Deck 12: Alkenes and Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/123
Play
Full screen (f)
Deck 12: Alkenes and Alkynes
1
In alkenes there are four groups bonded to the carbons which constitute the carbon-carbon double bond. Which of the following situations produces cis-trans isomerism?
A) when all four groups are the same
B) when the two groups on one atom are the same, but the two groups on the other atom are different from each other and also different from the groups on the first atom
C) when three of the four groups are the same, but the fourth group is different
D) none of these results in the existence of cis-trans isomerism
A) when all four groups are the same
B) when the two groups on one atom are the same, but the two groups on the other atom are different from each other and also different from the groups on the first atom
C) when three of the four groups are the same, but the fourth group is different
D) none of these results in the existence of cis-trans isomerism
D
2
Which of the following is true of cis and trans isomers?
A) they have the same structural connectivity
B) they have the same physical properties
C) both a and b
D) neither a nor b
A) they have the same structural connectivity
B) they have the same physical properties
C) both a and b
D) neither a nor b
A
3
Which of the following is true in the IUPAC system for naming alkenes?
A) The alkene is always named based on the longest carbon chain.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determines the number associated with the position of the double bond.
D) None of the above is correct.
A) The alkene is always named based on the longest carbon chain.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determines the number associated with the position of the double bond.
D) None of the above is correct.
D
4
Given an alkane, an alkene, and an alkyne, each of which contains two carbon atoms, which compound will contain most hydrogen atoms?
A) the alkane
B) the alkene
C) the alkyne
D) None, they all contain the same number of hydrogen atoms.
A) the alkane
B) the alkene
C) the alkyne
D) None, they all contain the same number of hydrogen atoms.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
5
In alkenes there are four groups bonded to the carbons which constitute the carbon-carbon double bond. Which of the following situations produces cis-trans isomerism?
A) when all four groups are the same
B) when the two groups bonded to each carbon atom are different from one another
C) when three of the four groups are the same, but the fourth group is different
D) None of these results in the existence of cis-trans isomerism.
A) when all four groups are the same
B) when the two groups bonded to each carbon atom are different from one another
C) when three of the four groups are the same, but the fourth group is different
D) None of these results in the existence of cis-trans isomerism.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
6
Given an alkane, an alkene, and an alkyne, each of which contains two carbon atoms, which compound will contain the fewest hydrogen atoms?
A) the alkane
B) the alkene
C) the alkyne
D) None, they all contain the same number of hydrogen atoms.
A) the alkane
B) the alkene
C) the alkyne
D) None, they all contain the same number of hydrogen atoms.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the characteristic feature of all alkenes?
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of at least one carbon-carbon double bond, and at least one carbon-carbon triple bond
D) the presence of a ring system
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of at least one carbon-carbon double bond, and at least one carbon-carbon triple bond
D) the presence of a ring system

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is true in the IUPAC system for naming alkenes?
A) The alkene is named based on the longest carbon chain which contains the double bond.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determines the number associated with the position of the double bond.
D) None of the above is correct.
A) The alkene is named based on the longest carbon chain which contains the double bond.
B) The position of the double bond is always given the highest possible number.
C) The position and identity of substituents determines the number associated with the position of the double bond.
D) None of the above is correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is true of ethylene?
A) It is not a very important industrial chemical.
B) It is present in large amounts in natural gas.
C) Its most important use is as a fuel.
D) The vast majority of ethylene is produced by thermal cracking.
A) It is not a very important industrial chemical.
B) It is present in large amounts in natural gas.
C) Its most important use is as a fuel.
D) The vast majority of ethylene is produced by thermal cracking.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
10
Given a non-cyclic alkane, a non-cyclic alkene, and a non-cyclic alkyne, each of which contains six carbon atoms, which compound will contain the fewest hydrogen atoms?
A) the alkane
B) the alkene
C) the alkyne
D) It is not possible to tell, without knowing how many double and/or triple bonds are present in the unsaturated compounds.
A) the alkane
B) the alkene
C) the alkyne
D) It is not possible to tell, without knowing how many double and/or triple bonds are present in the unsaturated compounds.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following structural features is never associated with cis-trans isomerism?
A) double bonds
B) ring systems
C) triple bonds
D) None, they are all associated with cis-trans isomerism.
A) double bonds
B) ring systems
C) triple bonds
D) None, they are all associated with cis-trans isomerism.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
12
For which class of compounds does the lack of free rotation around carbon-carbon bonds result in the existence of cis-trans isomerism?
A) alkanes
B) alkenes
C) alkynes
D) arenes
A) alkanes
B) alkenes
C) alkynes
D) arenes

