Deck 10: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/156
Play
Full screen (f)
Deck 10: Gases
1
What is the term for a gas that deviates from ideal behavior under conditions of low temperature and high pressure?
A)ideal gas
B)perfect gas
C)real gas
D)theoretical gas
E)none of the above
A)ideal gas
B)perfect gas
C)real gas
D)theoretical gas
E)none of the above
real gas
2
What is the term for a gas that obeys the kinetic theory under all conditions?
A)ideal gas
B)perfect gas
C)real gas
D)theoretical gas
E)none of the above
A)ideal gas
B)perfect gas
C)real gas
D)theoretical gas
E)none of the above
ideal gas
3
Which of the following is described by the equation: PV = nRT?
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)ideal gas law
E)none of the above
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)ideal gas law
E)none of the above
ideal gas law
4
Which of the following states that the volume and Kelvin temperature are directly proportional for a gas at constant pressure?
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following instruments measures atmospheric pressure?
A)aerometer
B)barometer
C)manometer
D)spectrophotometer
E)none of the above
A)aerometer
B)barometer
C)manometer
D)spectrophotometer
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
6
What principle states that equal volumes of gases,at the same temperature and pressure,contain equal numbers of molecules?
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above
A)Avogadro's theory
B)law of combining volumes
C)law of conservation of mass
D)law of constant composition
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
7
What is the term for a gas at 273 K and 760 mm Hg pressure?
A)atmospheric temperature and pressure
B)experimental temperature and pressure
C)ideal gas temperature and pressure
D)standard temperature and pressure
E)none of the above
A)atmospheric temperature and pressure
B)experimental temperature and pressure
C)ideal gas temperature and pressure
D)standard temperature and pressure
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
8
What is the term that describes the relationship between two variables such that when one variable doubles,the other variable doubles?
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following laws states that the pressure and Kelvin temperature are directly proportional for a gas at constant volume?
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
10
What is the term that refers to the frequency and energy of gas molecules colliding with the walls of the container?
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following states that the pressure exerted by a gas is inversely proportional to its volume and directly proportional to its Kelvin temperature?
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)combined gas law
E)none of the above
A)Boyle's law
B)Charles's law
C)Gay-Lussac's law
D)combined gas law
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
12
What is the term that describes the relationship between two variables such that when one variable doubles,the other variable halves?
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above
A)directly proportional
B)inversely proportional
C)reciprocally proportional
D)theoretically proportional
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
13
What is the term for the pressure exerted by the gas molecules in air?
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
14
What is the term for the pressure exerted by an individual gas in a mixture of two or more gases?
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following states that the pressure and volume are inversely proportional for a gas at constant temperature?
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
16
What is the theoretical temperature at which the kinetic energy of a gas is zero?
A)absolute zero
B)-100 °C
C)-273 °F
D)-273 K
E)none of the above
A)absolute zero
B)-100 °C
C)-273 °F
D)-273 K
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following states that the pressure exerted by a mixture of gases is equal to the sum of the individual gas pressures?
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above
A)Boyle's law
B)Charles's law
C)Dalton's law
D)Gay-Lussac's law
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
18
What is the term that describes gas molecules demonstrating ideal behavior?
A)gaseous state theory
B)ideal theory
C)kinetic theory
D)molecular theory
E)none of the above
A)gaseous state theory
B)ideal theory
C)kinetic theory
D)molecular theory
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
19
What is the term for gas molecules colliding with no change in total energy?
A)elastic collision
B)isokinetic collision
C)isothermal collision
D)molecular collision
E)none of the above
A)elastic collision
B)isokinetic collision
C)isothermal collision
D)molecular collision
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
20
What is the term for the constant R in the equation PV = nRT?
A)combined gas constant
B)ideal gas constant
C)real gas constant
D)universal gas constant
E)none of the above
A)combined gas constant
B)ideal gas constant
C)real gas constant
D)universal gas constant
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
21
If a steel scuba tank contains compressed air at 2250 psi,what is the pressure expressed in atmospheres?
A)2.96 atm
B)29.6 atm
C)75.3 atm
D)153 atm
E)3.31 x 104 atm
A)2.96 atm
B)29.6 atm
C)75.3 atm
D)153 atm
E)3.31 x 104 atm

