Deck 14: Alcohols, Ethers, and Thiols
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/122
Play
Full screen (f)
Deck 14: Alcohols, Ethers, and Thiols
1
What is the IUPAC name of the following compound? 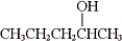
A) methyl propyl methanol
B) 2-pentanol
C) 1-methyl-1-butanol
D) 4-pentanol
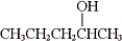
A) methyl propyl methanol
B) 2-pentanol
C) 1-methyl-1-butanol
D) 4-pentanol
B
2
Which of the following is the IUPAC name of butyl alcohol?
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
A
3
What are the approximate bond angles at the OH-bearing carbon atom of all alcohols?
A) 90°
B) 109°
C) 120°
D) 180°
A) 90°
B) 109°
C) 120°
D) 180°
B
4
Which of the following elements is present in thiols but not in alcohols?
A) C
B) O
C) P
D) S
A) C
B) O
C) P
D) S

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the IUPAC name of tert-butyl alcohol?
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the IUPAC name of sec-butyl alcohol?
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a complete and correct IUPAC name?
A) 1-isopropyl-3-cyclohexanol
B) 3-isopropyl-1-cyclohexanol
C) 3-isopropylcyclohexanol
D) cis-3-isopropylcyclohexanol
A) 1-isopropyl-3-cyclohexanol
B) 3-isopropyl-1-cyclohexanol
C) 3-isopropylcyclohexanol
D) cis-3-isopropylcyclohexanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following structures represents 3,3-dimethyl-1-butanol?
A)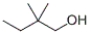
B)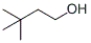
C)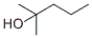
D)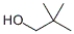
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name of the following compound? 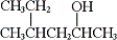
A) 2-ethyl-4-pentanol
B) 4-methyl-2-hexanol
C) 4-ethyl-2-pentanol
D) 3-methyl-5-hexanol
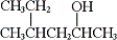
A) 2-ethyl-4-pentanol
B) 4-methyl-2-hexanol
C) 4-ethyl-2-pentanol
D) 3-methyl-5-hexanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
10
What is the geometry at the OH-bearing carbon atom of all alcohols?
A) linear
B) tetrahedral
C) trigonal planar
D) none of these
A) linear
B) tetrahedral
C) trigonal planar
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
11
What is the IUPAC name of the following compound? 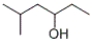
A) 2-methyl-4-hexanol
B) 5-methyl-3-hexanol
C) 3-hydroxy-5-methylhexane
D) 4-hydroxy-2-methylhexane

A) 2-methyl-4-hexanol
B) 5-methyl-3-hexanol
C) 3-hydroxy-5-methylhexane
D) 4-hydroxy-2-methylhexane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a glycol?
A) HOCH2CH2CH2OH
B)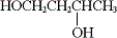
C) HOCH2CH2OH
D) None of these is a glycol.
A) HOCH2CH2CH2OH
B)
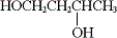
C) HOCH2CH2OH
D) None of these is a glycol.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is true?
A) All diols are glycols.
B) All glycols contain both a primary and a secondary alcohol.
C) All glycols are diols.
D) All glycols must have at least three carbon atoms.
A) All diols are glycols.
B) All glycols contain both a primary and a secondary alcohol.
C) All glycols are diols.
D) All glycols must have at least three carbon atoms.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is(are) glycols?
A) HOCH2CH2CH2CH2CH2OH
B)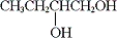
C) both a and b
D) neither a nor b
A) HOCH2CH2CH2CH2CH2OH
B)
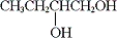
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following structures represents 4-methylcyclohexanol?
A)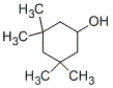
B)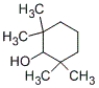
C)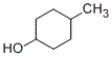
D)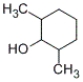
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds has an extremely disagreeable odor?
A) CH3CH2OH
B) CH3CH2OCH2CH3
C) CH3CH2SH
D) none of these
A) CH3CH2OH
B) CH3CH2OCH2CH3
C) CH3CH2SH
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a correct IUPAC name?
A) 1-isopropyl-1-cyclohexanol
B) 1-isopropylcyclohexanol
C) trans-1-isopropyl-1-cyclohexanol
D) none of these
A) 1-isopropyl-1-cyclohexanol
B) 1-isopropylcyclohexanol
C) trans-1-isopropyl-1-cyclohexanol
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the IUPAC name of isobutyl alcohol?
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
19
What is the IUPAC name of the following compound? 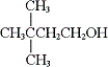
A) 2,2-dimethyl-4-butanol
B) 3,3-dimethyl-1-butanol
C) 1-tert-butyl-2-ethanol
D) 2-sec-butyl-1-ethanol
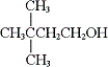
A) 2,2-dimethyl-4-butanol
B) 3,3-dimethyl-1-butanol
C) 1-tert-butyl-2-ethanol
D) 2-sec-butyl-1-ethanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the common name of 2-methyl-2-propanol?
A) sec-butyl alcohol
B) tert-butyl alcohol
C) isobutyl alcohol
D) isopropyl alcohol
A) sec-butyl alcohol
B) tert-butyl alcohol
C) isobutyl alcohol
D) isopropyl alcohol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is true of the alcohol functional groups in 1,3-butanediol?
A) One is primary and one is secondary.
B) One is primary and one is tertiary.
C) Both are primary.
D) Neither is primary.
A) One is primary and one is secondary.
B) One is primary and one is tertiary.
C) Both are primary.
D) Neither is primary.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following alcohols has the lowest boiling point?
A) 1-pentanol
B) 1-octanol
C) 1-hexanol
D) 1-heptanol
A) 1-pentanol
B) 1-octanol
C) 1-hexanol
D) 1-heptanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
23
Of the alcohols with the molecular formula C4H9OH, which has the highest boiling point?
A) 1-butanol
B) sec-butyl alcohol
C) tert-butyl alcohol
D) isobutyl alcohol
A) 1-butanol
B) sec-butyl alcohol
C) tert-butyl alcohol
D) isobutyl alcohol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
24
What is the color of pure ethylene glycol?
A) colorless
B) green
C) red
D) white
A) colorless
B) green
C) red
D) white

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a starting material in the synthesis of nitroglycerin?
A) ethylene glycol
B) 1,2-propanediol
C) 1,3-propanediol
D) 1,2,3-propanetriol
A) ethylene glycol
B) 1,2-propanediol
C) 1,3-propanediol
D) 1,2,3-propanetriol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is true about the polarities of the C−O and O−H bonds?
A) Neither the C−O nor the O−H bond is polar.
B) The C−O and O−H bonds are equally polar.
C) Both bonds are polar, but the C−O bond is less polar than the O−H bond.
D) Both bonds are polar, but the O−H bond is less polar than the C−O bond.
A) Neither the C−O nor the O−H bond is polar.
B) The C−O and O−H bonds are equally polar.
C) Both bonds are polar, but the C−O bond is less polar than the O−H bond.
D) Both bonds are polar, but the O−H bond is less polar than the C−O bond.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
27
Given that in stable diols the two hydroxyl groups are not attached to the same carbon atom, which of the following is the molecular formula of a stable diol in which both alcohol functional groups are tertiary?
A) C3H8O2
B) C4H10O2
C) C5H12O2
D) C6H14O2
A) C3H8O2
B) C4H10O2
C) C5H12O2
D) C6H14O2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is true of the alcohol functional groups in 2,3-butanediol?
A) One is secondary and one is tertiary.
B) Both are secondary.
C) Both are tertiary.
D) None of the above is true.
A) One is secondary and one is tertiary.
B) Both are secondary.
C) Both are tertiary.
D) None of the above is true.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following alcohols has the highest boiling point?
A) 1-pentanol
B) 1,5-pentanediol
C) 1,3,5-pentanetriol
D) 1-butanol
A) 1-pentanol
B) 1,5-pentanediol
C) 1,3,5-pentanetriol
D) 1-butanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following alcohols is most soluble in water?
A) 1-pentanol
B) 1,5-pentanediol
C) 1,3,5-pentanetriol
D) None, they are all equally soluble.
A) 1-pentanol
B) 1,5-pentanediol
C) 1,3,5-pentanetriol
D) None, they are all equally soluble.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is true of the alcohol functional groups in 2-methyl-1,2-propanediol?
A) One is primary and one is secondary.
B) One is primary and one is tertiary.
C) Both are primary.
D) Neither is primary.
A) One is primary and one is secondary.
B) One is primary and one is tertiary.
C) Both are primary.
D) Neither is primary.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
32
Of the alcohols with the molecular formula C4H9OH, which has the lowest boiling point?
A) 1-butanol
B) sec-butyl alcohol
C) tert-butyl alcohol
D) isobutyl alcohol
A) 1-butanol
B) sec-butyl alcohol
C) tert-butyl alcohol
D) isobutyl alcohol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
33
What is the maximum number of hydrogen bonds which can be formed by a single molecule of an alcohol which contains only one hydroxyl group?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds is most soluble in water?
A) 1-pentanol
B) propane
C) 1-propanol
D) trans-2-butene
A) 1-pentanol
B) propane
C) 1-propanol
D) trans-2-butene

