,dissolves in water,which of the following is formed in aqueous solution?
A)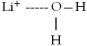
B)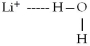
C)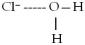
D)all of the above
E)none of the above
A)
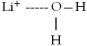
B)
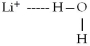
C)
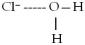
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
60
When potassium iodide,KI,dissolves in water,which of the following is formed in aqueous solution?
A)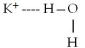
B)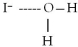
C)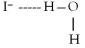
D)all of the above
E)none of the above
A)
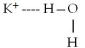
B)
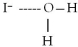
C)
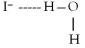
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
61
What is the molarity of a hydrochloric acid solution prepared by diluting 250.0 mL of 6.00 M HCl to a total volume of 2.50 L?
A)0.0600 M
B)0.250 M
C)0.600 M
D)2.50 M
E)6.00 M
A)0.0600 M
B)0.250 M
C)0.600 M
D)2.50 M
E)6.00 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following unit factors is related to a 0.500 M LiF solution?
A)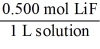
B)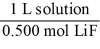
C)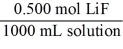
D)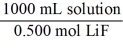
E)all of the above
A)
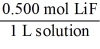
B)
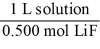
C)
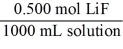
D)
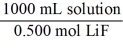
E)all of the above

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
63
What is the volume of 6.00 M nitric acid that contains 6.302 g of HNO₃ solute (63.02 g/mol)?
A)0.600 mL
B)16.7 mL
C)60.0 mL
D)167 mL
E)1670 mL
A)0.600 mL
B)16.7 mL
C)60.0 mL
D)167 mL
E)1670 mL

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
64
What is the molarity of a hydrochloric acid solution prepared by diluting 500.0 mL of 1.00 M HCl to a total volume of 2.50 L?
A)0.100 M
B)0.200 M
C)0.500 M
D)2.00 M
E)5.00 M
A)0.100 M
B)0.200 M
C)0.500 M
D)2.00 M
E)5.00 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
65
What is the mass of barium hydroxide (171.35 g/mol)dissolved in 0.500 L of 0.100 M Ba(OH)2 solution?
A)8.57 g
B)17.1 g
C)85.7 g
D)171 g
E)857 g
A)8.57 g
B)17.1 g
C)85.7 g
D)171 g
E)857 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
66
What is the molarity of a hydrochloric acid solution prepared by diluting 200.0 mL of 0.500 M HCl to a total volume of 1.00 L?
A)0.100 M
B)0.200 M
C)0.250 M
D)1.00 M
E)2.50 M
A)0.100 M
B)0.200 M
C)0.250 M
D)1.00 M
E)2.50 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following unit factors is not related to a 0.250 M KOH solution?
A)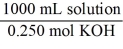
B)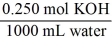
C)
D)
E)
A)
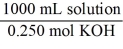
B)
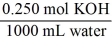
C)

D)

E)


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
68
What is the mass of water required to prepare 5.25 kg of 15.0% calcium nitrate solution?
A)0.788 kg
B)0.926 kg
C)4.46 kg
D)6.18 kg
E)29.8 kg
A)0.788 kg
B)0.926 kg
C)4.46 kg
D)6.18 kg
E)29.8 kg

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
69
What is the mass of water required to prepare 250.0 g of 10.0% copper(II)sulfate solution?
A)25.0 g
B)27.8 g
C)225 g
D)278 g
E)2250 g
A)25.0 g
B)27.8 g
C)225 g
D)278 g
E)2250 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
70
Permanent antifreeze contains ethylene glycol dissolved in water.What is the mass of ethylene glycol dissolved in 5.00 kg of a 40.0% solution?
A)2.00 kg
B)3.33 kg
C)7.5 kg
D)12.5 kg
E)200 kg
A)2.00 kg
B)3.33 kg
C)7.5 kg
D)12.5 kg
E)200 kg

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
71
What is the mass of nickel(II)nitrate (182.71 g/mol)dissolved in 25.0 mL of 0.100 M Ni(NO₃)2 solution?
A)0.250 g
B)0.457 g
C)4.00 g
D)45.7 g
E)457 g
A)0.250 g
B)0.457 g
C)4.00 g
D)45.7 g
E)457 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
72
What is the molarity of a glucose solution that contains 10.0 g of C₆H₁₂O₆ (180.18 g/mol)dissolved in 100.0 mL of solution?
A)0.00555 M
B)0.0555 M
C)0.555 M
D)1.80 M
E)18.0 M
A)0.00555 M
B)0.0555 M
C)0.555 M
D)1.80 M
E)18.0 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
73
What is the mass of water required to prepare 50.0 g of 10.0% sodium nitrate solution?
A)5.00 g
B)5.56 g
C)45.0 g
D)55.6 g
E)450 g
A)5.00 g
B)5.56 g
C)45.0 g
D)55.6 g
E)450 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
74
What is the molarity of a sucrose solution that contains 10.0 g of C12H₂2O11 (342.34 g/mol)dissolved in 100.0 mL of solution?
A)0.00292 M
B)0.0292 M
C)0.292 M
D)3.42 M
E)34.2 M
A)0.00292 M
B)0.0292 M
C)0.292 M
D)3.42 M
E)34.2 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
75
What is the volume of 12.0 M hydrochloric acid that contains 3.646 g of HCl solute (36.46 g/mol)?
A)1.20 mL
B)8.33 mL
C)83.3 mL
D)120 mL
E)833 mL
A)1.20 mL
B)8.33 mL
C)83.3 mL
D)120 mL
E)833 mL

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following unit factors is not related to a 0.100 M NaBr solution?
A)
B)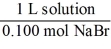
C)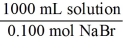
D)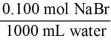
E)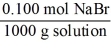
A)

B)
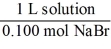
C)
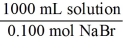
D)
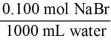
E)
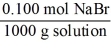

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
77
What is the mass of zinc acetate (183.49 g/mol)dissolved in 0.200 L of 0.500 M Zn(C₂H₃O₂)2 solution?
A)1.83 g
B)12.4 g
C)18.3 g
D)36.7 g
E)91.7 g
A)1.83 g
B)12.4 g
C)18.3 g
D)36.7 g
E)91.7 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
78
What is the volume of 3.00 M sulfuric acid that contains 9.809 g of H₂SO₄ solute (98.09 g/mol)?
A)0.300 mL
B)30.0 mL
C)33.3 mL
D)333 mL
E)3330 mL
A)0.300 mL
B)30.0 mL
C)33.3 mL
D)333 mL
E)3330 mL

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
79
What is the mass of solute dissolved in 50.0 g of 12.5% saline solution?
A)4.00 g
B)6.25 g
C)7.14 g
D)43.8 g
E)625 g
A)4.00 g
B)6.25 g
C)7.14 g
D)43.8 g
E)625 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
80
What is the molarity of a saline solution that contains 0.900 g NaCl (58.44 g/mol)dissolved in 100.0 mL of solution?
A)0.154 M
B)0.527 M
C)1.54 M
D)1.90 M
E)5.27 M
A)0.154 M
B)0.527 M
C)1.54 M
D)1.90 M
E)5.27 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck