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
22
If a gas pressure gauge reads 31.6 in.Hg,what is the pressure in millimeters of mercury?
A)1.24 mm Hg
B)12.4 mm Hg
C)80.3 mm Hg
D)719 mm Hg
E)803 mm Hg
A)1.24 mm Hg
B)12.4 mm Hg
C)80.3 mm Hg
D)719 mm Hg
E)803 mm Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following increases the pressure of a gas?
A)decreasing the volume
B)increasing temperature
C)increasing the number of molecules
D)all of the above
E)none of the above
A)decreasing the volume
B)increasing temperature
C)increasing the number of molecules
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
24
If a barometer reads 772 mm Hg,what is the atmospheric pressure expressed in pounds per square inch?
A)14.9 psi
B)30.4 psi
C)149 psi
D)3990 psi
E)39,900 psi
A)14.9 psi
B)30.4 psi
C)149 psi
D)3990 psi
E)39,900 psi

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is not an observed property of gases?
A)gases have a variable volume
B)gases expand to fill the container
C)gases compress infinitely
D)gases have a low density
E)gases mix uniformly
A)gases have a variable volume
B)gases expand to fill the container
C)gases compress infinitely
D)gases have a low density
E)gases mix uniformly

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is not an observed property of gases?
A)gases have a variable shape
B)gases expand uniformly
C)gases compress and liquefy
D)gases have a high density
E)gases mix uniformly
A)gases have a variable shape
B)gases expand uniformly
C)gases compress and liquefy
D)gases have a high density
E)gases mix uniformly

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following expresses standard atmospheric pressure?
A)29.9 in.Hg
B)76.0 cm Hg
C)760 mm Hg
D)14.7 psi
E)all of the above
A)29.9 in.Hg
B)76.0 cm Hg
C)760 mm Hg
D)14.7 psi
E)all of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
28
If a barometer reads 76.5 cm Hg,what is the atmospheric pressure expressed in millimeters of mercury?
A)0.765 mm Hg
B)7.65 mm Hg
C)76.5 mm Hg
D)765 mm Hg
E)7650 mm Hg
A)0.765 mm Hg
B)7.65 mm Hg
C)76.5 mm Hg
D)765 mm Hg
E)7650 mm Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is an observed property of gases?
A)gases have a variable shape
B)gases expand uniformly
C)gases compress uniformly
D)gases mix uniformly
E)all of the above
A)gases have a variable shape
B)gases expand uniformly
C)gases compress uniformly
D)gases mix uniformly
E)all of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
30
What is the term for the pressure exerted by gaseous molecules above a liquid in a sealed container when the rates of vaporization and condensation are equal?
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above
A)atmospheric pressure
B)gas pressure
C)partial pressure
D)vapor pressure
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is a unit of pressure equal to 1 mm Hg?
A)1 atm
B)1 kPa
C)1 psi
D)1 torr
E)none of the above
A)1 atm
B)1 kPa
C)1 psi
D)1 torr
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following does not express standard atmospheric pressure?
A)29.9 in.Hg
B)76 cm Hg
C)760 torr
D)29.9 psi
E)101 kPa
A)29.9 in.Hg
B)76 cm Hg
C)760 torr
D)29.9 psi
E)101 kPa

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following best describes the term vacuum?
A)attractive force
B)negative pressure
C)no gas molecules
D)suction force
E)none of the above
A)attractive force
B)negative pressure
C)no gas molecules
D)suction force
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
34
If a gas pressure gauge reads 15 mm Hg,what is the pressure in atmospheres?
A)0.020 atm
B)0.20 atm
C)15 atm
D)1100 atm
E)11,000 atm
A)0.020 atm
B)0.20 atm
C)15 atm
D)1100 atm
E)11,000 atm

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
35
If a barometer reads 535 mm Hg,what is the atmospheric pressure expressed in centimeters of mercury?
A)5.35 cm Hg
B)53.5 cm Hg
C)535 cm Hg
D)5350 cm Hg
E)53,500 cm Hg
A)5.35 cm Hg
B)53.5 cm Hg
C)535 cm Hg
D)5350 cm Hg
E)53,500 cm Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following does not express standard atmospheric pressure?
A)29.9 in.Hg
B)760 cm Hg
C)760 torr
D)14.7 psi
E)101 kPa
A)29.9 in.Hg
B)760 cm Hg
C)760 torr
D)14.7 psi
E)101 kPa