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following alcohols is least soluble in water?
A) 1-pentanol
B) 1-octanol
C) 1-hexanol
D) 2-propanol
A) 1-pentanol
B) 1-octanol
C) 1-hexanol
D) 2-propanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements about London dispersion forces is/are true?
A) London dispersion forces are stronger between molecules of 1-butanol than between molecules of 2-methyl-2-propanol.
B) London dispersion forces are weaker than hydrogen bonding interactions.
C) both a and b
D) neither a nor b
A) London dispersion forces are stronger between molecules of 1-butanol than between molecules of 2-methyl-2-propanol.
B) London dispersion forces are weaker than hydrogen bonding interactions.
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
37
What is the approximate strength of a hydrogen bond in alcohols?
A) less than 1 kcal/mol
B) 2 to 5 kcal/mol
C) 10-20 kcal/mol
D) more than 20 kcal/mol
A) less than 1 kcal/mol
B) 2 to 5 kcal/mol
C) 10-20 kcal/mol
D) more than 20 kcal/mol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is true of the alcohol functional groups in 1,3-propanediol?
A) One is primary and one is secondary.
B) One is primary and one is tertiary.
C) Both are primary.
D) Neither is primary.
A) One is primary and one is secondary.
B) One is primary and one is tertiary.
C) Both are primary.
D) Neither is primary.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following classes of compounds react with sodium hydroxide to give a water-soluble salt?
A) 1° alcohols
B) phenols
C) both a and b
D) neither a nor b
A) 1° alcohols
B) phenols
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements concerning the boiling points of isomeric alcohols is true?
A) Isomeric alcohols have the same boiling points.
B) The relative boiling points of isomeric alcohols are determined primarily by the different number of hydrogen bonds which they can form.
C)
C) The relative boiling points of isomeric alcohols are determined primarily by the different shapes of the molecules.
D) The relative boiling points of isomeric alcohols are determined equally by both b and
A) Isomeric alcohols have the same boiling points.
B) The relative boiling points of isomeric alcohols are determined primarily by the different number of hydrogen bonds which they can form.
C)
C) The relative boiling points of isomeric alcohols are determined primarily by the different shapes of the molecules.
D) The relative boiling points of isomeric alcohols are determined equally by both b and

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
41
Which of these responses describes the products formed by heating the following compound with concentrated sulfuric acid? 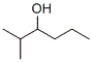
A) The major product is 2-methyl-2-hexene and the minor product is 2-methyl-3-hexene.
B) The major product is 2-methyl-3-hexene and the minor product is 2-methyl-2-hexene.
C) The only product formed is 2-methyl-1-hexene.
D) The major product is 2-methyl-2-hexene and the minor product is 2-methyl-1-hexene.