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
13
Cis-trans isomerism is associated with the presence of which of the following?
A) double bonds
B) ring systems
C) both a and b
D) neither a nor b
A) double bonds
B) ring systems
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
14
Which class of compounds is not widespread in nature, and has little importance in biochemistry?
A) alkanes
B) alkenes
C) alkynes
D) arenes
A) alkanes
B) alkenes
C) alkynes
D) arenes

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is true of the six atoms of ethylene?
A) Four atoms lie in the same plane, but two atoms are not in this plane.
B) All six atoms lie in the same plane.
C) The carbon atoms have a tetrahedral geometry so the C−C−H angles are approximately 109°.
D) none of the above
A) Four atoms lie in the same plane, but two atoms are not in this plane.
B) All six atoms lie in the same plane.
C) The carbon atoms have a tetrahedral geometry so the C−C−H angles are approximately 109°.
D) none of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds is a natural ripening agent for fruits?
A) ethane
B) ethylene
C) acetylene
D) none of these
A) ethane
B) ethylene
C) acetylene
D) none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the starting material derived from natural gas that is used for the production of ethylene?
A) acetylene
B) benzene
C) ethane
D) methane
A) acetylene
B) benzene
C) ethane
D) methane

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the characteristic feature of all alkynes?
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of at least one carbon-carbon double bond, and at least one carbon-carbon triple bond
D) the presence of a ring system
A) the presence of one or more carbon-carbon double bonds
B) the presence of one or more carbon-carbon triple bonds
C) the presence of at least one carbon-carbon double bond, and at least one carbon-carbon triple bond
D) the presence of a ring system

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
19
Which organic compound is produced in the largest amount by the chemical industry?
A) acetylene
B) benzene
C) ethane
D) ethylene
A) acetylene
B) benzene
C) ethane
D) ethylene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
20
A bond angle of approximately 120° is associated with the carbons in which functional group?
A) alkanes
B) alkenes
C) alkynes
D) none of these
A) alkanes
B) alkenes
C) alkynes
D) none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds, all of which have the molecular formula C6H12, has cis and trans isomers?
A) 2-methyl-1-pentene
B) 2-methyl-2-pentene
C) 3-methyl-2-pentene
D) They all have cis-trans isomers.
A) 2-methyl-1-pentene
B) 2-methyl-2-pentene
C) 3-methyl-2-pentene
D) They all have cis-trans isomers.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is not a correct IUPAC name for an alkene?
A) 1-methyl-1-butene
B) 2-methyl-1-butene
C) 3-methyl-1-butene
D) 2-methyl-2-butene
A) 1-methyl-1-butene
B) 2-methyl-1-butene
C) 3-methyl-1-butene
D) 2-methyl-2-butene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
23
A student wanted to use the branched groups, isobutyl, sec-butyl, and tert-butyl when naming alkenes, but was told not to. What is the correct IUPAC name for the compound the student called isobutylethene?
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
24
Cis-trans isomers do not exist for which of the following compounds, all of which have the molecular formula C3H4Br2?
A) 1,1-dibromopropene
B) 1,2-dibromopropene
C) 1,3-dibromopropene
D) They do not exist for any of these three molecules.
A) 1,1-dibromopropene
B) 1,2-dibromopropene
C) 1,3-dibromopropene
D) They do not exist for any of these three molecules.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following, all of which have the molecular formula C5H10, has cis and trans isomers?
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 2-pentene
D) none of them
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 2-pentene
D) none of them