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
37
If a gas pressure gauge reads 32.0 psi,what is the pressure in inches of mercury?
A)6.19 in.Hg
B)15.7 in.Hg
C)32.0 in.Hg
D)65.1 in.Hg
E)165 in.Hg
A)6.19 in.Hg
B)15.7 in.Hg
C)32.0 in.Hg
D)65.1 in.Hg
E)165 in.Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
38
What is the term for the technique of determining the volume of a gas by measuring the volume of water it displaces?
A)displacement method
B)volume method
C)volume by displacement
D)volume by difference
E)none of the above
A)displacement method
B)volume method
C)volume by displacement
D)volume by difference
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
39
If a vacuum pump reduces the pressure of a gas to 1.0 x 10-6 atm,what is the pressure expressed in millimeters of mercury?
A)1.3 x 10-9 mm Hg
B)1.3 x 10-8 mm Hg
C)3.0 x 10-5 mm Hg
D)7.6 x 10-5 mm Hg
E)7.6 x 10-4 mm Hg
A)1.3 x 10-9 mm Hg
B)1.3 x 10-8 mm Hg
C)3.0 x 10-5 mm Hg
D)7.6 x 10-5 mm Hg
E)7.6 x 10-4 mm Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
40
If an aluminum scuba tank contains compressed air at 2750 psi,what is the pressure expressed in inches of mercury?
A)92.0 in.Hg
B)187 in.Hg
C)5590 in.Hg
D)8.22 x 104 in.Hg
E)1.21 x 106 in.Hg
A)92.0 in.Hg
B)187 in.Hg
C)5590 in.Hg
D)8.22 x 104 in.Hg
E)1.21 x 106 in.Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
41
A 5.00 mL volume of methane gas is cooled from 60.0 °C to 30.0 °C.If the pressure remains constant,what is the final volume in milliliters?
A)2.50 mL
B)4.55 mL
C)5.00 mL
D)5.50 mL
E)10.0 mL
A)2.50 mL
B)4.55 mL
C)5.00 mL
D)5.50 mL
E)10.0 mL

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
42
If a volume of nitrogen gas at 452 K decreases from 100.0 mL to 50.0 mL,what is the final Kelvin temperature? Assume pressure remains constant.
A)177 K
B)226 K
C)362 K
D)723 K
E)904 K
A)177 K
B)226 K
C)362 K
D)723 K
E)904 K

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
43
A 5.00 L volume of ethane gas is heated from 298 K to 596 K.If the pressure remains constant,what is the final volume in liters?
A)2.50 L
B)4.58 L
C)5.00 L
D)5.46 L
E)10.0 L
A)2.50 L
B)4.58 L
C)5.00 L
D)5.46 L
E)10.0 L

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
44
If a volume of air at 375 K increases from 10.0 L to 15.0 L,what is the final Kelvin temperature? Assume pressure remains constant.
A)153 K
B)250 K
C)344 K
D)375 K
E)563 K
A)153 K
B)250 K
C)344 K
D)375 K
E)563 K

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following represents the graph of pressure versus volume for a gas at constant temperature? 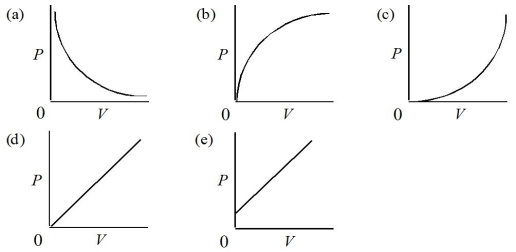
A)Graph (a)
B)Graph (b)
C)Graph (c)
D)Graph (d)
E)Graph (e)
A)Graph (a)
B)Graph (b)
C)Graph (c)
D)Graph (d)
E)Graph (e)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
46
A sample of krypton gas at 75.0 psi and 100 °C expands from 0.100 L to 0.450 L.Ifthe temperature remains constant,what is the final pressure in psi?
A)0.167 psi
B)3.38 psi
C)16.7 psi
D)75.0 psi
E)338 psi
A)0.167 psi
B)3.38 psi
C)16.7 psi
D)75.0 psi
E)338 psi