A) The major product is 2-methyl-2-hexene and the minor product is 2-methyl-3-hexene.
B) The major product is 2-methyl-3-hexene and the minor product is 2-methyl-2-hexene.
C) The only product formed is 2-methyl-1-hexene.
D) The major product is 2-methyl-2-hexene and the minor product is 2-methyl-1-hexene.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is the major product obtained from the dehydration of 2,3-dimethyl-3-pentanol?
A) 2-isopropyl-2-butene
B) 2,3-dimethyl-2-pentene
C) 3,4-dimethyl-2-pentene
D) none of these
A) 2-isopropyl-2-butene
B) 2,3-dimethyl-2-pentene
C) 3,4-dimethyl-2-pentene
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
43
How many different alkene products (counting cis and trans isomers of a compound as a single product) could be formed when 2,3-dimethyl-3-pentanol is dehydrated?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following alkenes will be the major product when 2-methylcyclohexanol is heated with concentrated sulfuric acid?
A)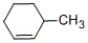
B)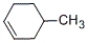
C)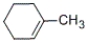
D)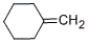
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the alcohols shown below will react when heated with concentrated sulfuric acid to give the following alkene as the major product? 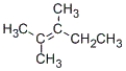
A)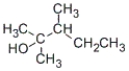
B)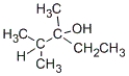
C) both a and b
D) neither a nor b

A)

B)

C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following alcohols will undergo acid-catalyzed dehydration most easily?
A) 1-butanol
B) 2-butanol
C) 2-methyl-2-propanol
D) ethanol
A) 1-butanol
B) 2-butanol
C) 2-methyl-2-propanol
D) ethanol

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
47
Dehydration of which type of alcohol can never result in a mixture of products?
A) 1°
B) 2°
C) 3°
D) None, mixtures can be obtained from any of these.
A) 1°
B) 2°
C) 3°
D) None, mixtures can be obtained from any of these.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
48
Which definition of oxidation is most useful when considering the oxidation of tertiary alcohols?
A) gain of oxygen
B) loss of electrons
C) loss of hydrogen
D) None of these, 3° alcohols are not oxidized.
A) gain of oxygen
B) loss of electrons
C) loss of hydrogen
D) None of these, 3° alcohols are not oxidized.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following describes the products formed by the dehydration of 2,2,4,4-tetramethyl-3-pentanol?
A) 2,2,4,4-tetramethyl-2-pentene is the only product.
B) 2,2,4,4-tetramethyl-3-pentene is the only product.
C) 2,2,4,4-tetramethyl-4-pentene is the only product.
D) There is no product, since 2,2,4,4-tetramethyl-3-pentanol does not undergo dehydration.
A) 2,2,4,4-tetramethyl-2-pentene is the only product.
B) 2,2,4,4-tetramethyl-3-pentene is the only product.
C) 2,2,4,4-tetramethyl-4-pentene is the only product.
D) There is no product, since 2,2,4,4-tetramethyl-3-pentanol does not undergo dehydration.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
50
Suppose you carried out two reactions in sequence. In the first reaction you hydrated 1-hexene and in the second reaction you dehydrated the product(s) of the first reaction? Which of the following would be the major final product?
A) 1-hexene
B) 2-hexene
C) 3-hexene
D) none of the above
A) 1-hexene
B) 2-hexene
C) 3-hexene
D) none of the above

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
51
Of 1-pentanol, 2-pentanol and 3-pentanol, which compound(s) can yield only a single alkene product when dehydrated?
A) only 1-pentanol
B) both 1-pentanol and 2-pentanol
C) both 1-pentanol and 3-pentanol
D) all of them
A) only 1-pentanol
B) both 1-pentanol and 2-pentanol
C) both 1-pentanol and 3-pentanol
D) all of them

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
52
In which class of compounds is the carbon at the functional group most highly oxidized?
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
53
In which class of compounds is the carbon at the functional group least highly oxidized?
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
54
Which of these compounds is obtained as the major product from the reaction of the following alkene with aqueous sulfuric acid? 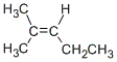
A)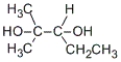
B)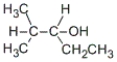
C)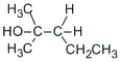
D)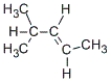