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following compounds, all of which have the molecular formula C6H12, does not have cis and trans isomers?
A) 4-methyl-2-pentene
B) 2-methyl-1-pentene
C) 3-methyl-2-pentene
D) None of them, they all have cis-trans isomers.
A) 4-methyl-2-pentene
B) 2-methyl-1-pentene
C) 3-methyl-2-pentene
D) None of them, they all have cis-trans isomers.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
27
A student named a particular compound 2-propyl-1-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound?
A) 2-propyl-1-butene
B) 2-ethyl-1-pentene
C) 3-methyl-2-pentene
D) none of these
A) 2-propyl-1-butene
B) 2-ethyl-1-pentene
C) 3-methyl-2-pentene
D) none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
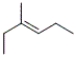
28
Which of the following structures corresponds to cis-3-methyl-2-hexene?
A)
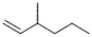
B)
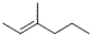
C)
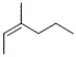
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is true of the common names of low molecular weight alkenes and alkynes?
A) The common names of all alkenes end in −ene, and those of alkynes end in −yne.
B) The common names of all alkenes and all alkynes end in −ene.
C) The common names of all alkenes and all alkynes end in −yne.
D) None of the above is correct.
A) The common names of all alkenes end in −ene, and those of alkynes end in −yne.
B) The common names of all alkenes and all alkynes end in −ene.
C) The common names of all alkenes and all alkynes end in −yne.
D) None of the above is correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
30
A student wanted to use the branched groups, isobutyl, sec-butyl, and tert-butyl when naming alkenes, but was told not to. What is the correct IUPAC name for the compound the student called sec-butylethene?
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these
A) 4-methyl-1-pentene
B) 3-methyl-1-pentene
C) 3,3-dimethyl-1-butene
D) none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is not a correct IUPAC name for an alkene?
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 2-methyl-3-butene
D) None, these are all correct IUPAC names.
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 2-methyl-3-butene
D) None, these are all correct IUPAC names.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
32
Cis-trans isomers exist for which of the following molecules, all of which have the molecular formula C3H5Br?
A) 1-bromopropene
B) 2-bromopropene
C) 3-bromopropene
D) none of these
A) 1-bromopropene
B) 2-bromopropene
C) 3-bromopropene
D) none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
33
What is the correct IUPAC name for the following compound? 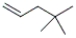
A) 2,2-dimethyl-4-pentene
B) 4,4-dimethyl-2-pentene
C) 4,4-dimethyl-1-pentene
D) isoheptene

A) 2,2-dimethyl-4-pentene
B) 4,4-dimethyl-2-pentene
C) 4,4-dimethyl-1-pentene
D) isoheptene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
34
What is the correct IUPAC name for the following compound? 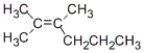
A) 2-methyl-3-propyl-2-butene
B) 1,1,2-trimethyl-1-pentene
C) 2,3-dimethyl-2-hexene
D) none of these

A) 2-methyl-3-propyl-2-butene
B) 1,1,2-trimethyl-1-pentene
C) 2,3-dimethyl-2-hexene
D) none of these

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
35
Cis-trans isomers exist for which of the following molecules, all of which have the molecular formula C5H10?
A) 1-pentene
B) 2-methyl-1-butene
C) 2-methyl-2-butene
D) 1,2-dimethylcyclopropane
A) 1-pentene
B) 2-methyl-1-butene
C) 2-methyl-2-butene
D) 1,2-dimethylcyclopropane

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is true in the IUPAC system for naming alkenes?
A) The alkene is always named based on the longest carbon chain.
B) The position of the double bond is always given the lowest possible number.
C) The position and identity of substituents determines the number associated with the position of the double bond.
D) None of the above is correct.
A) The alkene is always named based on the longest carbon chain.
B) The position of the double bond is always given the lowest possible number.
C) The position and identity of substituents determines the number associated with the position of the double bond.
D) None of the above is correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
37
What is the correct IUPAC name for the following compound? 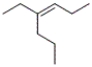
A) trans-3-propyl-3-hexene
B) cis-3-propyl-3-hexene
C) trans-4-ethyl-3-heptene
D) cis-4-ethyl-3-heptene