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following decreases the pressure of a gas?
A)decreasing the volume
B)increasing the temperature
C)increasing the number of gas molecules
D)all of the above
E)none of the above
A)decreasing the volume
B)increasing the temperature
C)increasing the number of gas molecules
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
48
A sample of propane gas occupies 625 cm3 at 20.0 °C and 750 torr.What is the final volume in cubic centimeters at -80.0 °C and 750 torr?
A)156 cm3
B)412 cm3
C)519 cm3
D)949 cm3
E)2500 cm3
A)156 cm3
B)412 cm3
C)519 cm3
D)949 cm3
E)2500 cm3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following decreases the pressure of a gas?
A)increasing the volume
B)increasing the temperature
C)increasing the number of gas molecules
D)all of the above
E)none of the above
A)increasing the volume
B)increasing the temperature
C)increasing the number of gas molecules
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
50
If the pressure of 125 cm3 of nitrogen gas at 100 °C decreases from 885 torr to 225torr,what is the final volume? Assume temperature remains constant.
A)0.318 cm3
B)4.92 cm3
C)31.8 cm3
D)492 cm3
E)4590 cm3
A)0.318 cm3
B)4.92 cm3
C)31.8 cm3
D)492 cm3
E)4590 cm3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
51
A sample of neon gas at 1.20 atm compresses from 0.250 L to 0.125 L.If the temperature remains constant,what is the final pressure in atm?
A)0.600 atm
B)1.00 atm
C)1.20 atm
D)2.40 atm
E)none of the above
A)0.600 atm
B)1.00 atm
C)1.20 atm
D)2.40 atm
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following represents the graph of volume versus Kelvin temperature for a gas at constant pressure? 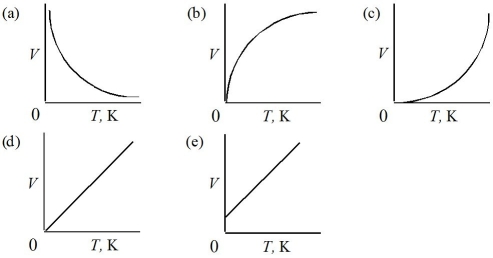
A)Graph (a)
B)Graph (b)
C)Graph (c)
D)Graph (d)
E)Graph (e)

A)Graph (a)
B)Graph (b)
C)Graph (c)
D)Graph (d)
E)Graph (e)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
53
A 5.00 L volume of methane gas is cooled from 298 K to 149 K.If the pressure remains constant,what is the final volume in liters?
A)2.50 L
B)4.58 L
C)5.00 L
D)5.46 L
E)10.0 L
A)2.50 L
B)4.58 L
C)5.00 L
D)5.46 L
E)10.0 L

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following decreases the pressure of a gas?
A)increasing the volume
B)decreasing the temperature
C)decreasing the number of gas molecules
D)all of the above
E)none of the above
A)increasing the volume
B)decreasing the temperature
C)decreasing the number of gas molecules
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following increases the pressure of a gas?
A)increasing the volume
B)decreasing the temperature
C)decreasing the number of gas molecules
D)all of the above
E)none of the above
A)increasing the volume
B)decreasing the temperature
C)decreasing the number of gas molecules
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
56
If the pressure of 1.50 L of hydrogen gas at 100 °C decreases from 0.500 atm to 0.115 atm,what is the final volume? Assume temperature remains constant.
A)0.345 L
B)0.652 L
C)1.50 L
D)3.45 L
E)6.52 L
A)0.345 L
B)0.652 L
C)1.50 L
D)3.45 L
E)6.52 L

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
57
If the pressure of 50.0 mL of oxygen gas at 100 °C increases from 735 mm Hg to 925 mm Hg,what is the final volume? Assume temperature remains constant.
A)39.7 mL
B)48.4 mL
C)50.0 mL
D)51.7 mL
E)62.9 mL
A)39.7 mL
B)48.4 mL
C)50.0 mL
D)51.7 mL
E)62.9 mL

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
58
A 40.0 mL volume of ethane gas is heated from 25.0 °C to 50.0 °C.If the pressure remains constant,what is the final volume in milliliters?
A)20.0 mL
B)36.9 mL
C)40.0 mL
D)43.4 mL
E)80.0 mL
A)20.0 mL
B)36.9 mL
C)40.0 mL
D)43.4 mL
E)80.0 mL