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is true of the hydration-dehydration equilibrium which exists between alcohols and alkenes under aqueous acidic conditions?
A) It lies so far in one direction that it can only be used for the preparation of alcohols.
B) It lies so far in one direction that it can only be used for the preparation of alkenes.
C) Reaction conditions can be adjusted so that the equilibrium can be used to prepare either alcohols or alkenes in high yields.
D) It is not useful for the preparation of either alcohols or alkenes.
A) It lies so far in one direction that it can only be used for the preparation of alcohols.
B) It lies so far in one direction that it can only be used for the preparation of alkenes.
C) Reaction conditions can be adjusted so that the equilibrium can be used to prepare either alcohols or alkenes in high yields.
D) It is not useful for the preparation of either alcohols or alkenes.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following describes the products formed by the dehydration of cis-2-methylcyclopentanol?
A) The only product is 1-methylcyclopentene.
B) The only product is 3-methylcyclopentene.
C) The major product is 1-methylcyclopentene and the minor product is 3-methylcyclopentene.
D) The major product is 3-methylcyclopentene and the minor product is 1-methylcyclopentene.
A) The only product is 1-methylcyclopentene.
B) The only product is 3-methylcyclopentene.
C) The major product is 1-methylcyclopentene and the minor product is 3-methylcyclopentene.
D) The major product is 3-methylcyclopentene and the minor product is 1-methylcyclopentene.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
57
How many different alkene products (counting cis and trans isomers of a compound as a single product) could be produced when 3-methyl-3-pentanol is dehydrated?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following describes the products formed by the dehydration of trans-4-methylcyclohexanol?
A) The only product is 4-methylcyclohexene.
B) The only product is 5-methylcyclohexene.
C) The major product is 4-methylcyclohexene and the minor product is 5-methylcyclohexene.
D) The major product is 5-methylcyclohexene and the minor product is 4-methylcyclohexene.
A) The only product is 4-methylcyclohexene.
B) The only product is 5-methylcyclohexene.
C) The major product is 4-methylcyclohexene and the minor product is 5-methylcyclohexene.
D) The major product is 5-methylcyclohexene and the minor product is 4-methylcyclohexene.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
59
When a primary alcohol is oxidized to a carboxylic acid, an intermediate product is an aldehyde. Which physical property of the aldehyde can be exploited if it is desired to obtain the aldehyde as the reaction product?
A) boiling point
B) molecular weight
C) solubility
D) any of these
A) boiling point
B) molecular weight
C) solubility
D) any of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
60
Which definition of oxidation is most useful when considering the oxidation of primary or secondary alcohols?
A) gain of oxygen
B) loss of electrons
C) loss of hydrogen
D) None of these, 1° and 2° alcohols are not oxidized.
A) gain of oxygen
B) loss of electrons
C) loss of hydrogen
D) None of these, 1° and 2° alcohols are not oxidized.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
61
What is the smallest number of carbon atoms which can be found in an unsymmetrical ether that contains only C, H and a single O atom?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following reagents was used in the original breath alcohol test used by law enforcement officials?
A) Cr3+
B) hydrogen peroxide
C) potassium dichromate
D) none of these
A) Cr3+
B) hydrogen peroxide
C) potassium dichromate
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is obtainable by the oxidation of tert-butyl alcohol?
A)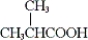
B)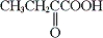
C) both a and b
D) neither a nor b
A)
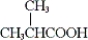
B)
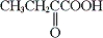
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following correctly gives the order of boiling points for the molecules propane, dimethyl ether, and ethanol?
A) ethanol > dimethyl ether > propane
B) ethanol > propane > dimethyl ether
C) propane > dimethyl ether > ethanol
D) propane > ethanol > dimethyl ether
A) ethanol > dimethyl ether > propane
B) ethanol > propane > dimethyl ether
C) propane > dimethyl ether > ethanol
D) propane > ethanol > dimethyl ether