A) trans-3-propyl-3-hexene
B) cis-3-propyl-3-hexene
C) trans-4-ethyl-3-heptene
D) cis-4-ethyl-3-heptene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
38
What is the name of the lightest hydrocarbon which has cis and trans isomers?
A) 1-butene
B) 2-butene
C) 1,2-dimethylcyclopropane
D) propene
A) 1-butene
B) 2-butene
C) 1,2-dimethylcyclopropane
D) propene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is not a correct IUPAC name for an alkene?
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 3-methyl-1-butene
D) None, these are all correct IUPAC names.
A) 2-methyl-1-butene
B) 2-methyl-2-butene
C) 3-methyl-1-butene
D) None, these are all correct IUPAC names.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
40
A student named a particular compound 2-ethyl-3-methyl-2-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound?
A) 2-ethyl-3-methyl-2-butene
B) 3,4-dimethyl-3-pentene
C) 2,3-dimethyl-2-pentene
D) 2,3-dimethyl-1-pentene
A) 2-ethyl-3-methyl-2-butene
B) 3,4-dimethyl-3-pentene
C) 2,3-dimethyl-2-pentene
D) 2,3-dimethyl-1-pentene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
41
How many stereoisomers are possible for 1,4,7-octatriene?
A) 2
B) 4
C) 8
D) 16
A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
42
Including cis-trans isomers, how many different alkenes have the molecular formula C5H10?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
43
Of the following alkenes, all of which have the molecular formula C5H10, which would you expect to have the highest boiling point?
A) 4-methyl-1-pentene
B) 4-methyl-2-pentene
C) 2,3-dimethyl-2-butene
D) 2-hexene
A) 4-methyl-1-pentene
B) 4-methyl-2-pentene
C) 2,3-dimethyl-2-butene
D) 2-hexene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
44
How many stereoisomers are possible for 1,4,6-octatriene?
A) 2
B) 4
C) 8
D) 16
A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is true of the non-cyclic portion of vitamin A?
A) It contains 4 double bonds, all in the cis configuration.
B) It contains 4 double bonds, all in the trans configuration.
C) It contains 4 double bonds, which alternate cis, trans, cis, trans.
D) None of the above is true.
A) It contains 4 double bonds, all in the cis configuration.
B) It contains 4 double bonds, all in the trans configuration.
C) It contains 4 double bonds, which alternate cis, trans, cis, trans.
D) None of the above is true.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
46
How many stereoisomers are possible for 2,4,6-nonatriene?
A) 2
B) 4
C) 8
D) 16
A) 2
B) 4
C) 8
D) 16

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following occurs when rhodopsin absorbs light?
A) The less stable 11,12-cis isomer is converted to the more stable trans isomer.
B) The less stable 11,12-trans isomer is converted to the more stable cis isomer.
C) The more stable 11,12-cis isomer is converted to the less stable trans isomer.
D) The more stable 11,12-trans isomer is converted to the less stable cis isomer.
A) The less stable 11,12-cis isomer is converted to the more stable trans isomer.
B) The less stable 11,12-trans isomer is converted to the more stable cis isomer.
C) The more stable 11,12-cis isomer is converted to the less stable trans isomer.
D) The more stable 11,12-trans isomer is converted to the less stable cis isomer.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
48
How many cycloalkenes have the molecular formula C5H8?
A) 7
B) 8
C) 9
D) 10
A) 7
B) 8
C) 9
D) 10

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
49
How many stereoisomers are possible for 2,4-heptadiene?
A) 2
B) 4
C) 7
D) 8
A) 2
B) 4
C) 7
D) 8

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is not a correct IUPAC name?
A) cis-2,3-dimethylcyclopentene
B) cis-3,4-dimethylcyclopentene
C) cis-3,5-dimethylcyclopentene
D) None, they are all correct.
A) cis-2,3-dimethylcyclopentene
B) cis-3,4-dimethylcyclopentene
C) cis-3,5-dimethylcyclopentene
D) None, they are all correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
51
How many cycloalkenes have the molecular formula C4H6?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
52
Stereoisomers exist for which of the following molecules, all of which have the molecular formula C5H8?
A) 1,3-pentadiene
B) 1,4-pentadiene
C) 2-methyl-1,3-butadiene
D) all of them
A) 1,3-pentadiene
B) 1,4-pentadiene
C) 2-methyl-1,3-butadiene
D) all of them

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
53
How many stereoisomers are possible for 1,4-heptadiene?
A) 2
B) 4
C) 7
D) 8
A) 2
B) 4
C) 7
D) 8

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following alkenes, all of which have the molecular formula C5H10, would you expect to have the lowest boiling point?
A) 4-methyl-1-pentene
B) 4-methyl-2-pentene
C) 2,3-dimethyl-2-butene
D) 2-hexene
A) 4-methyl-1-pentene
B) 4-methyl-2-pentene
C) 2,3-dimethyl-2-butene
D) 2-hexene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
55
What is the configuration of each of the alkenes shown below? 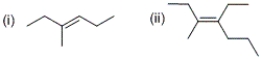
A) (i) cis (ii) cis
B) (i) trans (ii) trans
C) (i) cis (ii) trans
D) (i) trans (ii) cis
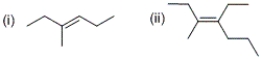
A) (i) cis (ii) cis
B) (i) trans (ii) trans
C) (i) cis (ii) trans
D) (i) trans (ii) cis