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
59
A sample of argon gas at 520 mm Hg expands from 0.150 L to 0.300 L.If the temperature remains constant,what is the final pressure in mm Hg?
A)260 mm Hg
B)520 mm Hg
C)760 mm Hg
D)1040 mm Hg
E)none of the above
A)260 mm Hg
B)520 mm Hg
C)760 mm Hg
D)1040 mm Hg
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following increases the pressure of a gas?
A)decreasing the volume
B)decreasing the temperature
C)decreasing the number of gas molecules
D)all of the above
E)none of the above
A)decreasing the volume
B)decreasing the temperature
C)decreasing the number of gas molecules
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
61
A sample of hydrogen bromide gas occupies 45.5 cm3 at 30.0 °C and 30.0 in.Hg.What is the final Celsius temperature if the volume is 45.5 cm3 at 15.0 in.Hg?
A)-121 °C
B)15 °C
C)60 °C
D)152 °C
E)333 °C
A)-121 °C
B)15 °C
C)60 °C
D)152 °C
E)333 °C

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
62
A sample of hydrogen sulfide gas occupies 205 mL at 25.0 °C and 319 mm Hg.If the volume remains constant,what is the final pressure of the gas at -75.0 °C?
A)106 mm Hg
B)182 mm Hg
C)212 mm Hg
D)480 mm Hg
E)957 mm Hg
A)106 mm Hg
B)182 mm Hg
C)212 mm Hg
D)480 mm Hg
E)957 mm Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
63
If a volume of nitrous oxide gas at 45.0 °C decreases from 50.0 mL to 25.0 mL,what is the final Celsius temperature? Assume pressure remains constant.
A)-114 °C
B)23 °C
C)159 °C
D)363 °C
E)636 °C
A)-114 °C
B)23 °C
C)159 °C
D)363 °C
E)636 °C

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
64
The pressure of a neon gas sample at 25 °C decreases from 1.00 atm to 0.500 atm.What is the final Celsius temperature if the volume remains constant?
A)-124 °C
B)149 °C
C)323 °C
D)422 °C
E)596 °C
A)-124 °C
B)149 °C
C)323 °C
D)422 °C
E)596 °C

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
65
A sample of nitrogen dioxide gas occupies 625 cm3 at 70.0 °C and 15.0 psi.What is the final Celsius temperature if the volume is 545 cm3 at 15.0 psi?
A)26 °C
B)61 °C
C)80 °C
D)120 °C
E)299 °C
A)26 °C
B)61 °C
C)80 °C
D)120 °C
E)299 °C

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
66
A sample of krypton gas at 3.50 atm is heated from 20.0 °C to 150.0 °C.If the volume remains constant,what is the final pressure?
A)0.467 atm
B)1.79 atm
C)2.42 atm
D)5.05 atm
E)26.3 atm
A)0.467 atm
B)1.79 atm
C)2.42 atm
D)5.05 atm
E)26.3 atm

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
67
A sample of ozone gas occupies 225 mL at 1.00 atm and 0 °C.If the volume of the gas is 625 mL at 25 °C,what is the pressure?
A)0.330 atm
B)0.360 atm
C)0.393 atm
D)2.54 atm
E)3.03 atm
A)0.330 atm
B)0.360 atm
C)0.393 atm
D)2.54 atm
E)3.03 atm

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
68
The pressure of sulfur trioxide gas at 25 °C increases from 0.500 atm to 1.00 atm.What is the final Celsius temperature if the volume remains constant?
A)-124 °C
B)149 °C
C)323 °C
D)422 °C
E)596 °C
A)-124 °C
B)149 °C
C)323 °C
D)422 °C
E)596 °C

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
69
If a volume of nitric oxide gas at 25.0 °C increases from 2.00 L to 3.00 L,what is the final Celsius temperature? Assume pressure remains constant.
A)-74 °C
B)17 °C
C)38 °C
D)174 °C
E)199 °C
A)-74 °C
B)17 °C
C)38 °C
D)174 °C
E)199 °C