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
65
What is the smallest number of hydrogen atoms which can be found in a noncyclic unsymmetrical ether that contains only C, H and a single O atom?
A) 3
B) 6
C) 8
D) 10
A) 3
B) 6
C) 8
D) 10

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
66
What is the approximate C−O−C bond angle in the cyclic ether ethylene oxide, C2H4O?
A) 60°
B) 90°
C) 109°
D) 120°
A) 60°
B) 90°
C) 109°
D) 120°

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
67
What is the smallest number of hydrogen atoms which can be found in a noncyclic ether?
A) 3
B) 6
C) 8
D) 10
A) 3
B) 6
C) 8
D) 10

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is obtained from the oxidation of sec-butyl alcohol with potassium dichromate in aqueous sulfuric acid?
A)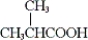
B)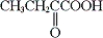
C) both a and b
D) neither a nor b
A)
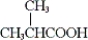
B)
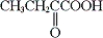
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
69
Ketones can be obtained by the oxidation of which of the following?
A) 1° alcohols
B) 2° alcohols
C) 3° alcohols
D) none of these
A) 1° alcohols
B) 2° alcohols
C) 3° alcohols
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
70
What color change is associated with the original breath alcohol test?
A) green to reddish orange
B) reddish orange to green
C) colorless to pink
D) None, the test is not based on a color change.
A) green to reddish orange
B) reddish orange to green
C) colorless to pink
D) None, the test is not based on a color change.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
71
If experimental conditions are carefully controlled, aldehydes can be obtained by the oxidation of which of the following?
A) 1° alcohols
B) 2° alcohols
C) 3° alcohols
D) all of these
A) 1° alcohols
B) 2° alcohols
C) 3° alcohols
D) all of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is the name of the smallest cyclic ether?
A) ethylene oxide
B) methylene oxide
C) tetrahydrofuran
D) none of these
A) ethylene oxide
B) methylene oxide
C) tetrahydrofuran
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
73
What is the common name for the following compound? 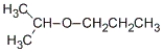
A) hexyl ether
B) propyl isopropyl ether
C) isopropyl propyl ether
D) none of these
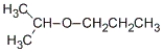
A) hexyl ether
B) propyl isopropyl ether
C) isopropyl propyl ether
D) none of these

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the products listed below can be obtained from the reaction of the following alcohol with sodium dichromate in aqueous sulfuric acid? 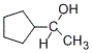
A)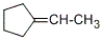
B)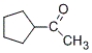
C)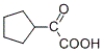
D)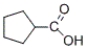

A)
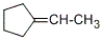
B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following would be the IUPAC name for the ether commonly called ethyl methyl ether?
A) ethoxymethane
B) ethoxyethane
C) methoxymethane
D) methoxyethane
A) ethoxymethane
B) ethoxyethane
C) methoxymethane
D) methoxyethane

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
76
What is the approximate C−O−C bond angle in noncyclic ethers?
A) 90°
B) 109°
C) 120°
D) 180°
A) 90°
B) 109°
C) 120°
D) 180°

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
77
What is the smallest number of carbon atoms which can be found in an ether?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following explains why the boiling points of ethers are more similar to those of comparable alkanes than to those of comparable alcohols?
A) Both ether molecules and alkane molecules are nonpolar.
B) Hydrogen bonding between ether molecules is weak.
C) Although ether molecules are polar they cannot form hydrogen bonds to other ether molecules.
D) None of the above is the correct explanation.
A) Both ether molecules and alkane molecules are nonpolar.
B) Hydrogen bonding between ether molecules is weak.
C) Although ether molecules are polar they cannot form hydrogen bonds to other ether molecules.
D) None of the above is the correct explanation.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
79
What is the name of the −OCH3 group?
A) methyloxy
B) methoxy
C) oxymethyl
D) oxymeth
A) methyloxy
B) methoxy
C) oxymethyl
D) oxymeth

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is obtained from the oxidation of isobutyl alcohol with potassium dichromate in aqueous sulfuric acid?
A)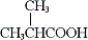
B)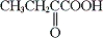
C) both a and b
D) neither a nor b
A)
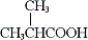
B)
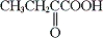
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck