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is not a correct IUPAC name?
A) 3-methylcyclopentene
B) 4-methylcyclopentene
C) 5-methylcyclopentene
D) None, they are all correct.
A) 3-methylcyclopentene
B) 4-methylcyclopentene
C) 5-methylcyclopentene
D) None, they are all correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
57
Including cis-trans isomers, how many different alkenes have the molecular formula C4H8?
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
58
Based on your knowledge of the properties of the analogous alkanes, which of the following is the lightest alkene that you would expect to be a liquid at room temperature?
A) propene
B) 1-pentene
C) 1-heptene
D) 1-nonene
A) propene
B) 1-pentene
C) 1-heptene
D) 1-nonene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is a correct IUPAC name?
A) 4-ethyl-1-methylcyclohexene
B) 5-ethyl-2-methylcyclohexene
C) 1-methyl-4-ethylcyclohexene
D) 5-methyl-2-ethylcyclohexene
A) 4-ethyl-1-methylcyclohexene
B) 5-ethyl-2-methylcyclohexene
C) 1-methyl-4-ethylcyclohexene
D) 5-methyl-2-ethylcyclohexene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
60
Cis-trans isomers exist for which of the following?
A) 1,2-dimethylcyclopentene
B) 2,3-dimethylcyclopentene
C) 3,5-dimethylcyclopentene
D) All of them exist.
A) 1,2-dimethylcyclopentene
B) 2,3-dimethylcyclopentene
C) 3,5-dimethylcyclopentene
D) All of them exist.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
61
Markovnikov's rule is useful in predicting the outcome for which of the following addition reactions of unsymmetrical alkenes?
A) hydration
B) bromination
C) both a and b
D) neither a nor b
A) hydration
B) bromination
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
62
Examine the following reaction. 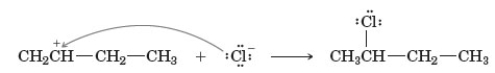 A B C Which of the following describes this reaction?
A B C Which of the following describes this reaction?
A) Involves a secondary carbocation.
B) A represents the nucleophile.
C) B represents the electrophile.
D) Produces a new carbon to carbon bond.
E) All of the above except d are correct.
 A B C Which of the following describes this reaction?
A B C Which of the following describes this reaction?A) Involves a secondary carbocation.
B) A represents the nucleophile.
C) B represents the electrophile.
D) Produces a new carbon to carbon bond.
E) All of the above except d are correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
63
When the following substance reacts with water in the presence of an acid, which of the following describes the reaction? 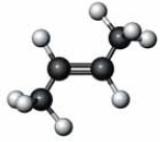
A) Two products are produced.
B) The product is an alcohol.
C) both a and b
D) neither a nor b

A) Two products are produced.
B) The product is an alcohol.
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the molecule represented below. 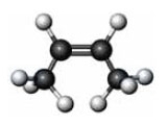 Which of the following is a characteristic of the substance represented here?
Which of the following is a characteristic of the substance represented here?
A) alkene
B) cis isomer
C) melting point different than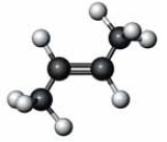
D) All of these are characteristic of this compound.
 Which of the following is a characteristic of the substance represented here?
Which of the following is a characteristic of the substance represented here?A) alkene
B) cis isomer
C) melting point different than

D) All of these are characteristic of this compound.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the alkyne counterpart of the following compound. 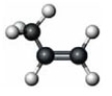 Which of the following would characterize this alkyne?
Which of the following would characterize this alkyne?
A) C3H6
B) CH3C CH
CH
C)
D) both a and b
E) a, b and c
 Which of the following would characterize this alkyne?
Which of the following would characterize this alkyne?A) C3H6
B) CH3C
 CH
CHC)

D) both a and b
E) a, b and c

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following statements is true about the solubility of alkenes?
A) All alkenes are soluble in water.
B) Long chain alkenes are soluble in water, but short chain alkenes are insoluble.
C) Long chain alkenes are insoluble in water, but short chain alkenes are soluble.
D) All alkenes are soluble in alkanes.
A) All alkenes are soluble in water.
B) Long chain alkenes are soluble in water, but short chain alkenes are insoluble.
C) Long chain alkenes are insoluble in water, but short chain alkenes are soluble.
D) All alkenes are soluble in alkanes.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following correctly characterizes the compound represented below? 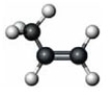
A) trans isomer
B) alkyne
C) at least one 120° bond angle
D) free rotation around all bonds

A) trans isomer
B) alkyne
C) at least one 120° bond angle
D) free rotation around all bonds

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following curved arrows correctly describes the first step of the reaction of propene with a hydrogen halide?
A)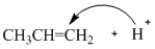
B)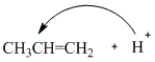
C)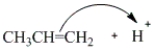
D) None of these are correct.
A)

B)

C)

D) None of these are correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following reactions involves the addition of different groups to the two ends of a double bond?
A) bromination
B) hydrogenation
C) both a and b
D) neither a nor b
A) bromination
B) hydrogenation
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
70
Markovnikov's rule is not useful for predicting the outcome of the reaction of hydrogen chloride with which of the following alkenes?
A) 1-butene
B) 2-butene
C) 2-methylpropene
D) 1-pentene
A) 1-butene
B) 2-butene
C) 2-methylpropene
D) 1-pentene

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following reactions involves the addition of two equivalent groups to the two ends of a double bond?
A) bromination
B) hydrogenation
C) both a and b
D) neither a nor b
A) bromination
B) hydrogenation
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the substance represented by the following model. 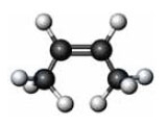 The physical properties of this substance would most closely represent the corresponding member of which functional group class?
The physical properties of this substance would most closely represent the corresponding member of which functional group class?
A) alkyne
B) alcohol
C) aldehyde
D) carboxylic acid
 The physical properties of this substance would most closely represent the corresponding member of which functional group class?
The physical properties of this substance would most closely represent the corresponding member of which functional group class?A) alkyne
B) alcohol
C) aldehyde
D) carboxylic acid

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following curved arrows correctly describes the second step in the reaction of propene with hydrogen bromide?
A)
B)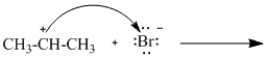
C)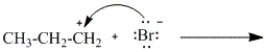
D)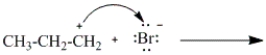
A)
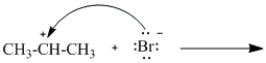
B)

C)
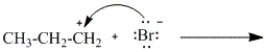
D)
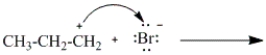

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
74
Markovnikov's rule is useful to predict the outcome of the reaction of hydrogen chloride with which of the following alkenes?
A) 1-butene
B) 2-butene
C) both a and b
D) neither a nor b
A) 1-butene
B) 2-butene
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following reactions involves the addition of different groups to the two ends of a double bond?
A) hydration
B) hydrogenation
C) both a and b
D) neither a nor b
A) hydration
B) hydrogenation
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following reactions involves the addition of different groups to the two ends of a double bond?
A) hydrogenation
B) hydrobromination
C) both a and b
D) neither a nor b
A) hydrogenation
B) hydrobromination
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the most characteristic reaction of alkenes?
A) addition
B) oxidation
C) reduction
D) substitution
A) addition
B) oxidation
C) reduction
D) substitution

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following reactions involves the addition of two equivalent groups to the two ends of a double bond?
A) hydration
B) hydrogenation
C) both a and b
D) neither a nor b
A) hydration
B) hydrogenation
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
79
What is the product of the reaction of the substance given below with hydrogen? 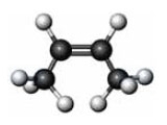
A) butyne
B) cis-butane
C) trans-butene
D) butane

A) butyne
B) cis-butane
C) trans-butene
D) butane

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following reactions involves the addition of two equivalent groups to the two ends of a double bond?
A) hydrogenation
B) hydrobromination
C) both a and b
D) neither a nor b
A) hydrogenation
B) hydrobromination
C) both a and b
D) neither a nor b

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck