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
70
If 5.00 L of argon gas is at 0.460 atm and -123 °C,what is the volume at STP?
A)4.19 L
B)4.94 L
C)5.06 L
D)5.49 L
E)5.97 L
A)4.19 L
B)4.94 L
C)5.06 L
D)5.49 L
E)5.97 L

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
71
A sample of carbon monoxide gas occupies 125 mL at 20.0 °C and 455 mm Hg.If the volume of gas is 55.5 mL at 898 mm Hg,what is the Celsius temperature?
A)-207 °C
B)-16 °C
C)18 °C
D)257 °C
E)1030 °C
A)-207 °C
B)-16 °C
C)18 °C
D)257 °C
E)1030 °C

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
72
The pressure of an air sample at 190 K increases from 415 mm Hg to 830 mm Hg.What is the final Kelvin temperature if the volume remains constant?
A)-42K
B)-166K
C)95K
D)380K
E)653K
A)-42K
B)-166K
C)95K
D)380K
E)653K

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
73
A sample of air at 7.50 atm is heated from 224K to 448K.If the volume remains constant,what is the final pressure?
A)3.75 atm
B)4.57 atm
C)6.15 atm
D)12.3 atm
E)15.0 atm
A)3.75 atm
B)4.57 atm
C)6.15 atm
D)12.3 atm
E)15.0 atm

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
74
If a 50.0 mL sample of xenon gas is at 0.921 atm and 27 °C,what is the volume of the gas at STP?
A)41.9 mL
B)49.4 mL
C)50.6 mL
D)54.9 mL
E)59.7 mL
A)41.9 mL
B)49.4 mL
C)50.6 mL
D)54.9 mL
E)59.7 mL

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
75
A sample of laughing gas occupies 0.250 L at 14.7 psi and -80.0 °C.If the volume of the gas is 0.375 L at 25.0 °C,what is the pressure?
A)6.35 psi
B)14.3 psi
C)15.1 psi
D)31.4 psi
E)34.0 psi
A)6.35 psi
B)14.3 psi
C)15.1 psi
D)31.4 psi
E)34.0 psi

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
76
If 7.75 L of radon gas is at 1.55 atm and -19 °C,what is the volume at STP?
A)4.65 L
B)5.37 L
C)8.33 L
D)11.2 L
E)12.9 L
A)4.65 L
B)5.37 L
C)8.33 L
D)11.2 L
E)12.9 L

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
77
A sample of air at 7.50 atm is cooled from 448K to 224K.If the volume remains constant,what is the final pressure?
A)3.75 atm
B)4.57 atm
C)6.15 atm
D)12.3 atm
E)15.0 atm
A)3.75 atm
B)4.57 atm
C)6.15 atm
D)12.3 atm
E)15.0 atm

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
78
A sample of xenon gas at 786 mm Hg is cooled from 100.0 °C to 50.0 °C.If the volume remains constant,what is the final pressure?
A)153 mm Hg
B)393 mm Hg
C)681 mm Hg
D)908 mm Hg
E)1570 mm Hg
A)153 mm Hg
B)393 mm Hg
C)681 mm Hg
D)908 mm Hg
E)1570 mm Hg

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
79
A sample of ammonia gas occupies 20.0 mL at 585 torr and 20.0 °C.If the volume of the gas is 50.0 mL at 50.0 °C,what is the pressure?
A)212 torr
B)258 torr
C)585 torr
D)1330 torr
E)1610 torr
A)212 torr
B)258 torr
C)585 torr
D)1330 torr
E)1610 torr

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following represents the graph of pressure versus Kelvin temperature for a gas at constant volume? 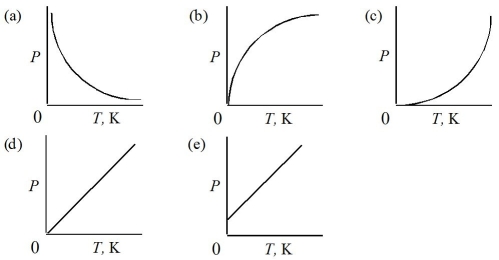
A)Graph (a)
B)Graph (b)
C)Graph (c)
D)Graph (d)
E)Graph (e)

A)Graph (a)
B)Graph (b)
C)Graph (c)
D)Graph (d)
E)Graph (e)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck


